Development of an Innovative Thermal Cracking of Mixed-Plastic Waste Without Catalyst as an Alternative Fuel Source
Corresponding email: janterps@unimed.ac.id
Published at : 18 Sep 2024
Volume : IJtech
Vol 15, No 5 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i5.6905
Simanjuntak, J.P., Zainon, M.Z., Zulkifli, N.W.M., Tambunan, B.H., Sihombing, J.L., Riduwan, 2024. Development of an Innovative Thermal Cracking of Mixed-Plastic Waste Without Catalyst as an Alternative Fuel Source. International Journal of Technology. Volume 15(5), pp. 1539-1549
| Janter P. Simanjuntak | Mechanical Engineering Department, State University of Medan, Jl. Willem Iskandar Pasar V Medan Estate, Medan 20221, North Sumatra, Indonesia |
| Mohd Zamri Zainon | Department of Mechanical Engineering, Faculty of Engineering, University of Malaya, 50603 Kuala Lumpur, Malaysia |
| Nurin Wahidah Bint Mohd Zulkifli | Department of Mechanical Engineering, Faculty of Engineering, University of Malaya, 50603 Kuala Lumpur, Malaysia |
| Bisrul H. Tambunan | Mechanical Engineering Department, State University of Medan, Jl. Willem Iskandar Pasar V Medan Estate, Medan 20221, North Sumatra, Indonesia |
| Junifa L. Sihombing | Department of Chemistry, Faculty of Mathematics and Natural Sciences, State University of Medan, Jl. Willem Iskandar Pasar V Medan Estate, Medan 20221, North Sumatra, Indonesia |
| Riduwan | Usaha Kecil Menengah, Program Kemitraan dan Bina Lingkungan PT. Kawasan Industri Medan, Medan 20371, North Sumatra, Indonesia |
The
growing consumption of plastic is a major contributor to the substantial
increase in waste, emphasizing the urgent need for the adoption of sustainable
and effective practices in management and resource reclamation. Therefore, this
study aimed to investigate the production of pyrolytic
distilled oil (PDO) from five types of mixed-plastic waste using
pyrolysis and distillation process without catalysts. Pyrolysis was conducted
at a temperature range of 300 to 450
with a fixed heating rate of 10
/min
for 60 minutes, while distillation was performed at 120
to 350
. The
distilled pyrolytic oil obtained was characterized using analytical techniques
with gas chromatography-mass spectrometry (GC-MS) and Carbon/Hydrogen/Nitrogen
(CHN) analyses to determine its chemical content and heating value. The effect
of pyrolysis and distillation temperature on product yield and hydrocarbon
content was also examined. The results showed that the predominant compounds
obtained were aromatic hydrocarbon groups, including styrene, benzene,
naphthalene, and xylene. Other compounds included non-aromatic hydrocarbon
groups, such as alkane and alkene. In addition, the chemical content was found
to be comparable to that of product obtained from the use of common pyrolysis
using catalysts. In this study, the
heating values observed were in the range of
30.835 – 33.004 MJ/kg. However, the heating value of the product
was still low and needed to be improved using another treatment. Comparison
with previous reports showed that temperature in this study was not significant
to the chemical content and heating value of the obtained distillate oil.
Low temperature; Non-catalyst; Plastic waste; Pyrolysis; Pyrolytic oil
Fossil fuels, such as coal, oil, and gas, are primary global energy sources that have been widely used for over a century. Despite the importance of these fuels, their extensive use has led to several detrimental effects, including climate change, air pollution, and resource depletion. Consequently, there is a pressing need to reduce their usage and support initiatives aiming for zero fossil fuels by 2050 (Holechek et al., 2022). These detrimental effects are likely to persist on Earth when decisive actions are not taken to mitigate human dependence. Climate change, caused by the greenhouse gases emitted from burning fossil fuel-derived energy, has significantly impacted the planet. The impacts include rising sea levels, more frequent and intense weather events, and increased extinction rates for plants and animals (Haines et al., 2006).
According to
previous studies, mitigating human reliance on fossil fuels necessitates a
transformative shift towards cleaner and more sustainable energy sources,
including wind, solar, and geothermal power (Owusu
and Asumadu-Sarkodie, 2016). This transition is expected to reduce
environmental impact and create new economic opportunities and jobs within the
renewable energy sector. Several studies have shown that continued dependence
on the use of fossil fuels is unsustainable and poses a significant threat to
Earth.
In line with
several reports, plastic waste has become a significant environmental concern
due to its widespread use, improper disposal, and slow degradation (Vriend et al., 2021). In addition, it
comprises discarded plastic, such as packaging, bags, bottles, straws, and
other single-use variants. The waste typically causes several environmental
problems, including land and water pollution, where it ends up in landfills,
taking centuries to degrade (Sari et al., 2022).
This condition often leads to its accumulation in landfills, contributing to
soil contamination and the release of harmful chemicals. The consequences
extend to marine pollution, where it accumulates in the oceans and water
bodies, posing a severe threat to marine life.
Compared to other countries, Indonesia
also needs a mix of energy sources to meet its future demands while reducing
its dependence on fossil fuels. This approach requires increasing the use of
renewable sources, improving energy efficiency, and exploring new technologies
to support a more sustainable system of producing liquid biofuel (Simanjuntak, Tambunan, and Sihombing, 2023; Simanjuntak et
al., 2022). In
Indonesia, two crucial sources of liquid fuel production are organic and
inorganic materials. Organic materials, typically sourced from plants or
biomass, serve as a highly environmentally friendly alternative and are often
referred to as green fuel.
A typical example of organic materials is palm oil, which serves as an
ideal and highly productive source of biofuel for internal combustion engines (Prihadiyono et al., 2022). Algae plants
also have the potential to serve as a renewable energy source due to their high
productivity and ability to grow in diverse environment (Sardi et al., 2022; Jamilatun et al., 2020). In
addition, inorganic
materials, such as plastic waste, have high productivity and can be used for
industrial and construction purposes. Various plastic materials can be
processed into liquid fuels with properties similar to diesel (Suhartono et al., 2023). The quality of
liquid fuels can be improved by mixing organic and inorganic materials (Kusrini et al., 2018). Plastic
has been reported to possess the potential to be used as building material,
thereby reducing concrete production costs. Previous reports showed that it
could also be used as aggregate substitute for cement in concrete. The
aggregate acts as filling materials that contribute to the structural strength
of concrete (Purnomo, Baskoro, and Muslim, 2023).
Plastic waste can be a potential source for
future energy mixes through the use of pyrolysis. This method comprises heating
the waste to high temperatures in the absence of oxygen, leading to the
breakdown of the constituent long polymer chains and the production of a liquid
fuel known as pyrolysis oil (Sharma et al., 2014).
The liquid product obtained can be used as a fuel substitute or blended with
bio-oil for internal combustion engines (Awang et
al., 2021), electricity generation, or feedstock for the production
of chemicals. Another effective method of using plastic waste is gasification (Ahmed and Gupta, 2010), which comprises heating
in the presence of oxygen and steam to produce a gas mixture called syngas.
This product can be burned for heat and electricity or processed into biofuels,
such as ethanol or methanol.
Based on
findings, pyrolysis and gasification have the potential to convert plastic into
useful energy sources while also reducing the amount of waste being accumulated
in landfills or the environment. However, there are also some challenges
associated with its use as an energy source. These include potential emissions
of harmful pollutants during the conversion process, the need for careful
handling to prevent contamination with other materials, and the cost of
building and operating the necessary infrastructure (Verma
et al., 2016).
According to
previous studies, pyrolysis is a promising solution for addressing the issue of
mixed-plastic waste. This process comprises the thermal decomposition of
organic materials in an oxygen-deprived environment, leading to the production
of valuable by-products, including fuel, gases, and char (Papari, Bamdad, and Berruti, 2021). Pyrolysis of mixed-plastic waste holds
significant potential as it can generate a valuable energy source, serving as a
sustainable alternative to fossil fuels. This transition not only mitigates
greenhouse gas emissions but also contributes to the advancement of the
circular economy (Siddiqui and Redhwi, 2009).
However, it presents inherent challenges, such as the substantial variability
in substrate composition and the imperative need for optimal operating
conditions to ensure the production of high-quality fuels (Kasar, Sharma, and Ahmaruzzaman, 2020; Al-Salem et
al., 2017). The
complexity of managing diverse waste streams necessitates comprehensive study
efforts to explore the technical feasibility, economic viability, and
environmental impact associated with the pyrolysis process.
Plastic
waste holds great promise in the petrochemical industry through the pyrolysis
process, leading to the release of several valuable industrial materials. In
this study, data from previous research by Soni et al., (2021) is
referenced, which demonstrates that plastic waste, categorized by its recycling
code, contains a range of chemical components.
The values
presented represent the proximate analysis of a single sample obtained from
different types. The high volatile fraction and low ash content showed that
co-pyrolysis process can contribute to optimal pyrolysis production, yielding a
high heating value for fuels.
The current trend in plastic pyrolysis studies focuses
on achieving high yields of products with high quality and low energy
consumption, collectively referred to as high efficiency. This efficiency can
be enhanced by using catalysts, which allow for lower operational temperatures (Peng et al., 2022). However, most studies
employing catalysts have been conducted on individual types of plastic. The use
of these materials presents challenges, such as feeding issues and difficulties
in process control. Despite the drawbacks, the primary benefit of using
catalysts is the higher production of bio-oil (Kim,
2004).
Previous reports have explored pyrolysis without catalysts, particularly when
processing single types of plastic. These reports have demonstrated the
possibility of achieving comparable yields at moderate temperatures (Miskolczi et al., 2004).
2.1. Materials
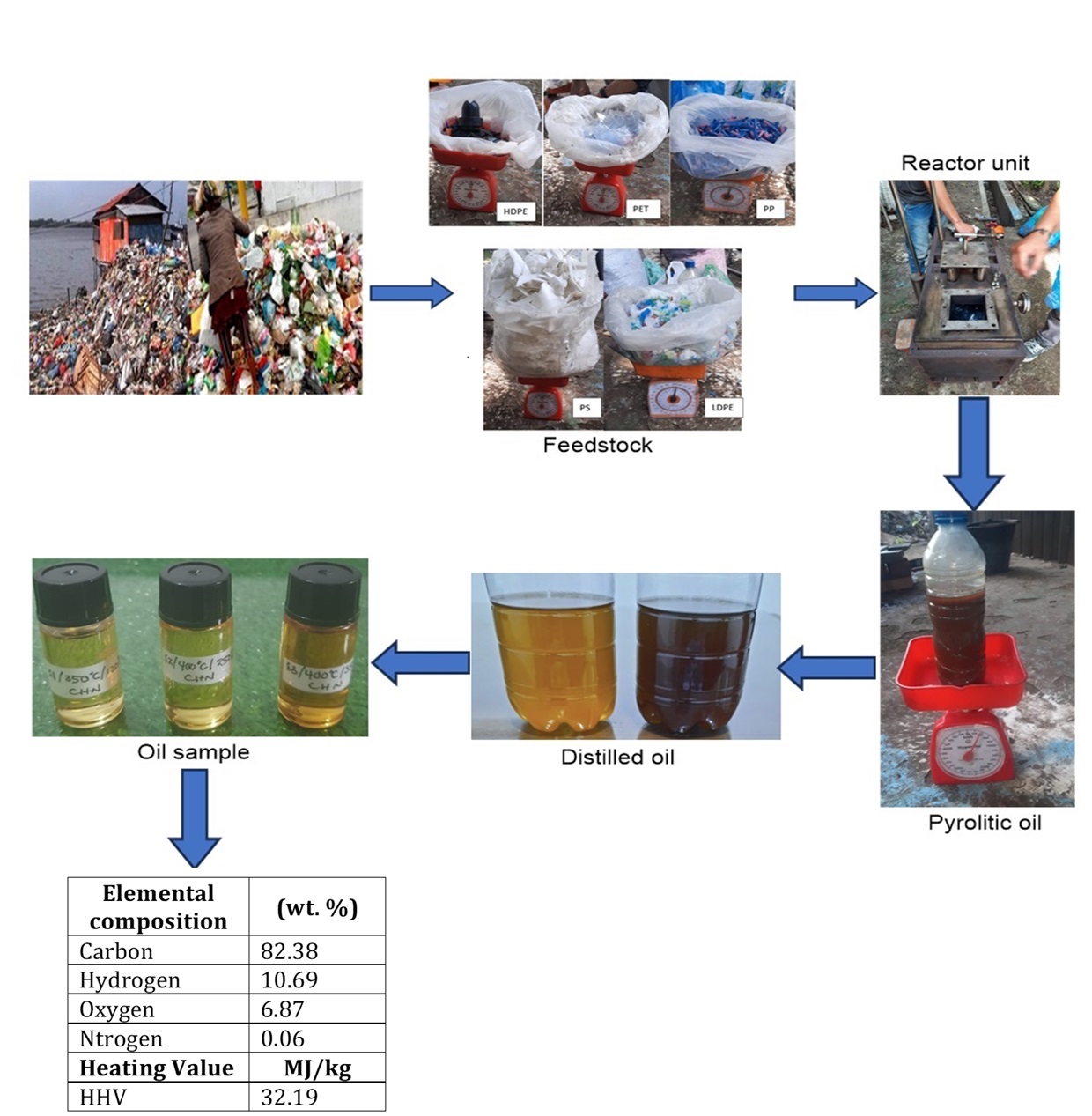
This study involved the utilization of
a mixed feedstock comprising five types of plastic waste. The plastic materials
were collected
from the trash bin around the study site, which was sorted, cleaned, and dried
before being put into the pyrolysis apparatus. The feedstock included polyethylene
terephthalate (PET), low-density polyethylene (LDPE), high-density polyethylene
(HDPE), polypropylene (PP), and polystyrene (PS) derived from mineral beverage water bottles, grocery plastic bags, detergent bottle caps, beverage pipettes, and food wrapping of Styrofoam
respectively. The feedstock was chopped manually to achieve a standardized size of
approximately 3 cm x 3 cm. The proximate analysis of the material used was
presented in Table 1.
Table 1 Proximate
Analysis of Plastic (%)
|
Plastics |
C |
H |
N |
S |
O |
Cl |
|
HDPE |
85.40 |
15.04 |
0.00 |
0.52 |
2.77 |
0.14 |
|
PVC |
38.53 |
5.04 |
0.013 |
0.176 |
0.00 |
56.25 |
|
PS |
92.59 |
8.13 |
0.00 |
0.00 |
2.24 |
0.00 |
|
LDPE |
77.60 |
21.55 |
0.00 |
0.003 |
0.00 |
0.00 |
|
PET |
77.00 |
13.00 |
0.20 |
n.a |
5.00 |
n.a |
2.2. Method
Approximately 200 grams of each type of
plastic was introduced into the pyrolysis reactor and heated to the desired
temperature without oxygen using an LPG combustor. During the heating process,
the mixed plastic was degraded into lighter molecules in the form of vapor.
After passing through the condenser, the vapor reached its saturation
temperature and condensed into liquid. Subsequently, a distillation process was
used to separate the liquids according to the density of molecules which
resulted in pyrolytic distilled oil (PDO), exhibiting properties similar to
fossil-based liquid fuels.
Operating temperature for this process
ranged from 300 to 450 , while the heating rate and residence
time were 10
and
60 minutes, respectively. The schematic of the process is presented in Figure
1. The procedure began with pyrolysis, followed by the cooling of the pyrolytic
vapor in the condenser until all vapor transformed into condensate. After pyrolysis process was
completed, the reactor was allowed to cool down and the condensate was
extracted from the condenser. In addition, distillation was conducted within the
same reactor, generating pyrolytic oil as the final product.
The type of distillate or pyrolytic oil
obtained was based on the operating temperature utilized during the process. A total of six samples were
prepared for characteristic testing using only CHN and GC-MS methods to analyze the
product yield in terms of chemical and molecule components. According to Ghodke (2021), there were six methods for characterizing
pyrolytic oil. However, in this study, the CHN-GCMS was selected due to the
cost-effectiveness and time consumed during the characterization process using
multiple methods.
Pyrolysis process comprised
the thermal decomposition of inorganic materials in the absence of oxygen.
During the process, the plastic feedstock was subjected to decomposition based
on the application of heat. The absence of oxygen prevented combustion, leading
to the formation of various products, including gases, liquids or PPO, and
solids. These products could then be further processed or refined for various
applications, such as fuel production, chemical synthesis, or waste management.
The equation (1) below shows a simplified representation of pyrolysis reaction.
Figure 1 Schematic pyrolysis system of five types of plastic waste
The
plastic pyrolytic oil (PPO) product, derived from pyrolysis of a mixture of
five types of plastic, occurred within a temperature range of 300 to 450 oC. The
procedure yielded predominantly liquid oil, constituting an intricate
amalgamation of hydrocarbons, along with a solid residue identified as char.
This char primarily consisted of mixed carbon, and the quantity generated was
dependent on the type of plastic and the specific pyrolysis conditions
employed. The yield of each product was intricately linked to several factors,
including the composition of the mixed plastic, the temperature range applied,
the heating rate, and various other parameters. In addition, the choice of the
pyrolysis reactor and the conditions under which the procedure was conducted
significantly influenced the final product outcomes.
In this study, the yield of PPO from the five
mixed plastics reached 45% by weight of the feedstock at a temperature of
approximately 400oC. This finding was consistent with the results of other
reports, who achieved a yield of up to 69% when pyrolyzing mixed plastic at
approximately 500oC (Genuino et al., 2022).
Figure 2 shows the appearance of pyrolytic distilled products (PDO), revealing
a strong dependence on the applied distillation temperature. A total of six
samples (S1-S6) each with a volume of 10 ml, and subjected to different
pyrolysis and distillation temperature, were tested. The specific conditions
for each sample were 1) S1 pyrolyzed at 350 oC and distilled at 120 oC,
2) S2 pyrolyzed at 400 oC and distilled at 250 oC, 3) S3
pyrolized at 400 oC and distilled at 350 oC, 4) S4
pyrolyzed at 300 oC without distillation from the gasoline-like
port, 5) S5 pyrolyzed at 300 oC without distillation from the
diesel-like port, and 6) S6 pyrolyzed at 380 oC and distilled at 180 oC. Lower distillation temperature (<200 oC) led to the production
of products with bright colors, while higher temperature (>200 oC) produced a
yellowish hue. This observation was in line with the findings of a previous
report (Wiriyaumpaiwong and Jamradloedluk, 2017).
3.1. Chemical characterization
of plastic distilled oil
The chemical characterization
of the plastic distilled oil was conducted at the National Study and Innovation
Agency, Indonesia (BRIN). The samples were subjected to total CHN
(carbon/hydrogen/nitrogen) and analyzed using an Exeter Analytical (Chelmsford,
MA) CE-440 Elemental Analyzer. Subsequently, the oxygen content was determined
through mass balance closure. Table 2 presented the elemental composition and
heating value of the PDO obtained from the five mixed types of plastic. Table 3
provided a comparison of the results with those from another study that used a
catalyst. The chemical content was found
to be comparable to the common pyrolysis processes using catalysts. However, it
was noteworthy that the heating value was lower compared to the report by Sharma et al. (2014). This disparity could be
attributed to the higher oxygen content in the PDO produced in this study.
Table 2 Elemental composition and heating value of PDO of five
mixed plastics (wt %)
|
Sample Name |
Carbon (%) |
Hydrogen (%) |
Nitrogen (%) |
Oxygen (%) |
HHV (MJ/kg) |
|
S1 |
85.633 |
10.540 |
0.019 |
3.808 |
33.004 |
|
85.171 |
10.385 |
0.077 |
4.367 |
32.768 | |
|
S2 |
80.302 |
10.123 |
0.061 |
9.514 |
31.221 |
|
79.029 |
10.081 |
0.041 |
10.849 |
30.835 | |
|
S3 |
82.951 |
11.521 |
0.052 |
5.476 |
32.903 |
|
83.325 |
11.545 |
0.054 |
5.076 |
33.025 | |
|
S4 |
82.190 |
10.032 |
0.078 |
7.700 |
31.692 |
|
82.133 |
10.244 |
0.060 |
7.563 |
31.818 | |
|
S5 |
81.278 |
10.680 |
0.057 |
7.985 |
31.869 |
|
81.607 |
10.785 |
0.067 |
7.541 |
32.032 | |
|
S6 |
83.266 |
11.328 |
0.061 |
5.345 |
32.863 |
|
81.719 |
11.071 |
0.043 |
7.167 |
32.255 | |
|
Average |
82.384 |
10.695 |
0.056 |
6.866 |
32.190 |
|
Carbon
(%) |
Hydrogen
(%) |
Nitrogen
(%) |
Oxygen
(%) |
HHV
(MJ/kg) |
Reference |
|
81.24 |
14.69 |
0.13 |
3.95 |
34.54 |
Quesada et al.
(2019) |
|
74.90 |
1.70 |
2.78 |
8.6 |
32.12 |
Santella et al. (2016) |
|
82.38 |
10.69 |
0.06 |
6.87 |
32.19 |
This work (Non-catalyst) |
3.2. Gas
chromatography-mass spectroscopy (GC–MS)
Figures 3 and 4 depicted the molecular
fraction successfully recorded by GC-MS for the six samples (S1–S6). The data
exhibited a consistent and relatively similar pattern, suggesting that the
impact of temperature on pyrolysis products was not highly significant. The
GC-MS analysis revealed two distinct hydrocarbon groups, which were aromatics,
including benzene, toluene, xylene, styrene, and naphthalene, and a
non-aromatic group comprising C1-C10 alkanes, C11-C20 alkanes, and C1-C10
alkenes. Table 4 provided comparative data on pyrolysis results with and
without the use of a catalyst. From Table 4 it was evident that the pyrolysis
process without a catalyst had a similar product of liquid hydrocarbon with
pyrolysis without a catalyst.
According to these data, the PDO
yields were predominantly composed of aromatic hydrocarbons. The GC–MS analysis
revealed that the pyrolysis liquid oils from the 6 samples mainly consisted of
aromatic hydrocarbons with a minor presence of aliphatic hydrocarbon compounds
similar to the findings of Miandad et al.
(2017). Specifically, styrene, derived from the cracking of polystyrene
(PS), was the dominant aromatic compound, which was consistent with the study
conducted by Shah and Jan (2015). The production of aromatics
from the pyrolysis of polyethylene (PE) could involve Diels-Alder reaction and
dehydrogenation mechanisms (Miandad et al., 2019).
This study identified a high percentage of BTX components (benzene, toluene,
xylene), styrene monomers, and other mono-aromatic compounds, highlighting
their potential application in the petrochemical industry. In addition, these
mono-aromatic compounds could be blended into the gasoline pool to enhance the
fuel's octane number (Jaafar et al., 2022).
Table 4 Comparison of the chemical composition of liquid products identified by GC–MS
|
Component name | |||||
|
Naphthalene |
Benzene |
Toluene |
Xylene |
Styrene |
References |
|
17.20 |
28.8 |
28.80 |
n.a |
n.a |
Aisien,
Otuya, and Aisien (2021) *) |
|
0.21 |
1.10 |
1.49 |
n.a |
64.31 |
Shah
and Jan (2015) *) |
|
0.63 |
4.00 |
15.30 |
8.80 |
24.50 |
Miandad
et al. (2017) *) |
|
2.90 |
4.50 |
24.00 |
3.40 |
54.0 |
Onwudili,
Insura, and Williams, (2009) **) |
|
2.5 |
5.28 |
10 |
1.75 |
30 |
This work**) |
*) Catalyst
*) Non-catalyst
For polystyrene, the
raw material’s highly aromatic nature resulted in the predominant production of
aromatic products during pyrolysis. Conversely, for PET, LDPE, and HDPE, the
generated oil mainly comprised aliphatic hydrocarbons, including alkanes and
alkenes (Budsaereechai, Hunt, and Ngernyen, 2019).
The hydrocarbon groups ranging from C4–C11 represented light fraction petroleum
fuels, while carbon numbers from C12–C20 indicated medium fraction diesel
fuels. The thermal degradation of polyalkene mixed plastics occurred through
random cutting, producing a diverse range of hydrocarbon fragments with varying
carbon atom numbers. The weakest C-C bonds in the polyalkene structure
underwent random cutting reactions during degradation, leading to the formation
of carbon double bonds (C=C) in the resulting structure. Consequently, the
pyrolysis oil obtained showed a notable concentration of alkenes. This thermal
degradation process, induced by random cutting reactions, resulted in the
formation of a diverse array of hydrocarbon species. However, due to the
presence of CH3 side chains in the PET structure, several
hydrocarbons were also formed alongside those observed in the pyrolysis of
other polyalkenes (Siddiqui and Redhwi, 2009)."
The pyrolysis oil content, dominated by aromatic monobenzene, as well as non-aromatic alkanes and C1-C10 alkenes, indicated that plastic samples had undergone a cracked process to produce short-chain hydrocarbons. Furthermore, these compounds also produced content, such as Benzoic acid, n-Pentadecanol, 1-Tetradecanol, and some >C21 components, including Behenic alcohol, and Hentriacontane (Carbonic acid, decyl undecyl ester). The heavy oil content of compounds with >C21 was primarily obtained from the degradation of HDPE, which was mostly found in sample S6.
In
conclusion, valuable industrial products could be obtained from five mixed
plastic waste through pyrolysis and distillation processes without a catalyst.
This waste included polyethylene terephthalate (PET), low-density polyethylene
(LDPE), high-density polyethylene (HDPE), polypropylene (PP), and polystyrene
(PS) from mineral water bottles, grocery bags, detergent bottle caps, beverage
pipette, and Styrofoam food wrapping respectively. The chemical content was
found to be comparable to the common pyrolysis utilizing a catalyst. The
heating values achieved ranged from 30.835 to 33.004 MJ/kg. However, this
heating value was relatively low and required improvement through the
oxygenation process or blending with biodiesel, kerosene, and other fuels with higher
heating values.
The authors
are grateful tp the Directorate General of Higher Education, Ministry of
Education, for financial support, which was facilitated by the Institute for
Study and Community Service Universitas Negeri Medan, Directorate of Study,
Technology, and Community Service, Report, Innovation, and Culture, under
contract number SP DIPA No. 023.17.1.690523/2023 4th revision, March
31, 2023. In addition, the authors are grateful to 2 final-year diploma students,
and workshop employees for their valuable contribution to this study.
| Filename | Description |
|---|---|
| R2-CE-6905-20240917141713.pdf | --- |
Ahmed, I., Gupta, A., 2010. Chemical Energy
Recovery from Polystyrene Using Pyrolysis and Gasification. In: 48th
AIAA Aerospace Sciences Meeting Including the New Horizons Forum and Aerospace
Exposition, p. 804
Aisien, E.T., Otuya, I.C., Aisien, F.A., 2021. Thermal and Catalytic
Pyrolysis of Waste Polypropylene Plastic Using Spent FCC Catalyst. Environmental
Technology & Innovation, Volume 22, p. 101455
Al-Salem, S.M., Antelava, A., Constantinou, A.,
Manos, G., Dutta, A., 2017. A Review on Thermal and Catalytic Pyrolysis of
Plastic Solid Waste (PSW). Journal of Environmental Management, Volume
197, pp. 177–198
Awang, M.S.N., Mohd Zulkifli, N.W., Abbas, M.M.,
Amzar Zulkifli, S., Kalam, M.A., Ahmad, M.H., Mohd Yusoff, M.N.A., Mazlan, M.,
Daud, W.M.A.W., 2021. Effect of Addition of Palm Oil Biodiesel in Waste Plastic
Oil on Diesel Engine Performance, Emission, and Lubricity. ACS Omega,
Volume 6(33), pp. 21655–21675
Budsaereechai, S., Hunt, A.J., Ngernyen, Y., 2019.
Catalytic Pyrolysis of Plastic Waste for the Production of Liquid Fuels for
Engines. RSC Advances, Volume 9(10), pp. 5844–5857
Genuino, H.C., Ruiz, M.P., Heeres, H.J., Kersten,
S.R.A., 2022. Pyrolysis of Mixed Plastic Waste (DKR-350): Effect of Washing
Pre-treatment and Fate of Chlorine. Fuel Processing Technology, Volume
233, p. 107304
Ghodke, P.K., 2021. High-Quality Hydrocarbon Fuel
Production from Municipal Mixed Plastic Waste Using a Locally Available
Low-Cost Catalyst. Fuel Communications, Volume 8, p.100022
Haines, A., Kovats, R.S., Campbell-Lendrum, D.,
Corvalán, C., 2006. Climate Change and Human Health: Impacts, Vulnerability,
and Mitigation. The Lancet, Volume 367(9528), pp. 2101–2109
Holechek, J.L., Geli, H.M., Sawalhah, M.N., Valdez,
R., 2022. A Global Assessment: Can Renewable Energy Replace Fossil Fuels by
2050? Sustainability, Volume 14(8), p. 4792
Jaafar, Y., Abdelouahed, L., El Hage, R., El
Samrani, A., Taouk, B., 2022. Pyrolysis of Common Plastics and Their Mixtures
to Produce Valuable Petroleum-like Products. Polymer Degradation and
Stability, Volume 195, p. 109770
Jamilatun, S., Budhijanto, Rochmadi, Yuliestyan,
A., Aziz, M., Hayashi, J., Budiman, A., 2020. Catalytic Pyrolysis of Spirulina
platensis Residue (SPR): Thermochemical Behavior and Kinetics. International
Journal of Technology, Volume 11(3), pp. 522–531
Kasar, P., Sharma, D.K., Ahmaruzzaman, M., 2020.
Thermal and Catalytic Decomposition of Waste Plastics and Its Co-processing
with Petroleum Residue Through Pyrolysis Process. Journal of Cleaner
Production, Volume 265, p. 121639
Kim, J.S., 2004. Pyrolysis of Plastic Wastes Using
the Non-Catalytic Hamburg-Process and a Catalytic Process Using the
Cycled-Spheres-Reactor. Environmental Engineering Research, Volume 9,
pp. 31–37
Kusrini, E., Supramono, D., Degirmenci, V.,
Pranata, S., Bawono, A.A., Ani, F.N., 2018. Improving the Quality of Pyrolysis
Oil from Co-firing High-density Polyethylene Plastic Waste and Palm Empty Fruit
Bunches. International Journal of Technology, Volume 9(7), pp. 1498–1508
Miandad, R., Barakat, M.A., Rehan, M., Aburiazaiza,
A.S., Ismail, I.M.I. Nizami, A.S., 2017. Plastic Waste to Liquid Oil Through
Catalytic Pyrolysis Using Natural and Synthetic Zeolite Catalysts. Waste
Management, Volume 69, pp. 66–78
Miandad, R., Rehan, M., Barakat, M.A., Aburiazaiza,
A.S., Khan, H., Ismail, I.M., Dhavamani, J., Gardy, J., Hassanpour, A., Nizami,
A.S., 2019. Catalytic Pyrolysis of Plastic Waste: Moving Toward Pyrolysis Based
Biorefineries. Frontiers in Energy Research, Volume 7, p. 27
Miskolczi, N., Bartha, L., Deak, G., Jover, B.,
2004. Thermal Degradation of Municipal Plastic Waste for Production of
Fuel-like Hydrocarbons. Polymer Degradation and Stability, Volume 86(2),
pp. 357–366
Onwudili, J.A., Insura, N., Williams, P.T., 2009.
Composition of Products from The Pyrolysis of Polyethylene and Polystyrene in a
Closed Batch Reactor: Effects of Temperature and Residence Time. Journal of
Analytical and Applied Pyrolysis, Volume 86(2), pp. 293–303
Owusu, P.A., Asumadu-Sarkodie, S., 2016. A Review
of Renewable Energy Sources, Sustainability Issues and Climate Change
Mitigation. Cogent Engineering, Volume 3(1), p. 1167990
Papari, S., Bamdad, H., Berruti, F., 2021.
Pyrolytic Conversion of Plastic Waste to Value-added Products and Fuels: A
review. Materials, Volume 14(10), p. 2586
Peng, Y., Wang, Y., Ke, L., Dai, L., Wu, Q., Cobb,
K., Zeng, Y., Zou, R., Liu, Y., Ruan, R., 2022. A Review on Catalytic Pyrolysis
of Plastic Waste to High-value Products. Energy Conversion and Management,
Volume 254, p. 115243
Prihadiyono, F.I., Lestari, W.W., Putra, R., Aqna,
A.N.L., Cahyani, I.S., Kadja, G.T.M., 2022. Heterogeneous Catalyst based on
Nickel Modified into Indonesian Natural Zeolite in Green Diesel Production from
Crude Palm Oil. International Journal of Technology, Volume 13(4), pp.
931–943
Purnomo, H., Baskoro, H., Muslim, F., 2021. Stress
and Strain Behavior of Confined Lightweight Concrete using Sand Coated
Polypropylene Coarse Aggregate. International Journal of Technology,
Volume 12, pp. 1261–1272
Quesada, L., Calero, M., Martin-Lara, M.A., Perez,
A., Blazquez, G., 2019. Characterization of Fuel Produced by Pyrolysis of
Plastic Film Obtained of Municipal Solid Waste. Energy, Volume 186,
p.115874
Santella, C., Cafiero, L., De Angelis, D., La
Marca, F., Tuffi, R., Ciprioti, S.V., 2016. Thermal and Catalytic Pyrolysis of
A Mixture of Plastics from Small Waste Electrical and Electronic Equipment
(WEEE). Waste Management, Volume 54, pp. 143–152
Sardi, B., Ningrum, R.F., Ardianyah, V.A.,
Qadariyah, L., Mahfud, M., 2022. Production of Liquid Biofuels from Microalgae
Chlorella sp. via Catalytic Slow Pyrolysis. International Journal of
Technology, Volume 13(1), pp. 147–156
Sari, M.M., Andarani, P., Notodarmojo, S., Harryes,
R.K., Nguyen, M.N., Yokota, K., Inoue, T., 2022. Plastic Pollution in the
Surface Water in Jakarta, Indonesia. Marine Pollution Bulletin, Volume
182, p. 114023
Shah, J., Jan, M.R., 2015. Effect of Polyethylene
Terephthalate on The Catalytic Pyrolysis of Polystyrene: Investigation of The
Liquid Products. Journal of the Taiwan Institute of Chemical Engineers,
Volume 51, pp. 96–102
Sharma, B.K., Moser, B.R., Vermillion, K.E., Doll,
K.M., Rajagopalan, N., 2014. Production, Characterization and Fuel Properties
of Alternative Diesel Fuel from Pyrolysis of Waste Plastic Grocery Bags. Fuel
Processing Technology, Volume 122, pp. 79–90
Siddiqui, M.N., Redhwi, H.H., 2009. Pyrolysis of
Mixed Plastics for the Recovery of Useful Products. Fuel Processing
Technology, Volume 90(4), pp. 545–552
Simanjuntak, J.P., Al-attab, K.A., Daryanto, E.,
Tambunan, B.H., 2022. Bioenergy as an Alternative Energy Source: Progress and
Development to Meet the Energy Mix in Indonesia. Journal of Advanced
Research in Fluid Mechanics and Thermal Sciences, Volume 97, pp. 85–104
Simanjuntak, J.P., Tambunan, B.H., Sihombing, J.L.,
2023. Potential of Pyrolytic Oil from Plastic Waste as an Alternative Fuel
Through Thermal Cracking in Indonesia: A Mini Review to Fill the Gap of the
Future Research. Journal of Advanced Research in Fluid Mechanics and Thermal
Sciences, Volume 102(2), pp. 196–207
Soni, V.K., Singh, G., Vijayan, B.K., Chopra, A.,
Kapur, G.S., Ramakumar, S.S.V., 2021. Thermochemical Recycling of Waste
Plastics by Pyrolysis: A Review. Energy & Fuels, Volume 35(16), pp.
12763–12808
Suhartono, Romli, A., Hari Prabowo, B., Kusumo, P.,
Suharto, 2023. Converting Styrofoam Waste into Fuel Using a Sequential Pyrolysis
Reactor and Natural Zeolite Catalytic Reformer. International Journal of
Technology, Volume 14(1), pp. 185–194
Verma, R., Vinoda, K.S., Papireddy, M., Gowda,
A.N.S., 2016. Toxic Pollutants from Plastic Waste-A Review. Procedia
Environmental Sciences, Volume 35, pp. 701–708
Vriend, P., Hidayat, H., van Leeuwen, J., Cordova,
M.R., Purba, N.P., Lohr, A.J., Faizal, I., Ningsih, N.S., Agustina, K., Husrin,
S., Suryono, D.D., 2021. Plastic Pollution Research in Indonesia: State of
Science and Future Research Directions to Reduce Impacts. Frontiers in
Environmental Science, Volume 9, p. 187
Wiriyaumpaiwong, S.,
Jamradloedluk, J., 2017. Distillation of Pyrolytic Oil Obtained from Fast
Pyrolysis of Plastic Wastes. Energy Procedia, Volume 138, pp. 111–115
