Performance Comparison of Heterogeneous Catalysts based on Natural Bangka Kaolin for Biodiesel Production by Acid and Base Activation Processes
Corresponding email: eny.k@ui.ac.id
Published at : 24 Dec 2024
Volume : IJtech
Vol 15, No 6 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i6.7023
Kusrini, E., Rifqi, A., Usman, A., Wilson, L., Degirmenci, V., Aidil Adhha Abdullah, M., Sofyan, N., 2024. Performance Comparison of Heterogeneous Catalysts based on Natural Bangka Kaolin for Biodiesel Production by Acid and Base Activation Processes. International Journal of Technology. Volume 15(6), pp. 1994-2008
| Eny Kusrini | 1. Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus Baru UI, Depok 16424, Indonesia 2. Green Product and Fine Chemical Engineering Research Group, Laboratory |
| Abi Rifqi | Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus Baru UI, Depok 16424, Indonesia |
| Anwar Usman | Department of Chemistry, Faculty of Science, Universiti Brunei Darussalam, Jalan Tungku Link, Gadong BE1410, Negara Brunei Darussalam |
| Lee Wilson | Department of Chemistry, University of Saskatchewan 110 Science Place, Room 156 Thorvaldson Building, Saskatoon, SK S7N 5C9, Canada |
| Volkan Degirmenci | School of Engineering, University of Warwick, Library Road, CV4 7AL, Coventry, UK |
| Mohd Aidil Adhha Abdullah | Chemical Science Program, Faculty of Science and Marine Environment, Universiti Malaysia Terengganu, 21030 Kuala Nerus, Terengganu, Malaysia |
| Nofrijon Sofyan | Department of Metallurgical and Materials Engineering, Universitas Indonesia, Kampus Baru UI, Depok 16424, Indonesia |
This study is aimed to evaluate e?ciency low-cost natural kaolin from Bangka island as a catalyst to produce biodiesel (fatty acid methyl ester, FAME) via the transesterification reaction using the cooking oil as a model of free fatty acids (FFA) source. Heterogeneous catalysts of the natural kaolin were prepared by an activation process using base (1-2 M NaOH) and acid (1 M HCl) solution. The base and acid activated kaolin are labelled as Kb1M, Kb2M, and Ka1M, respectively. The quality of biodiesel was analyzed according to the SNI 04-7182-2015 method, the American Society for Testing and Materials (ASTM) D 6751, and the Europäische Norm (EN) 14214, while the composition of biodiesel was determined using gas chromatography-mass spectrometry (GC-MS) analysis. Among the activated kaolin, the Kb2M showed the best heterogenous catalyst performance, producing 96.3% methyl ester with a yield of 69.4%. The highest FAME conversion was achieved using the Kb1M catalyst at 79.1% with a mole ratio of cooking oil to methanol being 1:3, whereas the lowest FAME conversion, 72.0%, was obtained using the Kb2M catalyst with a mole ratio of cooking oil to methanol being 1:6. Overall, the Kb2M showed the best efficient catalyst, while the Ka1M showed the lowest catalytic performance.
Acid; Base; Biodiesel; Heterogenous Catalyst; Kaolin
Exploring new catalyst materials to meet the growing demand for renewable and fossil energy has become increasingly important for energy production. Catalysts play a crucial role in reducing activation energy and shifting the position of chemical equilibrium, allowing reactions to reach completion and produce the desired product. For the case of biodiesel production, homogeneous base catalysts such as sodium hydroxide (NaOH) or potassium hydroxide (KOH), and also alkoxide solutions are employed. However, homogeneous catalysts are reported to affect corrosion in the reactor, and also present challenges for catalyst recycling (Yang et al., 2017). On the other hand, heterogeneous catalysts have also been explored to reduce the cost of biodiesel production (Carmo-Jr et al., 2009).
Heterogeneous
catalysts possess different phases between reactants and products. These
catalysts have various advantages such as being environmentally compatible,
non-corrosive, easy to separate from reactants, and facile to regenerate (Guan et al., 2009). It is noted that
heterogeneous catalysts can promote the transesterification of triglycerides
for biodiesel production (Aziz et al., 2017;
Yan et al., 2010). Efforts to reduce the cost production of
biodiesel rely on the availability of abundant and cheap raw material
catalysts, sources of free fatty acid, proper reactor design, method, and type
of reaction process (transesterification or esterification).
Natural
kaolin occurs over several regions of Indonesia, such as West Kalimantan, South
Kalimantan, Bangka Belitung, Sulawesi, and Java with a total deposit of
approximately 66.21 million tons (Subari, Wenas and
Suripto, 2008). Natural Bangka kaolin has been used as an adsorbent for
the adsorptive removal of antibiotic rifampicin from an aqueous solution (Majid et al., 2023). On the other hand,
the application of Indonesian kaolin from Bangka has also been reported as an
adsorbent for adsorption of negatively charged acid blue 25 and acid 1 (Asbollah et al., 2022). Natural kaolin has
also been reported as a catalyst for transesterification reactions (Ali et al., 2018; ??ng,
Chen, and Lee, 2017). With its high
surface area, porosity, and composition, along with its low cost, the natural
kaolin highlights its potential as an effective raw catalyst material. For
instance, graphene oxide (GO) enriched natural kaolinite clay as catalyst for
biodiesel production has been reported by Syukri et
al. (2020). GO is a
single atomic layer of graphite oxide that has a high specific surface area
with a complex mixture of oxygen at the edges and basal planes (Kusrini et al., 2020a; Nasrollahzadeh et al.,
2014). GO has a Bronsted
acid side which is important for esterifying the free fatty acid (FFA) content
in oil (Atadashi et al., 2013).
It was reported that metal
oxide/GO composites increased the mechanical strength of heterogeneous
catalysts (Marso et al., 2017).
To
increase the quality and ability of natural Bangka kaolin as a heterogeneous
catalyst for biodiesel production, activation of kaolin using base and acid
treatments is needed to enhance the active sites of the natural kaolin. This
process is able to enlarge its surface area and remove impurities on the
surface of natural kaolin. Base treatment for the activation can improve
crystallinity of the natural kaolin (Belver, Bañares-Muñoz,
and Vicente, 2002). The catalyst that was activated using base treatment
showed 4,000 times faster than those found for the acid catalyst under a
similar amount of catalyst for the transesterification reaction (Fukuda, Kondo, and Noda, 2001). New catalyst
materials for energy production and/or other applications have attracted many
researchers to address the need for clean energy (Arnas,
Whulanza, and Kusrini, 2024; Whulanza & Kusrini, 2024).
Biodiesel
is an alkyl ester with other non-toxic compounds. It is a mixture of long-chain
fatty acid methyl esters (FAMEs) or ethyl esters (FAEEs). Biodiesel can be
produced by either the transesterification of animal fats, vegetable oils, or
used cooking oil or esterification of free fatty acids (FFAs) (Dang, Chen and Lee, 2013) in the present of
alcohol compound such as methanol (CH3OH) or ethanol (C2H5OH).
Usually, production of biodiesel through esterification and/or
transesterification reactions that are assisted by an acid or base catalyst (Maneerung et al., 2016). Biodiesel can be
obtained by a transesterification reaction, whereas the green diesel as the second
generation of diesel can be produced through hydrodeoxygenation reaction (Aisyah et al., 2023). Apart from containing esters,
vegetable oils and animal fats also contain small amounts of FFA. The presence
of FFA in the transesterification reaction with an alkali catalyst needs to be
considered. The maximum FFA content in vegetable oil when using a base catalyst
is approximately 3%, if it exceeds this level the reaction cannot occur, as reported
by Atadashi et al. (2013). This is because free fatty acids will
react with an alkali catalyst to form soap. Thus, before the
transesterification reaction was carried out, the oil must be first pretreated
to reduce the FFA content. After the transesterification reaction was complete,
two products will be obtained, namely biodiesel (methyl ester) and glycerol.
Although
sources of biodiesel can be vegetable oils such as palm oil, coconut oil, corn
oil, soybean oil, sunflower seed oil, and rapeseed oil, non-edible oils such as
jatropha curcas, pongamina pinnata, sea mango, palanga, and/or tallow oil are
more preferred (Leung, Wu and Leung, 2010). Many countries use vegetable oils as
the main ingredient for making biodiesel because the properties of the
biodiesel produced are close to those of diesel fuel (Gui et al., 2008). Biodiesel has
advantages compared to diesel fuel from petroleum. The advantages of biodiesel
are an environmentally friendly fuel because it produces much better emissions
(free sulfur and smoke number), and higher cetane number (>50). Thus, the
combustion efficiency of biodiesel is better than that of crude oil, displays
lubricating properties for engine pistons, and improves vehicle life, safe
storage, and transport, including its non-toxic and biodegradable properties (Balat and Balat, 2010). Biodiesel is an ideal
fuel for the transportation industry because it can be used in various diesel
engines, including agricultural machines.
Thus, to
observe the potential of Bangka kaolin as a heterogeneous catalyst for
biodiesel production, this natural kaolin was activated using a base solution (1–2
M NaOH) and in acid (1 M HCl) media. Both heterogeneous catalysts were
evaluated and tested for biodiesel production, where cooking oil was used as a
source of free fatty acid (FFA) for biodiesel production. Kaolin was chosen as
the model clay for the development of the e?cacious heterogeneous catalyst in
production of biodiesel through the catalyzed transesterification reaction.
2.1.
Materials
Natural
kaolin was originated from Bangka, Belitung Island, Indonesia. Palm cooking oil
is a commercial product containing FFA <2%. NaOH and HCl were purchased from
Merck (Germany). All the chemicals were used without any further purification.
2.1.1. Pretreatment of natural kaolin
50 g of Bangka
natural kaolin (150 mesh) was mixed with 400 mL of distilled water and stirred
using a magnetic stirrer until the mixture became homogeneous. The natural
kaolin was then separated from this mixture using the centrifugation technique.
The clean natural kaolin was dried in an oven at 105°C for 2 h. Furthermore, a
dried natural kaolin was calcined at 500°C for 6 h to form a metakaolin. Then,
a metakaolin was used to produce a heterogeneous catalyst in subsection 2.3.
2.2. Activated heterogeneous catalysts using base and acid
treatments
Each sample of
metakaolin (3.7 g) was mixed with at 1 M or 2 M NaOH solution, along with
magnetic stirring at 500 rpm for 6 h at room temperature. Then, the base
metakaolin obtained by using 1 M and 2 M NaOH in the respective order were
separated from the NaOH solution using a centrifugation process for 10 minutes
at a speed of 2500 rpm. The solid base metakaolin was washed using distilled
water, and it was dried in an oven at 105°C for 2 h. Each base metakaolin
product was calcined in the furnace at 500°C for 6 h. Finally, both
heterogeneous catalysts were produced and named as follows: base-activated
kaolin using 1 M NaOH (Kb1M) and base-activated kaolin 2 M NaOH (Kb2M). A
similar procedure was used to produce acid-activated kaolin using 1M HCl solution,
which was named as acid-activated kaolin 1 M (Ka1M).
2.3. Performance test of heterogeneous catalysts for
biodiesel production
The palm cooking oil
was used as a model source of oil. This oil was heated at 65°C. A mixture of
methanol and catalyst with a ratio of 1:3 was added into the heated cooking oil
and stirred using magnetic stirring at 500 rpm and 60°C for 60 minutes. The
ratio of catalyst to cooking oil is 3 wt.%. Then, the mixture was put in the
separated funnel and kept for 6 h until 2 layers formed, where the biodiesel
(fatty acid methyl ester, FAME) product was obtained. FAME was washed using a
warm water until the color of the water was clean. A product of biodiesel was
heated at 110°C to remove the remaining water. Finally, the biodiesel product
was kept for further characterization according to the SNI 04-7182-2015 method.
2.4. Characterizations
where L is crystallite size, is
wavelength of diffraction light (0.15406 nm),
full width at half maximum
(FWHM) in radians, K is constant (0.89), and
is diffraction angle.
The results of the
transesterification reaction, namely FAME were analyzed using GCMS. The quality of FAME was
determined using the SNI quality standards 04-7182-2015 method, the American
Society for Testing and Materials (ASTM) D 6751, and the Europaische Norm (EN)
14214, including density, viscosity, and FAME %. The FAME yield was calculated
using an equation that was reported by Soetaredjo et
al. (2011). FAME % yield and % conversion based on the GC-MS
analysis were also calculated using Equations 2 and 3;
3.1. Preparation of kaolin to metakaolin
Pre-treatment of kaolin
aims to reduce impurities and eliminate the water content present in natural
kaolin. No color change was observed in the kaolin before and after calcination
at 500°C. At a calcination temperature of 500°C, the bond between the hydroxyl groups
attached to the octahedral alumina on the kaolin surface weakens and breaks
which is called pre-dehydroxylation. A dehydroxylation reaction occurred, where
the hydroxyl group bonds in natural kaolin absorb energy which then decomposes.
Removal of nearly all hydroxyl groups from natural kaolin causes the crystal
structure of kaolin to break down, which then becomes amorphous. This amorphous
phase of kaolin is called a metakaolin. A comparison of the color of kaolin
(150 mesh), heated in an oven and after calcination at 500°C is shown in Figure 1A-C.
Figure 1 Preparation of (A) natural kaolin 150 mesh, (B) kaolin after oven
heating, and (C) kaolin after calcination (metakaolin)
3.2. The physical properties of activated
Kaolin
Comparison of the physical properties of activated kaolin using 1M or
2M NaOH, the kaolin was separated from the NaOH solution after washing with
distilled water. The dried kaolin and the calcined kaolin (metakaolin) are
shown in Figure 2A-E.
All
heterogeneous catalysts were produced, namely Kb1M, Kb2M, and Ka1M. The
addition of NaOH and HCl aims to dissolve silica and alumina from natural
kaolin. NaOH acts as a metallizer and a base agent because the kaolin structure
forms an excess negative charge on the aluminum ion in order to support cations
that are needed outside the framework to neutralize its surface charge. The
addition of NaOH as a mineralizer in the synthesis of kaolin catalysts due to
the capacity of water as a solvent at high temperatures is often unable to
dissolve substances in the crystallization process. The activation process can
enlarge the pore size and open the pores of natural kaolin. On the other hand,
the calcination process at the activation stage aims to ensure the formation of
crystals on the kaolin surface. In general, the reaction mechanisms are
illustrated in Equations (4) and (5);
Figure 2 Comparison of the physical properties of (A) activated kaolin using NaOH
(1M and 2M), (B) kaolin that was separated from the NaOH solution, (C) washing
of kaolin using aquadest, (D) dried kaolin, and (E) calcined kaolin
(metakaolin)
3.3. FTIR spectral characterization
FTIR characterizations were
only carried out for 2 types of heterogeneous catalysts, namely Kb1M and Kb2M.
The FTIR spectra of catalysts Kb1M and Kb2M are shown in Figure 3(A-B). Both FTIR spectra are not similar
since the intensity of the absorption peaks show variability, as noted for the
weak band at 3648 cm-1, strong bands at 1032; 1012 cm-1
for Kb1M, and 3366 cm-1, 1051; 1032 cm-1 for Kb2M. The
Kb1M catalyst has an absorption peak at 704 cm-1, showing the
asymmetric stretching of Si-O-Si and Al-O-Al. The absorption bands at 1012 cm-1
and 1032 cm-1 indicate the presence of O-Si-O and O-Al-O asymmetric
stretching vibrations of the aluminosilicate framework. One of the
characteristics of zeolite A is having double rings which is revealed by the
absorption for the IR band at ??550 cm-1. The peaks at 1456 cm-1
and 1507 cm-1 relate to the bending vibration of the Si-O-Si group.
The band at 3648 cm-1 was observed for Kb1M, while the peak at 3366
cm-1 was observed for KB2M. This peak is assigned for the vibration
of the hydroxyl (O-H) group. The IR band at 2972 cm-1 shows the
Al-O-Na vibration. An IR band at 688 cm-1 shows the symmetrical Si-O
and Al-O vibrations for the Kb2M catalyst.
For
comparison purposes, the FTIR spectra of Kb1M and Kb2M are quite different than
the FTIR spectra of zeolite A, as reported by Kusrini
et al. (2024a; 2024b). Generally, the vibrational bands of
zeolite A appeared at 2175, 1989, and 879 cm-1, where the absorption
peak at 879 cm-1 was attributed to the internal vibrations of Si-O
and Al-O bonds and the asymmetric stretching within the tetrahedral zeolite
structure (Kusrini et al., 2024a; Kusrini et
al., 2024b).
Figure 3 FTIR spectra of (A) Kb1M and (B) Kb2M catalyst materials
3.4. XRD characterization
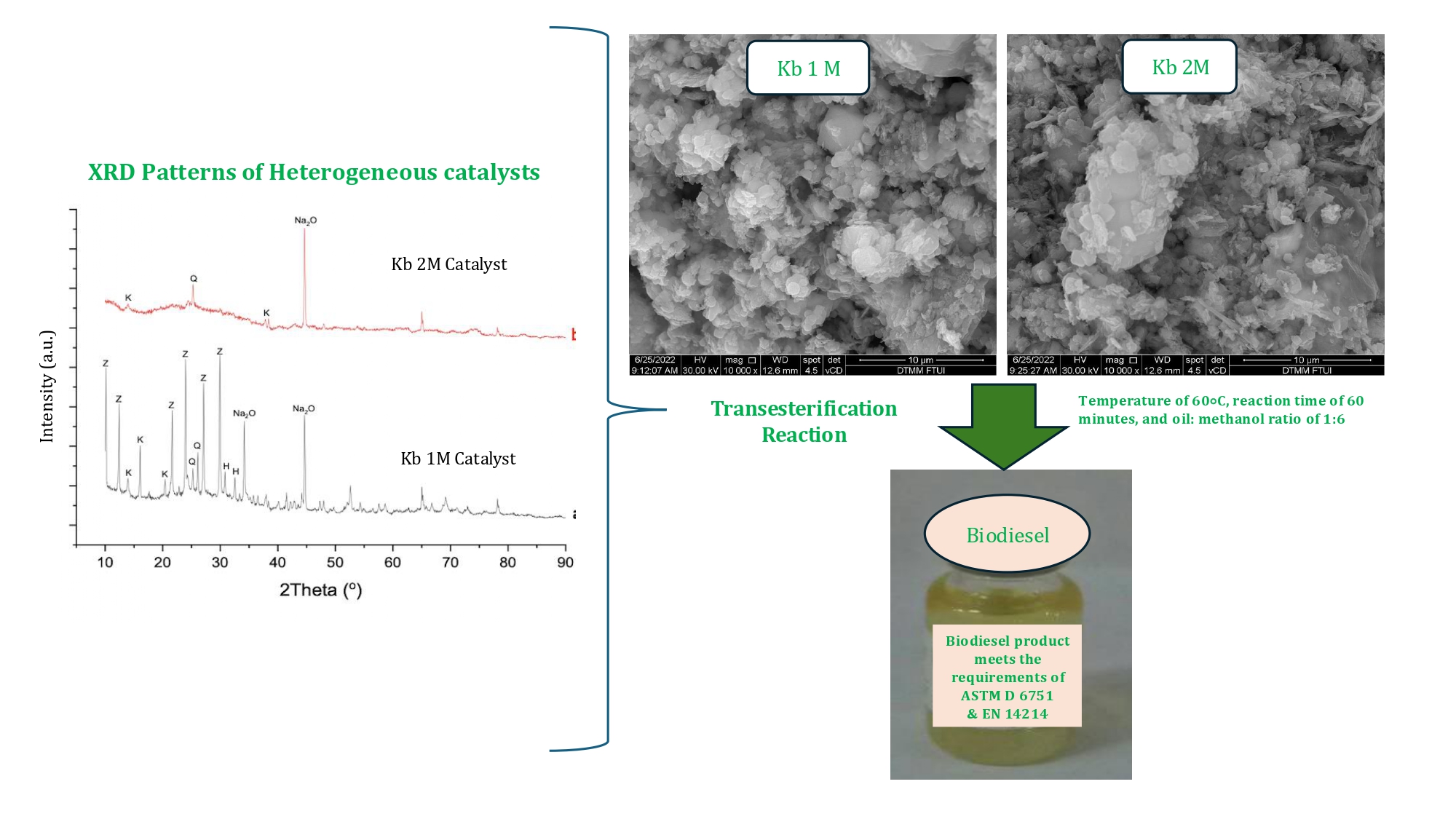
XRD patterns of
heterogeneous catalysts (Kb1M and Kb2M) are shown in Figure 4(A-B). Based on
Figure 4(A), the XRD results of the Kb1M catalyst showed the appearance of XRD
signatures at values of 10.15°; 12.44°; 21.65°; 23.98°; 27.1°; and 29.94°, which provide support of the formation of zeolite A. By comparison,
the XRD result in Figure 4(B) for Kb2M showed the appearance of a band at
25.28°, which indicates that quartz was still present. The dominant crystal
structure is kaolin, while the Q peak in the form of quartz (SiO2)
is a crystalline phase only slightly observed in the XRD pattern, where the
peak of SiO2 occurs at
26.61°. The formation of zeolite A is
not supported by the Kb2M catalyst. Based on a calculation
using the Debye-Scherrer equation (Equation 1), we obtained the crystallite sizes of the Kb1M and Kb2M catalysts. The crystallite size of Kb1M is larger (41.73 nm) compared to Kb2M (23.64 nm), as shown in Table 1. This size is smaller than those found by Kusrini et al. (2024b) with crystallite
size of 49.22 nm and crystallinity of NaA
is 99.73%.
Figure 4 XRD patterns of the heterogeneous catalyst materials:
(A) Kb1M, and (B) Kb2M, where K (kaolinite), Q (quart), and Z (zeolite A) are
indicated
Table 1 Comparison of heterogeneous catalyst crystal size using
Debye-Scherrer Equation
3.5. SEM-EDX studies
Scanning electron microscopy (SEM) characterization
was carried out to determine the morphology and surface structure of the
catalysts. Based on Figure 5, the morphology of the Kb1M catalyst showed
uniform cube-shaped crystals for zeolite A. In this study, the aim to obtain e?cient catalysts from lost-cost natural
kaolin, not to synthesize purposely the zeolites. However, the kaolin-derived
catalysts possessed the characteristics of Na-zeolite A (NaA). This SEM
image showed that zeolite A particles are cubic in shape with sharp edges. It is similar
reported by Kusrini et al. (2024b), where the NaA
has the morphology of a perfect cube with sharp cube edges, while zeolite A or
Linde Type-A (LTA) is the result of the synthesis of alumina silicate minerals
which have a cube morphology with blunter cube ends.
Figure 5 SEM images
of the catalyst materials: (A) Kb1M and (B) Kb2M with 20,000×
magnification
From this
study, the Kb1M catalyst has sharp edges with a cubic shape that resembles the
NaA material reported by Kusrini et al. (2024a; 2024b). It is also similar to the
results reported by Farghali, Abo-Aly, and Salaheldin (2021) where zeolite A has a uniform cube
shape with an average particle diameter of 1 The SEM results for the Kb2M catalyst showed a typical morphology of
kaolinite in the form of a group of layered hexagonal sheets with heterogeneous
sizes and a few typical zeolite cube crystals. This morphology can be
attributed to an incomplete calcination process or imperfect grinding of the
hexagonal sheets into cube crystals, and may also be influenced by the
concentration of NaOH.
The EDX spectra of both heterogeneous catalysts of Kb1M and Kb2M showed
the elements present on the sample surface, including C, O, Na, Al, and Si (Table 2). The presence of C in Kb2M may be due to
impurities and/or other precursors. It is similar observed with NaA that
reported by Kusrini et al. (2024a). The
formation of heterogeneous catalysts using pre-treatment, calcination, and
activation stages using NaOH solution ranged from 1–2 M, which gave rise to the
formation of zeolite A. With the addition of NaOH, the Si/Al ratio of the Kb1M
and Kb2M catalysts varied from 1.154 to 1.246 (see Table 2). The zeolite A
morphology and the Si/Al ratio are comparable with results reported by Kusrini et al. (2020b). Na-zeolite A (NaA) reported
by Kusrini et al. (2024b), the Si/Al
rasio is 1.007. However, according Kusrini et
al. (2024a), the Si/Al ratio is 2.469. The Si/Al ratio of zeolite A
can differ due to the NaOH concentration, pre-treatment, size of kaolin, and
the sequence of synthetic steps that follow the hydrothermal and calcination
techniques.
Table 2 Compariosn of EDX compositional characterization of the heterogeneous catalysts
(Kb1M and Kb2M) and NaA
|
Material |
Element (At %) |
Ratio |
Reference | ||||
|
|
C |
O |
Na |
Al |
Si |
Si/Al |
|
|
Kb1M |
- |
46.89 |
11.96 |
18.32 |
22.83 |
1.246 |
|
|
Kb2M |
06.53 |
48.21 |
10.87 |
15.97 |
18.43 |
1.154 |
|
|
NaA |
- |
39.49 |
9.21 |
13.46 |
14.76 |
1.097 |
Kusrini et al.
(2020b) |
|
NaA |
3.147 |
40.217 |
11.303 |
12.96 |
32.003 |
2.469 |
Kusrini et al.
(2024a) |
|
NaA |
- |
39.49 |
9.21 |
13.46 |
13.55 |
1.007 |
Kusrini et al.
(2024b) |
3.6. Production of
Biodiesel via a Transesterification Reaction
The
Kb1M, Kb2M, and Ka1M materials as heterogeneous catalysts for biodiesel
production using transesterification reaction were studied in detail. The acid number of cooking oil is
0.56 mg KOH/g. This value shows that the cooking oil as a model can be used
directly to produce biodiesel via the transesterification reaction. If the
value of acid numbers is high that can indicate high FFA levels, which can
inhibit the biodiesel formation process. This can also lead to a saponification
reaction. The variation of mole ratio of cooking oil:methanol used in the
transesterification reaction are 1:3 and 1:6 to obtain the best ratio of
cooking oil:methanol for biodiesel production. Furthermore, the palm cooking
oil was heated at a temperature of 65°C to remove the water content contained
in the oil. High water content can cause the reaction to undergo
saponification, which causes a reduction of the methyl ester yield and
challenges for separating glycerol from the methyl ester, including an increase
in viscosity and emulsion formation. The catalyst content of 3 wt.% was used
for the biodiesel production.
Initially, the process for the
production of biodiesel was started by mixing heated cooking oil, methanol, and
respective catalysts Kb1M or Kb2M in a reactor at a temperature of 60°C during
a reaction time of 60 minutes. After the transesterification reaction, the
process was complete, and the mixture was transferred to a separatory funnel to
separate the formed phases. After separation from the separatory funnel, the
biodiesel product was washed with warm water until the color of the water was
no longer cloudy. Washing with the warm water prevents the precipitation of
saturated methyl esters and the formation of emulsions. It is noted that the heterogeneous catalyst exhibits better reusability, multiple
cycles, and easier separation compared to the homogenous catalyst. The
major causes of catalyst deactivation can be occurred if the leaching of active
sites, clogging of pore spaces of catalyst, the multiple cycles, and thus the
performance of heterogeneous catalyst, were reduced. This condition is comparable
with the biodiesel production that reported by Dang,
Chen and Lee (2013). The palm
oil produced the yield approximately 95% with the operation condition for
biodiesel production after 2 h of reaction at 63°C (Dang, Chen and Lee, 2013). Almost 90% of triglycerides in palm oil was converted
to biodiesel after 6 h of reaction at 50°C.
The temperature reaction in this study is similar to biodiesel
production from palm fatty acid distillate (PFAD) using ZrO2/bagasse
fly ash catalyst with FFA esterification conversion of 90.6% as reported by Rahma and Hidayat (2023). They reported that the
reaction temperature of 60°C, ratio of methanol to palm fatty acid
distillate of 10:1, catalyst loading of 10 wt.% of PFAD, and reaction time of 2
h.
3.7. GC-MS
characterization
GC-MS
analysis was carried out to determine the composition of methyl esters
contained in a biodiesel product. The identified methyl esters were compared
with standard references, based on the respective retention time data which was
confirmed by mass spectrometry from the GCMS results with a cooking
oil:methanol mole ratio (1:3) and (1:6) (see Table 3). Kb1M catalyst produced
35.1% area of methyl ester at a mole ratio (1:3) and 86.5% at a mole ratio
(1:6). As shown in Table 3, the Kb2M catalyst can produce 96.3% of the area of
methyl ester with a mole ratio of cooking oil:methanol (1:6). Meanwhile, the
Ka1M catalyst did not produce any area (0% based on GCMS results) for the
methyl ester. This indicates that the Ka1M material cannot be used as a
catalyst for biodiesel production via transesterification reaction since the
acid cannot change FFA to FAME. Kaolin as catalysts in biodiesel production via
transesterification reaction of vegetable oils in excess of methanol has been also
reported by Dang,
Chen, and Lee (2013). However, the conversion efficiencies were
approximately 2.2 and 2.6%, respectively, for soybean oil and palm oil after 40
h of transesterification reaction (Dang, Chen, and Lee (2013).
As shown in Table 3, it can be seen that the
highest FAME yield was 69.4% using Kb2M at a ratio of cooking oil: methanol
(1:6). The best and optimum use of cooking oil:methanol mole ratio is at 1:6
ratio because it produces a large FAME yield. When compared to a 1:3 ratio, the
FAME yield was 63.2% using Kb1M at a ratio of cooking oil:methanol (1:6) and
27.8% at a ratio of cooking oil:methanol (1:3). It is also comparable procedure
with the transesterification reaction of blended oils at 60°C for 1 h, and the
mole ratios of oil:methanol varied 1:3, 1:6, 1:9, 1:12, and 1:15 that reported
by Wahyono et al. (2022). The oil:
methanol mole ratios of 1:6 produced the best yield of 92.99% with the
conversion of 99.58% mass according to the GCMS results (Wahyono et al., 2022). Additionally, metakaolin was
activated using a 1 M HCl solution. This process does not result in the
production of FAME since the acid catalyst does not generate any fatty acids.
Table 3 Comparison of yield and conversion of FAME with a variable mole ratio of
oil: methanol 1:6 and 1:3.
|
Type of catalyst |
The mole ratio cooking oil:
methanol (1:6) | |||
|
|
Yield
FAME (%) |
Weight
(g) |
Conversion (%) |
Weight
(g) |
|
Kb1M |
63.16 |
48.93 |
73.03 |
48.93 |
|
Kb2M |
69.39 |
21.61 |
72.03 |
21.61 |
|
Ka1M |
0 |
|
75.89 |
|
|
|
The mole ratio cooking oil:
methanol (1:3) | |||
|
|
Yield
FAME (%) |
Weight
(g) |
Conversion (%) |
Weight
(g) |
|
Kb1M |
27.80 |
53 |
79.10 |
53 |
The
highest FAME conversion rate (79.1%) was achieved using Kb1M with a mole ratio
of 1:3. On the other hand, the lowest FAME conversion rate was 72.0% using Kb2M
with a mole ratio of 1:6. However, it is important to note that this conversion
value alone does not determine the quality of the resulting FAME. This
conclusion is supported by the GCMS results. It is also worth mentioning that
transesterification reactions do not always yield biodiesel. ??ng, Chen and Lee (2017) reported the conversion
yield of triolein to biodiesel increased up to 94.3% when the aging for time preparation
of the zeolite Linde Type A (LTA)-kaolin catalysts were extended from 6 to 48 hours.
The excess methanol for production of biodiesel using triglycerides as source is
one of optimum condition chosen.
The
physical properties of biodiesel products were analyzed, according to the
Indonesian National Standard (SNI) number 7182:2015 method for biodiesel. Table
4 shows the density and viscosity of the synthetic biodiesel that is
accompanied by standard data from SNI No. 7182:2015.
Table 4 Comparison of the physical
properties of biodiesel using an oil: methanol ratio of 1:6
|
Parameter |
Catalyst Type |
SNI 04-7182-2015 |
ASTM D 6751 |
EN 14214 | |
|
Kb1M |
Kb2M |
|
| ||
|
Density (kg/m3) |
895.2 |
898.1 |
850-890 |
900 |
860–900 |
|
Kinematic Viscosity
at 40°C mm2/s (CST) |
31.85 |
38.65 |
2.3-6.0 |
5.0 |
3.50–5.0 |
|
Acid Value (mg
KOH/g) (max) |
2.4 |
2.2 |
0.4 |
0.5 |
0.50 |
|
Total glycerol (%
mass) (max) |
Not determined |
Not determined |
0.24 |
0.24 |
0.25 |
|
Methyl ester (%
mass, min) |
86. |
96.3 |
96.5 |
|
96.5 |
The cooking oil employed has a density of 899 kg/m3 and a
viscosity of 48.62 cSt. A minor decrease in density occurs that was caused by
the reaction of triglycerides in cooking oil with methanol. Thus, the
triglycerides were converted into methyl esters. The characteristics of biodiesel products from
this study were compared with the biodiesel quality standards of the American
Society for Testing and Materials (ASTM) D 6751, Europäische Norm (EN)
14214 (European Committee for Standardization,
2002), and SNI 7182 (The National
Standardization Agency of Indonesia, 2015). The viscosity of the
biodiesel produced from the synthesis is relatively high and falls outside the
acceptable range according to the SNI standard. However, the biodiesel products
meet the requirements of ASTM D 6751 and EN 14214, primarily due to their acceptable
density properties and percentage of methyl ester by mass. The high viscosity
value is caused by the incomplete transesterification reaction and the large
number of FFA with long carbon chains in the biodiesel product. The high
mechanical viscosity of biodiesel probably occurs due to incomplete glycerol
separation.
Comparison of the biodiesel
produced by the heterogeneous catalysts (Kb1M and Kb2M) are shown in Figure 6.
The Kb2M catalyst showed the highest catalytic performance, which produced a
methyl ester area of 96.3% with a yield of 69.4%.
On the other hand, the oxide
lanthanides composites such as CaO/La2O3, MgO/La2O3,
and CaO/CeO2 were also reported and used as heterogeneous catalysts
for biodiesel production. In this sense,
the lanthanides as critical minerals and have many applications for petroleum
productions, energy, catalyst,
and antiamoebic activity (Kusrini et al.,
2024c).
These oxides can increase the catalytic activity and stability, as they have much better
resistance towards FFA in biodiesel reactions (Santoro et al., 2016). Additionally, the clay was
also reported as a catalyst which can improve for distributing lanthanides into
the clay. It can be useful for increasing the number of active sites on a
catalyst, thus the contact between the reactants and the catalyst will be
greater and product formation will be faster. Biodiesel is one of renewable
energy option that can be further produced to fulfil the need of energy in
Indonesia. This fuel is economically viable and environmentally friendly (Ebrahimi et al., 2024). These factors were
attributed to the high energy density, biodegradability, a reliable supply
chain, and non-toxicity. The main component of biodiesel is methyl esters that
can derived from various biomass sources including non-edible oil, edible, waste,
plant oils and/or and discarded cooking oils. This innovation can be more
promising as Indonesia has enormous abundant resources for biodiesel production
to reach the Golden Indonesian in 2045 and to reduce the energy crisis. This
will become more pronounced by
utilizing
different kinds of heterogeneous catalysts found locally as
resources to provide a renewable
energy including biodiesel, jet fuel and/or
biogasoline as an alternative
energy now and in the future.
Figure 6 Comparison of biodiesel produced by
heterogeneous catalysts of Kb1M and Kb2M. Operation condition at 60°C, reaction
time of 60 minutes, and oil:methanol ratio of 1:6
In this study, application of low-cost natural kaolin as a catalyst to produce biodiesel (fatty acid methyl ester, FAME) via the transesterification reaction using the cooking oil as a model of free fatty acids (FFA) has been evaluated. Natural kaolin was calcined and activated using base (NaOH) and acid (HCl) additives in solution to produce heterogeneous catalysts that were used for biodiesel production. Unexpectedly, the activation leads the formation of zeolite A, so that the kaolin-derived catalysts possessed the characteristics of zeolite NaA. The Kb1M catalyst has NaA morphology, whereas the Kb2M catalyst shows partial morphology of zeolite A that still maintains the hexagonal sheets that are typical of kaolinite. The quality of biodiesel products does not fulfil the standard SNI 04-7182-2015, except for the methyl ester area, according to the results of this study. However, the biodiesel products are acceptable for ASTM D 6751 and EN 14214 mainly for the density properties and % mass of methyl ester. In future, the Kb2M catalyst can be optimized further to obtain the best catalyst and meet the SNI 04-7182-2015 standards across all parameters. On the other hand, further investigation can be continued to obtain the biodiesel and having favorable properties for ASTM D 6751 and EN 14214, thus it could be leveraged in industrial biodiesel production. The Kb2M catalyst has the highest catalytic performance, which produced a methyl ester area of 96.3% with a yield of 69.4%. Future research would be exploring alternative activation methods and scaling up production of biodiesel using kaolin, graphene, lanthanides and/or their composites as catalysts for commercial applications.
The authors thank to International Indexed Publication (Publikasi Terindeks Internasional, PUTI) Q1 grant 2023—2024, NKB-508/UN2.RST/HKP.05.00/2023 from Universitas Indonesia (UI) for their financial support.
Aisyah, A.N., Ni’maturrohmah, D., Putra,
R., Ichsan, S., Kadja, G.T.M, Lestari, W.W., 2023. Nickel Supported on
MIL-96(Al) as an Efficient Catalyst for Biodiesel and Green Diesel Production
from Crude Palm Oil. International Journal of Technology, Volume 14(2), pp. 276–289. https://doi.org/10.14716/ijtech.v14i2.5064
Ali, B., Yusup, S., Quitain, A.T., Alnarabiji, M.S., Kamil, R.N.T.,
Kida, T., 2018. Synthesis of Novel Graphene Oxide/bentonite bi-functional
Heterogeneous Catalyst for One-pot Esterification and Transesterification
Reactions. Energy Conversion and Management, Volume 171, pp.1801–812. https://doi.org/10.1016/j.enconman.2018.06.082
Arnas, Whulanza, Y., Kusrini, E., 2024. Unveiling
Innovations Across Multidisciplinary Horizons. International Journal of
Technology. Volume 15(2), pp. 240–246.
https://doi.org/10.14716/ijtech.v15i2.6933
Asbollah,
M.A., Sahid, M.S.M., Padmosoedarso, K.M., Mahadi A.H., Kusrini E., Hobley, J.,
Usman A., 2022. Individual and Competitive Adsorption of Negatively Charged
Acid Blue 25 and Acid Red 1 onto Raw Indonesian Kaolin Clay. Arabian
Journal for Science and Engineering, Volume 47(5), pp.
6617–6630. https://doi.org/10.1007/s13369-021-06498-3
Atadashi,
I.M., Aroua, M.K., Aziz, A.A., Sulaiman, N.M.N., 2013. The Effects of Catalysts
in Biodiesel Production: A Review. Journal of Industrial and
Engineering Chemistry, Volume 19(1), pp. 14–26. https://doi.org/10.1016/j.jiec.2012.07.009
Aziz,
M.A.A., Puad, K., Triwahyono, S., Jalil, A.A., Khayoon, M.S., Atabani, A.E.,
Ramli, Z., Majid, Z.A., Prasetyoko, D., Hartanto, D., 2017. Transesterification
of Croton Megalocarpus Oil to Biodiesel Over WO3 Supported on Silica
Mesoporous-macroparticles Catalyst. Chemical Engineering Journal,
Volume 316, pp. 882–892.
https://doi.org/10.1016/j.cej.2017.02.049
Balat,
M., Balat, H., 2010. Progress in Biodiesel Processing. Applied Energy,
Volume 87(6), pp.1815–1835.
https://doi.org/10.1016/j.apenergy.2010.01.012
Belver,
C., Bañares-Muñoz, M.A., Vicente, M., 2002. Chemical Activation of a Kaolinite
under Acid and Base Conditions. Chemistry of Materials, Volume
14(5), pp. 2033–2043.
https://doi.org/10.1021/cm0111736
Carmo-Jr,
A.C., de Souza, L.K., da Costa, C.E., Longo, E., Zamian, J.R., da Rocha Filho,
G.N., 2009. Production of Biodiesel by Esterification of Palmitic Acid Over
Mesoporous Aluminosilicate Al-MCM-41. Fuel, Volume 88(3), pp. 461–468. https://doi.org/10.1016/j.fuel.2008.10.007
Dang,
T.H., Chen, B.-H., Lee, D.-J., 2017. Optimization of Biodiesel Production from
Transesterification of Triolein Using Zeolite LTA Catalysts Synthesized from
Kaolin Clay. Journal of the Taiwan Institute of Chemical Engineers, Volume
79, pp. 14–22. http://dx.doi.org/10.1016/j.jtice.2017.03.009
Dang,
T.H., Chen, B.-H., Lee, D.-J., 2013. . Application of Kaolin-Based Catalysts in
Biodiesel Production via Transesterification of Vegetable Oils in Excess
Methanol. Bioresource Technology, Volume 145, pp. 175–181. http://dx.doi.org/10.1016/j.biortech.2012.12.024
Ebrahimi,
A., Haghighi, M., Ghasemi, I., Bekhradinassab, E., 2024. Design of Highly
Recoverable Clay-Foundation Composite of Plasma-Treated Co3O4/Kaolin to Produce
Biodiesel from Low-Cost Oil. Fuel, Volume 366, p. 131267.
https://doi.org/10.1016/j.fuel.2024.131267
European
Committee for Standardization (CEN), 2002. Europäische Norm (EN) 14214:2002.
Automotive Fuels - Fatty Acid Methyl Esters (FAME) for Diesel Engines
Requirements and Test Methods. Brussels, Belgium
Farghali,
M.A., Abo-Aly, M.M., Salaheldin, T.A., 2021. Modified Mesoporous
Zeolite-A/reduced Graphene Oxide Nanocomposite for Dual Removal of Methylene
Blue and Pb2+ ions from Wastewater. Inorganic Chemistry
Communications, Volume 126, p. 108487.
https://doi.org/10.1016/j.inoche.2021.108487
Fukuda, H., Kondo, A., Noda, H., 2001. Biodiesel Fuel Production by
Transesterification of Oils. Journal of Bioscience and Bioengineering,
Volume 92(5), pp. 405–416.
https://doi.org/10.1016/S1389-1723(01)80288-7
Guan,
J., Yang, G., Zhou, D., Zhang, W., Liu, X., Han, X., Bao, X., 2009. The
Formation Mechanism of Mo-methylidene Species Over Mo/HBeta Catalysts for
Heterogeneous Olefin Metathesis: A Density Functional Theory Study. Journal
of Molecular Catalysis A: Chemical, Volume 300(1-2), pp. 41–47. https://doi.org/10.1016/j.molcata.2008.10.044
Gui, M.M., Lee, K.T., Bhatia, S., 2008. Feasibility of
Edible Oil vs. Non-edible Oil vs. Waste Edible Oil as Biodiesel
Feedstock. Energy, Volume 33(11), pp. 1646–1653.
https://doi.org/10.1016/j.energy.2008.06.002
Kusrini, E., Alhamid, M.I., Wulandari, D.A.,
Fatkhurrahman, M., Shahrin, E.W.E.S., Shahri, N.N.M., Usman, A., Prasetyo,
A.B., Sufyan, M., Rahman, A., Khoirina Dwi Nugrahaningtyas, K.D., Santosa, S.J., 2024a. Simultaneous
Adsorption of Multicomponent Lanthanide Ions on Pectin Encapsulated Zeolite A. EVERGREEN
Joint Journal of Novel Carbon Resource Sciences & Green Asia Strategy, Volume 11(1), pp. 371–378. https://doi.org/10.5109/7172296
Kusrini, E., Usman, A., Ayuningtyas, K., Wilson, L.D.,
Putra, N., Prasetyo, A.B., Rahman, A., Nugrahaningtyas, K.D., Santosa, S.J.,
Noviyanti, A.R., 2024b. Fragrance Carrier Based on Zeolite-A Modified Bentonite
as a Controlled Release System in Air Freshener Applications. Sains
Malaysiana, Volume 53(10), pp. 3499-3509. http://doi.org/10.17576/jsm-2024-5310-22
Kusrini, E., Hashim, F., Aziz, A., Hassim, M.F.N., Usman, A., Wilson, L.D., Negim, E.S., Prasetyo, A.B., 2024c. Potential of
Critical Mineral and Cytotoxicity Activity of Gadolinium Complex as Anti-amoebic
Agent by Viability Studies and Flow Cytometry Techniques. AUIQ Complementary
Biological System, Volume 1, pp. 1–10. https://doi.org/10.70176/3007-973X.1010
Kusrini, E., Oktavianto, F., Usman, A., Mawarni, D.P.,
Alhamid, M.I, 2020a. Synthesis, Characterization, and Performance of Graphene
Oxide and Phosphorylated Graphene Oxide as Additive in Water-based Drilling
Fluids. Applied Surface Science, Volume 506, p. 145005.
https://doi.org/10.1016/j.apsusc.2019.145005
Kusrini, E.,
Mualim, N.M., Usman, A., Setiawan, M.D.H., Rahman, A., 2020b. Synthesis,
Characterization and Adsorption of Fe3+, Pb2+ and Cu2+
Cations using Na-Zeolite A Prepared from Bangka Kaolin, In: AIP Conference Proceedings, Volume 2255, p. 060013. https://doi.org/10.1063/5.0014317
Kusrini, E., Mualim, N.M., Rahman, A., Usman, A.,
2020c. Application
of Activated Na-zeolite as a Water Softening Agent to Remove Ca2+
and Mg2+ Ions from Water. In: AIP Conference Proceedings, Volume 2255, p. 060012. https://doi.org/10.1063/5.0014314
Leung, D.Y., Wu, X., Leung,
M.K.H., 2010. A Review on Biodiesel Production using Catalyzed
Transesterification. Applied Energy, Volume 87(4), pp. 1083–1095. https://doi.org/10.1016/j.apenergy.2009.10.006
Majid, A.F.A., Dewi, R., Shahri, N.N.M., E., Shahrin, W.E.S., Kusrini,
E., Shamsuddin, N., Lim, J.-W., Thongratkaew, S., Faungnawakij, K., Usman, A.,
2023. Enhancing Adsorption Performance of Alkali Activated Kaolinite in the
Removal of Antibiotic Rifampicin from Aqueous Solution. Colloids and Surfaces A:
Physicochemical and Engineering Aspects. Volume 676, p. 132209.
https://doi.org/10.1016/j.colsurfa.2023.132209
Maneerung, T., Kawi, S., Dai, Y.,
Wang, C., 2016. Sustainable Biodiesel Production via Transesterification of
Waste Cooking Oil by using CaO Catalysts Prepared from Chicken Manure. Energy
Conversion and Management, Volume 123, pp. 487–497.
https://doi.org/10.1016/j.enconman.2016.06.071
Marso, T.M.M., Kalpage, C.S., M.Y. Udugala-Ganehenege,
M.Y., 2017. Metal modified graphene oxide composite catalyst for the production
of biodiesel via pre-esterification of Calophyllum inophyllum oil. Fuel, Volume
199, pp. 47-64. https://doi.org/10.1016/j.fuel.2017.01.004
Nasrollahzadeh,
M., Jaleh, B., Jabbari, A., 2014. Synthesis, Characterization and Catalytic
Activity of Graphene Oxide/ZnO Nanocomposites. RSC Advances, Volume
4(69), p. 36713. https://doi.org/10.1039/C4RA05833J
Rahma,
F.N., Hidayat, A., 2023. Biodiesel Production from Free Fatty Acid using ZrO2/Bagasse
Fly Ash Catalyst. International Journal of Technology. Volume 14(1), pp.
206–218.
https://doi.org/10.14716/ijtech.v14i1.4873
Santoro, S., Kozhushkov, S.I., Ackermann, L., Vaccaro,
L., 2016. ChemInform Abstract: Heterogeneous Catalytic Approaches in C-H
Activation Reactions. ChemInform, Volume 47(32).
https://doi.org/10.1039/C6GC00385K
Soetaredjo,
F.E., Ayucitra, A., Ismadji, S., Maukar, A.L., 2011. KOH/Bentonite Catalysts
for Transesterification of Palm Oil to Biodiesel. Applied Clay Science,
Volume 53(2), pp. 341–346. https://doi.org/10.1016/j.clay.2010.12.018
Subari, R.I.F. Wenas, R.I.F., Suripto., 2008. Industrial
Minerals in West Kalimantan and Their Utilization for Ceramic Products. Indonesian
Mining Journal, Volume 11(12), pp. 27 – 37.
Syukri,
S., Ferdian, F., Rilda, Y., Putri, Y.E., Efdi, M., Septiani, U. 2020. Synthesis
of Graphene Oxide Enriched Natural Kaolinite Clay and Its Application for Biodiesel
Production. International Journal of Renewable Energy Development,
10(2), pp. 307–315.
https://doi.org/10.14710/ijred.2021.32915
The
National Standardization Agency of Indonesia (BSN), 2015. Standar Nasional
Indonesia 7182:2015 Biodiesel (Indonesian National Standard 7182: 2015
Biodiesel). Indonesia, pp. 1–88.
Yan, S.,
DiMaggio, C., Mohan, S., Kim, M., Salley, S.O., Ng., K.S., 2010. Advancements
in Heterogeneous Catalysis for Biodiesel Synthesis. Topics in Catalysis,
Volume 53, pp. 721–736. https://doi.org/10.1007/s11244-010-9460-5
Yang,
B., Leclercq, L., Clacens, J.M., Nardello-Rataj, V., 2017. Acidic/amphiphilic
Silica Nanoparticles: New Eco-friendly Pickering Interfacial Catalysis for
Biodiesel Production. Green Chemistry, Volume 19(19), pp. 4552–4562.
https://doi.org/10.1039/C7GC01910F
Wahyono, Y., Hadiyanto, M.A., Budihardjo, Y.H., Baihaqi, R.A., 2022.
Multifeedstock Biodiesel Production from a Blend of Five Oils through
Transesterification with Variation of Moles Ratio of Oil: Methanol. International
Journal of Technology, Volume 13(3), pp. 606–618. https://doi.org/10.14716/ijtech.v13i3.4804
Whulanza, Y., Kusrini, E., 2024. Shaping a Sustainable Future: The Convergence of Materials Science, Critical Minerals and Technological Innovation. International Journal of Technology, Volume 15(1), pp. 1–7. https://doi.org/10.14716/ijtech.v15i1.6934
