Study of Solvent and Catalyst in Diimide Biphasic Hydrogenation System of Natural Rubber
Corresponding email: m.chalid@ui.ac.id
Published at : 05 Feb 2024
Volume : IJtech
Vol 15, No 2 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i2.6683
Winarto, D.A., Liza, C., Fathurrohman, M.I., Masa, A., Chalid, M., 2024. Study of Solvent and Catalyst in Diimide Biphasic Hydrogenation System of Natural Rubber. International Journal of Technology. Volume 15(2), pp. 414-424
| Dody Andi Winarto | 1. Green Polymer Technology Laboratory, Department of Metallurgical & Materials Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, Depok 16424, West Java, Indonesia, 2. Badan |
| Chandra Liza | National Research and Innovation Agency, Building #460, Puspiptek Area, South Tangerang 15314, Banten, Indonesia |
| Mohamad Irfan Fathurrohman | Indonesian Rubber Research Institute, Jl. Salak no 1., Bogor 16151, West Java, Indonesia |
| Abdulhakim Masa | Rubber Engineering & Technology Program, International College, Prince of Songkla University, Hat Yai, Songkhla, 90110, Thailand |
| Mochamad Chalid | 1. Green Polymer Technology Laboratory, Department of Metallurgical & Materials Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, Depok 16424, West Java, Indonesia, 2. Cente |
In Indonesia, solid rubber is the most common form of natural rubber (NR)
intermediates because it is easy to store and transport. However,
hydrogenation, a process aimed at improving the quality of NR by addressing its
vulnerability to environmental factors due to the presence of carbon double
bonds, is typically carried out under relatively mild conditions using latex
material. This study explores a biphasic hydrogenation approach using solid NR
dissolved in a solvent and a hydrogen source (hydrazine hydrate and hydrogen
peroxide) mixed in water. The choice of solvents, catalysts, and the
water-to-solvent volume ratio were examined for their impact on hydrogenation.
Characterization was conducted using Fourier Transform Infrared (FTIR) spectroscopy
as a qualitative indicator of hydrogenation. Meanwhile, Nuclear Magnetic
Resonance 1H-NMR spectroscopy is used to measure the degree of
hydrogenation. The results indicate that partial hydrogenation was successful
using toluene as a solvent and ethylene diamine tetra acetic acid diammonium
copper (Cu-EDTA) as a catalyst, with variations in water volume influencing the
degree of hydrogenation. A degree of hydrogenation of 7.69% was achieved using
15 mL of water. The thermal properties of hydrogenated NR remain comparable to
the original material, with improved heat resistance. This biphasic
hydrogenation method offers the potential to enhance the properties of NR in
various applications.
Biphasic hydrogenation; Diimide; FTIR; Natural Rubber; NMR; Thermal analysis
Natural rubber plays a vital role in various aspects of human life, with applications in fields such as medical equipment and industry (Kim et al., 2020; Saengdee, Phinyocheep, and Daniel, 2020; Inoue and Nishio, 2007). Despite its significance, natural rubber contains carbon double bonds (Cifriadi, Chalid, and Puspitasari, 2017; Piya-areetham, Prasassarakich, and Rempel, 2013) that make it less resistant to oxidation, heat, ozone, and other environmental factors (Ngudsuntear et al., 2022). However, this limitation can be overcome by chemically modifying it, potentially making it a substitute for the thermally resistant Ethylene Propylene Diene Monomer (EPDM) rubber (Taksapattanakul et al., 2019; Inoue and Nishio, 2007).
Hydrogenation can
be employed to enhance the quality of natural rubber (Saengdee,
Phinyocheep, and Daniel, 2020; Phinyocheep, 2014; Winters et al., 2002). In this process, solid natural rubber is
dissolved in a solvent and then reacted with hydrogen gas, with the help of a
catalyst (Ha et al., 2016; Ha et al.,
2015; Mahittikul, Prasassarakich, Rempel, 2009; Inoue and Nishio, 2007). Notably, this process demands the use
of organic solvents and containers capable of withstanding high pressure,
necessitating strict safety precautions. Moreover, these processes involve
expensive catalysts and recycling challenges. While hydrogenation can be
achieved using aryl-sulfonyl hydrazide thermolysis at temperatures above 100°C,
it is accompanied by chain degradation and side reactions.
A milder method to
hydrogenate natural rubber is by utilizing latex under conditions of low
temperature and atmospheric pressure (Cifriadi,
Chalid, and Puspitasari, 2017; Veni and Ma'zam, 2010).
This process involves the use of hydrazine hydrate as a hydrogen donor in
combination with hydrogen peroxide. Several studies have proven the success of
this approach, although some side reactions may cause cross-linking before
vulcanization takes place (Cifriadi,
Chalid, and Puspitasari, 2017; Hinchiranan, Prasassarakich, and Rempel, 2006).
Although latex
offers certain advantages as a raw material, one of its main disadvantages lies
in the need for stabilization during transportation to the processing site to
avoid coagulation (Winarto et al., 2023).
This stabilization process adds complexity and cost to the overall production
process. Moreover, transporting latex poses a challenge as it requires a
substantial amount of water due to its bulkiness and weight, leading to
additional logistical issues and potential environmental concerns. In contrast,
solid natural rubber obtained by coagulating latex is the primary intermediate
product produced by the Indonesian rubber industry (Yuningtyas,
Hakim, and Novianti, 2019), presenting a more manageable and widely used
alternative.
The objective of this research is to utilize a biphasic hydrogenation method that involves dissolving solid natural rubber in a solvent. This method also includes the use of a hydrogen source, namely hydrazine hydrate and hydrogen peroxide, which is mixed in water. The goal is to take advantage of the abundance of solid rubber and enhance its quality. The success of the hydrogenation process was evaluated through Fourier Transform Infrared (FTIR) spectral analysis and Nuclear Magnetic Resonance (NMR) spectroscopy. Additionally, the thermal properties of the resulting products were analyzed using Differential scanning calorimetry (DSC) and Thermogravimetric analysis (TGA).
The natural rubber crepe was obtained from the Research Center for Rubber Technology, Indonesia. Toluene, xylene, and n-hexane were obtained from Mallinckrodt Chemical. Hydrazine hydrate (80% solution in water) for synthesis, ethylene diamine tetraacetic acid diammonium copper (Cu-EDTA) salt solution 0.025 M EDTA-Cu (NH4)2 CAS-No 67989-88-2 Supelco and copper (II) sulfate p.a. anhydrous were products of Sigma-Aldrich, and hydrogen peroxide 35% was products of Merck. Aquadest was provided by Polymer Laboratory BRIN, which was buffer-added so that its pH was 9.
2.2. Biphasic hydrogenation of natural rubber
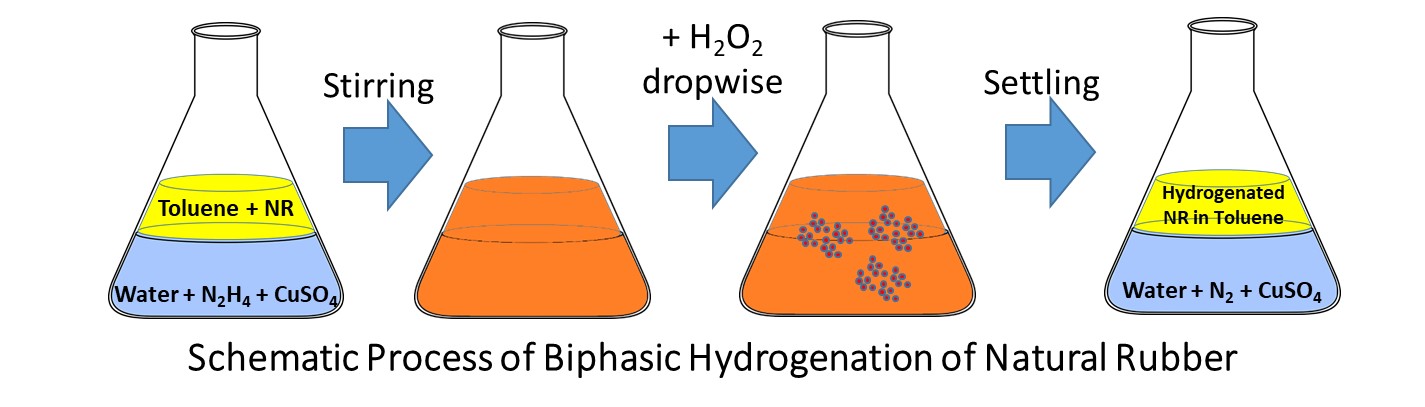
Natural rubber crepe (0.5 g) was dissolved in 50 mL of the solvent. In a separate container, hydrazine hydrate and copper sulfate were dissolved in 20 mL of aquadest at pH 9 and then poured into a container where natural rubber dissolved. They were mixed and stirred using a magnetic stirrer for 10 minutes at 500 rpm. Then, 2.914 mL hydrogen peroxide was put into the mixture drop by drop. The reaction was stirred for 2 hours and occurred under biphasic conditions, with a concentration ratio [C=C]:[N2H4/H2O2] of 1:12. The solution was left for more than 2 hours until the water and solvent were separated. The solution from the solvent side was taken out and put into a 250 mL boiling flask. The solvent was removed by rotary evaporation. The remaining material was characterized later. The scheme of the process is shown in Figure 1 below.
Figure 1 Schematic process of hydrogenation of natural rubber
In this work, the solvent volume used was fixed, at 50 mL. Several solvents, rubber contents, types of catalysts, and acidities of the water used were varied. The experimental conditions are listed in Table 1.
2.3. Characterization
2.3.1. Fourier Transform Infrared Spectroscopy
An analysis of the IR spectrum was performed using the Agilent Cary 630 FTIR Spectrometer, specifically employing the Attenuated Total Reflectance (ATR) method. The spectral range was from 4000 cm-1 to 650 cm-1, with sample scans 32 and a resolution of 4 cm-1.
2.3.2. Nuclear Magnetic Resonance Spectroscopy
1H-NMR and 13C-NMR spectroscopy was analyzed using JNM-ECZ500RlS 1. The sample was diluted in CDCl3. Measurements were performed at 500 MHz. The 1H-NMR was done with a repetition time of 6.75 seconds, 24 scans, and 13C-NMR was done with a repetition time of 2.83 seconds, 1044 scans.
2.3.3. Differential Scanning Calorimetry
Glass transition temperature Tg was performed using Perkin Elmer DSC8000. The sample was put into an aluminum crucible of about 10 milligrams. From RT, the sample was cooled to -100°C and then heated to 20°C at a heating/cooling rate of 10°C/min. Measurement was done in nitrogen condition (50mL/min).
2.3.4. Thermogravimetry Analysis
Thermogravimetry Analysis was performed using Netzsch TG 209 F3 Tarsus. The sample was put into an alumina crucible of about 20 milligrams. The temperature program was conducted from 30°C to 600 °C with a heating rate of 10°C/min. Measurement was done in nitrogen condition (50mL/min).
Natural rubber (NR) possesses a predominantly cis-1,4-polyisoprene molecular structure (Widiyati and Poernomo, 2018; Phinyocheep, 2014) as depicted in Figure 2. It contains a tri-substituted C=C bond with two CH2 substituents and one CH3 substituent (Smith, 2022). With this structure, NR has FTIR spectra, as shown in Figure 3 (a). It contains both methyl and methylene groups; hence, there are three peaks between 2840 and 3000 cm-1. The unsaturated C–H stretch of natural rubber is at 3022 cm-1, the C=C stretch is at 1664 cm-1, and the C–H wag is seen at 830 cm-1. As a note, through this paper, we use the code PHNR as partially hydrogenated natural rubber, and it is shown the spectral difference between NR and PHNR in Figure 3.
Figure 3 FTIR spectra of NR (a) and PHNR (b)
The chemical reactivity exhibited by the
carbon-carbon double bond (C=C) within the isoprene repeating unit may be
considered as C=C of alkenes. The hydrogenation reaction of natural rubber
using diimide as a hydrogen donor occurs according to equation (1), which is a
redox type of reaction (Taksapattanakul, 2016; Lin, 2005). R1HC=CHR2 denotes NR, and R1HC–CHR2 denotes
PHNR. It is expected that the C=C double bond will decrease, and the C–C bonds and CH2
substituents will increase. This is reflected in the FTIR spectra of PHNR as
depicted in Figure 3 (b), where the intensity of transmission of C=C stretch is
at 1654 cm-1, and C–H wag is at 834 cm-1 increase.
3.1. Solvent screening for hydrogenation NR
In the case of the presence of a C=C double
bond in natural rubber and metal ions as catalysts, reaction (3) predominates.
The use of this catalyst will increase the reaction for the formation of
diimide, and Cu2+ is a suitable ion for use in hydrogenation using
diimide (Mahittikul,
Prasassarakich, Rempel, 2007).
Unlike hydrogenation with the latex system
where copper ions are in 3 locations (water medium, polymer particle surface,
and inside of the particles) (Lin, 2005),
copper ions in the biphasic hydrogenation system exist solely within the water
medium, and will only interact with the polymer when stirring is initiated. If
this copper ion is only in the water medium, there will be no C=C reduction;
therefore, stirring is very important. The reaction was run at 500 rpm.
Figure 5 FTIR spectra of PHNR using (a) EDTA and (b) CuSO4
3.3. Solvent of catalyst used in the hydrogenation of NR
After analyzing the 1H-NMR spectra of hydrogenated natural rubber, we observed a decrease in intensity at the peak of 5.2 ppm. At the same time, a new peak appeared at 0.8 ppm, and there was a noticeable increase in signals at 1.1 ppm. These changes can be attributed to the presence of saturated –CH2 and –CH3 groups in the hydrogenated NR . The appearance of –CH2 was expected from the hydrogenation reaction and was also observed in the spectra FTIR in Figure 3 due to the reduction of C=C in NR. Based on these findings, the degree of hydrogenation resulting from the biphasic hydrogenation reaction of NR, with varying amounts of water used, is presented in Table 2. The degree of hydrogenation was calculated following the method presented by Taksapattanakul (Taksapattanakul, 2016).
Figure 6 FTIR spectra of NR (a) compared
with PHNR using water (b) 15 mL, (c) 20 mL, and (d) 25 mL
Figure 7 1H-NMR spectra of NR (a) compared with PHNR using water (b) 15 mL
Table 2 Degree of hydrogenation of PHNR
|
|
Degree of hydrogenation % |
|
NR |
0 |
|
PHNR using Water 15 mL |
7.69 |
|
PHNR using Water 20 mL |
6.25 |
|
PHNR using Water 25 mL |
5.96 |
3.4. Thermal Properties of PHNR
Figure 9 DSC thermogram of NR (a) compared with PHNR using water (b) 15 mL, (c) 20 mL, and (d) 25 mL
Figure 10 Thermogram of NR compared with PHNR using water 25 mL
Figure 11 Effect of water used in biphasic hydrogenation and Tg of the PHNR
Table 3 Thermal characteristics of hydrogenated NR
|
|
Tg (°C) |
Degradation temp (°C) |
|
NR |
-65.0 |
360.1 |
|
PHNR using Water 15 mL |
-64.4 |
362.5 |
|
PHNR using Water 20 mL |
-65.3 |
363.5 |
|
PHNR using Water 25 mL |
-64.9 |
371.4 |
The biphasic
hydrogenation of natural rubber using hydrazine hydrate and hydrogen peroxide
was successfully conducted. Hydrogenation was confirmed by 13C-NMR,
and the degree of hydrogenation was calculated using 1H-NMR. The
highest degree of hydrogenation of the product from the biphasic hydrogenation
was 7.69%, achieved using 15 mL of water. The glass transition temperature (Tg)
of the product was comparable to that of pre-hydrogenated natural rubber but
improves its heat resistance. Nevertheless, the attained highest degree of
hydrogenation (7.69%) may be considered relatively modest. Therefore, future
research of biphasic hydrogenation should focus on exploring alternative
reaction conditions to increase the degree of hydrogenation, as higher levels
might be preferable for specific applications.
We acknowledge the management of BRIN
for providing funding for this research through the "Degree by
Research" program, as per contract number KepKaLIPI No. 59/H/2020.
| Filename | Description |
|---|---|
| R1-MME-6683-20230925215508.docx | Figure 3. Spectra FTIR |
| R1-MME-6683-20230925215536.docx | Figure 4. Spectra FTIR |
| R1-MME-6683-20230925215608.docx | Figure 5. Spectra FTIR |
| R1-MME-6683-20230925215641.docx | Figure 6. Spectra FTIR |
Arnold, J.C., 2003. Environmental Effects on
Crack Growth in Polymers. In: Comprehensive Structural Integrity. Oxford:
Pergamon
Cifriadi, A., Chalid, M., Puspitasari, S., 2017.
Characterization of Hydrogenated Natural Rubber Synthesized by Diimide Transfer
Hydrogenation. International Journal of Technology, Volume 8(3), pp. 448–457
Dyson, P.J., Jessop, P.G., 2016. Solvent Effects
in Catalysis: Rational Improvements of Catalysts via Manipulation of Solvent Interactions.
Catalysis Science and Technology, Volume 6(10), pp. 3302–3316
Ha, N.T., Kaneda, K., Naitoh, Y., Fukuhara, L.,
Kosugi, K., Kawahara, S., 2015. Preparation and Graft-copolymerization of Hydrogenated
Natural Rubber in Latex Stage. Journal of Applied Polymer Science, Volume
132(34)
Ha, N.T., Kosugi, K., Kawahara, S., Nghia, P.,
2016. Mechanism of Heterogeneous Hydrogenation of Natural Rubber in Latex. KGK
Kautschuk Gummi Kunststoffe, Volume 69, pp. 71–76
Hansen, C.M., 2007. Hansen Solubility Parameters:
A User's Handbook, In: Chemical Rubber Company (CRC)
Hinchiranan, N., Prasassarakich, P., Rempel, G.,
2006. Hydrogenation of Natural Rubber in the Presence of OsHCl (CO)(O2)(PCy3)2
: Kinetics and Mechanism. Journal of Applied Polymer Science, Volume
100(6), pp. 4499–4514
Inoue, S.I., Nishio, T., 2007. Synthesis and
Properties of Hydrogenated Natural Rubber. Journal of Applied Polymer
Science, Volume 103(6), pp. 3957–3963
Kim, D.Y., Park, J.W., Lee, D.Y., Seo, K.H.,
2020. Correlation between the Crosslink Characteristics and Mechanical
Properties of Natural Rubber Compound via Accelerators and Reinforcement. Polymers,
Volume 12(9), p. 2020
Lin, X., 2005. Hydrogenation of unsaturated
polymers in latex form. Ph.D. Ph.D. Thesis, University of Waterloo
Mahittikul, A., Prasassarakich, P., Rempel, G.L.,
2006. Hydrogenation of Natural Rubber Latex in the Presence of
OsHCl(CO)(O2)(PCy3)2. Journal of Applied Polymer Science, Volume 100(1),
pp. 640–655
Mahittikul, A., Prasassarakich, P., Rempel, G.L.,
2007. Diimide Hydrogenation of Natural Rubber Latex. Journal of Applied
Polymer Science, Volume 105(3), pp. 1188–1199
Mahittikul, A., Prasassarakich, P., Rempel, G.L.,
2009. Hydrogenation of natural rubber latex in the presence of [Ir
(cod)(PCy3)(py)] PF6. Journal of Molecular Catalysis A: Chemical, Volume
297(2), pp. 135–141
Muhammad Adlim, O. M., Suhendrayatna
Suhendrayatna, Ismail Ismail, Noor Hana Hanif Abu Bakar. (2021). Photocatalytic
Degradation of Skim-Latex-Vapor Odor Using Iron-Doped Zinc Oxide. International
Journal of Technology, 12(4), 291-319. doi:https://doi.org/10.14716/ijtech.v12i4.4227
Ngudsuntear, K., Limtrakul, S., Vatanatham, T., Arayapranee,
W., 2022. Mechanical and Aging Properties of Hydrogenated Epoxidized Natural
Rubber and Its Lifetime Prediction. American Chemical Society (ACS) Omega,
Volume 7(41), pp. 36448–36456
Phinyocheep, P., 2014. Chemical Modification of Natural
Rubber (NR) for Improved Performance. Chemistry, Manufacture and Applications
of Natural Rubber, pp. 68–118
Phinyocheep, P., Pasiri, S., Tavichai, O., 2003.
Diimide Hydrogenation of Isoprene–Styrene Diblock Copolymers. Journal of
Applied Polymer Science, Volume 87, pp. 76–82
Piya-Areetham, P., Prasassarakich, P., Rempel,
G.L., 2013. Organic Solvent-free Hydrogenation of Natural Rubber Latex and Synthetic
Polyisoprene Emulsion Catalyzed by Water-soluble Rhodium Complexes. Journal
of Molecular Catalysis A: Chemical, Volume 372, pp. 151–159
Saengdee, L., Phinyocheep,
P., Daniel, P., 2020. Chemical Modification of Natural Rubber in Latex Stage
for Improved Thermal, Oil, Ozone and Mechanical Properties. Journal of
Polymer Research, Volume 27, pp. 1–13
Samran, J., 2005. A Study of Non-catalytic Hydrogenation
of Natural Rubber. Ph.D. Thesis, Universite Du Maine
Samran, J., Phinyocheep, P., Daniel, P., Derouet,
D., Buzare, J.Y., 2004. Spectroscopic Study of Di-imide Hydrogenation of Natural
Rubber. Macromolecular Symposia,
Wiley Online Library, Volume 216(1), pp. 131–144
Smith, B., 2022. The Infrared Spectra of Polymers
IV: Rubbers. Spectroscopy, Volume 37, pp. 8–12
Taksapattanakul, K., 2016. Thermoplastic
Vulcanizates Based on Hydrogenated Natural Rubber/Polypropylene Blends. Ph.D.
Thesis, Le Mans
Taksapattanakul, K., Tulyapitak, T., Phinyocheep,
P., Ruamcharoen, P., Ruamcharoen, J., Daniel, P., 2019. Hydrogenated Natural Rubber
as an Alternative Replacement to Ethylene-Propylene-Diene-Monomer (EPDM) Rubber
in Terms of Thermal-oxidative Degradation Properties. Polymer Science,
Series B, Volume 61, pp. 567–573
Veni, B., Ma'zam, M., 2010. Hydrogenated Natural Rubber
from Different Types of Preserved Latex. Journal of Rubber Research, Volume
13, pp. 103–109
Widiyati, C., Poernomo, H., 2018. Design of a
Prototype Photoreactor UV-LEDs for Radiation Vulcanization of Natural Rubber
Latex. International Journal of Technology, Volume 9(1), pp. 291–319
Winarto, D.A., Liza, C., Pemuji, A., Chalid, M.,
2023. Feasibility Study of Latex Stability for Free Solvent Hydrogenation to
Natural Rubbers. In: International
Conference on Chemical Science and Engineering, pp. 27–35
Winters, R., Heinen, W., Verbruggen, M.A.L.,
Lugtenburg, J., Van Duin, M., De-Groot, H.J.M., 2002. Solid-State 13C
NMR Study of Accelerated-Sulfur-Vulcanized 13C-Labeled ENB-EPDM. Macromolecules,
Volume 35, pp. 1958–1966
Yuningtyas, C.V., Hakim, D.B., Novianti, T.,
2019. Integration of the Indonesian Natural Rubber Market with the World
Market. Journal of Rubber Research, Volume 37(2), pp. 139–150
