Synthesis, Characterization, and Application of Poly(Styrene-Co-Glycidyl Methacrylate) as Reactive Diluents to Epoxy Resin
Corresponding email: da_ainakulova@kbtu.kz
Published at : 10 Jul 2024
Volume : IJtech
Vol 15, No 4 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i4.6920
Tulegenkyzy, A.D., Megat-Yusoff, P.S.M., Al Azzam, K.M., Kairatovna, B.L., Goyal, A., Eshmaiel, G., Negim, E.-S., Samy, M., Ravindran, B., 2024. Synthesis, Characterization, and Application of Poly(Styrene-Co-Glycidyl Methacrylate) as Reactive Diluents to Epoxy Resin. International Journal of Technology. Volume 15(4), pp. 903-916
| Ainakulova Dana Tulegenkyzy | School of Materials Science and Green Technologies, Kazakh-British Technical University, St. Tole bi 59, 050000, Almaty, Kazakhstan |
| Puteri Sri Melor Megat-Yusoff | Mechanical Engineering Department, Universiti Teknologi PETRONAS, Bandar Seri Iskandar 32610, Perak, Malaysia |
| Khaldun Mohammad Al Azzam | Department of Chemistry, Faculty of Science, The University of Jordan, 11942 Amman, Jordan |
| Bekbayeva Lyazzat Kairatovna | National Nanotechnology Open Laboratory, Al-Faraby Kazakh National University, 71, Al-Faraby av., 050040, Almaty, Kazakhstan |
| Arpit Goyal | Civil Engineering Department, Thapar Institute of Engineering and Technology, Patiala, Punjab, India- 147001 |
| Ganjian Eshmaiel | Concrete Corrosion Tech LTD, 12 Humphrey Middlemore Drive, Birmingham, England B17 0JN |
| El-sayed Negim | 1 School of Materials Science and Green Technologies, Kazakh-British Technical University, St. Tole bi 59, 050000, Almaty, Kazakhstan 2 School of Petroleum Engineering, Satbayev University, 22 Satbay |
| Moshera Samy | Polymers and Pigments Department, National Research Centre, 33 El Bouhoth St., Dokki, Giza 12622, Egypt |
| Balasubramani Ravindran | Department of Environmental Energy and Engineering, Kyonggi Unversity, Youngtong-Gu, Suwon 16277, Gyeonggi-Do, South Korea |
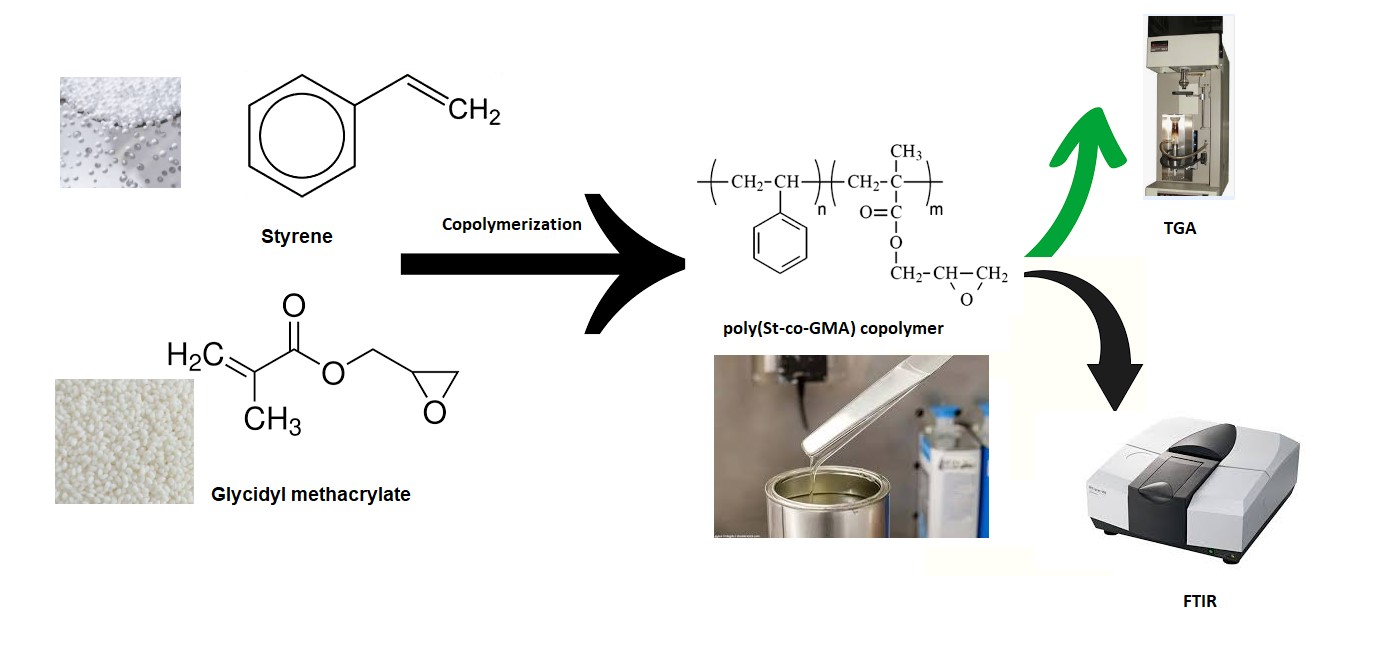
Anticorrosion coatings
are specialized products designed to protect metal surfaces from costly and
dangerous corrosion, acting as barriers against corrosive organisms. Therefore,
this study examined the physical and mechanical properties of epoxy resin in
the presence of reactive diluents. Reactive diluents were synthesized based on
styrene (St) and glycidyl methacrylate (GMA) through the free radical method
using benzoyl peroxide (BPO) as a catalyst with various feed ratios of St and
GMA. Diluents were characterized by Fourier Transform Infrared (FTIR)
spectroscopy and Thermogravimetric Analyzer (TGA). Epoxy resins and hardener
were at a ratio of 1:1, 1:0.5, and 1:0.3, respectively. The results showed that
reactive diluents [Poly(St-co-GMA)] had an excellent dilution effect on
epoxy resin with a decrease in epoxy resin viscosity from 8592 mPa-s to 1656,
680, and 430 mPa-s. Furthermore, the adhesion, tensile, and hardness properties
contained 70% GMA and 30% St at a hardener ratio of 1:0.3. The increase in the mechanical properties was attributed
to the reaction between modified epoxy resins with hardener.
Epoxy; Hardener; Mechanical properties; Reactive diluents; Styrene
Corrosion is a natural process that causes metal surfaces to degrade and fail, posing potential costs and hazards. These surfaces can be protected with anticorrosion coatings, which serve as barriers against corrosive organisms (Ainakulova et al., 2023; Ningrum et al., 2023; Devianto et al., 2023; Riyanto et al., 2023). Various anticorrosion coatings offer unique properties and application methods, among which the organic types are potentially helpful in preventing corrosion on metal surfaces (Msekh et al., 2018; Fu and Cheng, 2011). Some recently improved methods include epoxy polymers, characterized by excellent adhesion, chemical resistance, and durability properties. Most epoxy resins comprised aromatic rings and two heteroatoms, namely P, O, N, and S (Dagdag et al., 2020; Makhlouf, 2014). Hardener or cross-linking agent plays an important role in curing epoxy resins at room temperature. The mechanical properties of these resins depend on the type and characteristics of hardener, such as concentration and temperature (Najuma and Lity, 2014; Jain et al., 2006). Furthermore, reliable analytical methods that efficiently measure the groups in epoxy resins are crucial for synthesis and product quality control. The different application methods require low viscosities, particularly in coating industries. Meanwhile, reducing epoxy resin viscosities often entailed using organic solvents, which can harm both health and the environment (Pineda et al., 2016). The preliminary study focused on the exploration of alternative methods, such as reactive diluents rather than organic solvents. These diluents typically contained oxirane ring compounds, namely glycidyl methacrylate, benzyl glycidyl ether, 1, 4-butanediol diglycidyl ether, etc (Jingyu, Haichao, and Guoxin, 2022; Tran et al., 2020). Polymers with epoxide groups, such as glycidyl methacrylate (GMA), are categorized as epoxy resins (Tzoumani et al., 2022) and have been widely used in a variety of applications, including surface coatings, electrical laminates, adhesives, and molding compounds (Pramanik, Mendon, and Rawlins, 2012; Teh et al., 2007; Wang et al., 2005). Epoxy groups in polymers significantly impact the effective performance in diverse applications (Maruyama, 2001), which enable GMA-based copolymers to enhance coating adhesion, barrier properties, corrosion protection, and service life. Meanwhile, free radical solution polymerization offered advantages such as block copolymers with monomers (Asha et al., 2019). Azzahari et al. (2012) used free radical polymerization in toluene with BPO as an initiator to produce new copolymers from various feed compositions of GMA and tetrahydrofurfuryl acrylate (THFA). The thermal stability of copolymers rises as the THFA content increases. Srikanth et al. (2007) carried out a research to determine the methods needed to produce copolymers from different feed ratios of N-(acryloyloxymethyl) benzotriazole (AMBT) and GMA using the free radical solution polymerization process. Epoxy groups in acrylate anticorrosion coatings produced densely packed, cross-linked coatings, which enhanced corrosion protection due to the stronger chemical bonds. The result improved corrosion protection and prolonged service life for the coated metal substrates. Therefore, this study aimed to prepare Poly(St-co-GMA) copolymers as reactive diluents to reduce viscosity of epoxy resin as well as improve the physical and mechanical properties, including adhesion and thermal characteristics in the presence of an amine-based hardener at a ratio of 1:1, 1:0.5 and 1:0.3. During copolymerization process with GMA, styrene (St) played a critical role by increasing the rate of polymerization and improving the mechanical properties of epoxy resins such as thermal, mechanical, and chemical resistance (Mehmet, 2000). The prepared Poly(St-co-GMA) copolymers were characterized using Fourier Transform Infrared (FTIR) spectroscopy and Thermogravimetric Analyzer (TGA).
2.1. Materials
Epoxy resins ELM-NG 1000
and hardener ELM-NG 34H were supplied by Elcos Marketing LLP, Kazakhstan.
ELM-NG 1000 had an epoxy value ranging from 5.25 to 5.5 eq/Kg, with a weight
per epoxide, viscosity, and density of 182 g/eq, 8500 mPa-s at 25oC
and 1.16 gm/cm3, respectively. Meanwhile, ELM-NG
34H had an amine value and viscosity of 298 mgKOH/g and 254 mPa-s at 25oC.
Major chemicals, namely glycidyl methacrylate (GMA97%), St, xylene, and
benzoyl peroxide (BPO) were purchased from Sigma-Aldrich and used as received.
2.2. Synthesis of Poly(St-co-GMA) copolymer
Copolymerization
process included combining St and GMA with different feed monomer compositions,
namely M1= 70/30, M2= 50/50, and M3 = 30/70, using the free radical
polymerization method in the presence of xylene as a solvent. The solution
medium was put into a 500 mL three-necked flask system equipped with a stirrer,
reflux condenser, and thermometer. The catalyst BPO was added to the flask
while stirring mechanically at 500 to 600 rpm, maintaining a temperature of 65
2.3. Characterization of Poly[St-co-GMA]
copolymer
The
prepared Poly[St-co-GMA] copolymer was characterized using ALPHA FTIR
spectroscopy, Bruker. FTIR spectroscopy and TGA were used to identify and study
the functional groups of copolymers and the thermal properties, respectively.
The per-dried copolymers were tested using Perkin Elmer TGA (TGA/SDTA851e,
METTLER TOLEDO, Switzerland). Furthermore, the measurements were carried out at
room temperature and heating rates of 900°C and 10°C/min under the atmosphere.
2.4. Tests
The viscosity (mPa-s) of
epoxy resins and reactive diluents were measured at room temperature using a
Brookfield viscometer, according to ISO 12058-1 (ISO,
2018), at a speed of 5 and 50 rpm. Meanwhile,
epoxy value (eq/Kg) and weight per epoxide g/eq of resins were determined using
the titration method at room temperatures according to ASTM D1652. The
properties of reactive diluents and modified epoxy resins are shown in Tables 1
and 2, respectively.
Table 1 Properties of reactive diluents.
|
Reactive
diluents |
M1 |
M2 |
M3 |
Test
method |
|
Viscosity,
mPa-s |
10 |
14 |
35 |
ISO
12058-1 |
|
Epoxy
value, eq/Kg |
0.31-0.33 |
0.35-0.37 |
0.40-0.43 |
ASTM
D1652 |
|
Weight
per epoxide, g/eq |
130.5 |
138.6 |
142.5 |
ASTM
D1652 |
2.5. Mixing ratio
Epoxy
resins (E0, 90 %) were mixed with reactive diluents (10%) labeled M1, M2, and
M3 using a stirring stick or spatula for 10 minutes, which led to the formation
of epoxy resins denoted as EM1, EM2, and EM3. Additionally, the properties of
these epoxy resins are shown in Table 2.
ELM-NG
34H is a low-viscosity modified cycloaliphatic that functions as hardener when
mixed with epoxy resins in different ratios of 1.0:1.0, 0.5:1.0, and 0.3:1.0. To ensure proper blending, mix the
ingredients slowly and deliberately, making sure to scrape the container for
thorough incorporation.
Finally, apply the epoxy mixture to metal and concrete substrates, and allow it
to cure naturally at room temperature.
Table 2 Properties of epoxy resin mixed with reactive
diluents.
|
Modified
epoxy with reactive diluents |
EM1 |
EM2 |
EM3 |
Test
method |
|
Viscosity,
mPa-s |
430 |
680 |
1656 |
ISO
12058-1 |
|
Epoxy
value, eq/Kg |
4.4 –
4.9 |
4.10 –
4.8 |
4.1 –
5.2 |
ASTM
D1652 |
|
Weight
per epoxide, g/eq |
195 |
210 |
230 |
ASTM
D1652 |
2.6. Film preparation
Epoxy resin (E0) and mixtures with reactive diluents were mixed in a beaker with different hardener ratios of 1:1, 1:0.5, and 1:0.3 to ensure homogeneity. The resulting mixtures were poured into steel molds, forming specimens with dimensions of 7 mm x 7 mm x 7 mm, and allowed to dry at room temperature for 6 days (Negim et al., 2011). The process of mixing and casting is shown in Figure 1.
Figure 1 The process of mixing and casting for film
preparation.
2.7. Mechanical Tests
Steel
films of 12 cm x 6 cm x 1 mm were provided to evaluate mechanical properties
and chemical durability during curing. A cylindrical Mandrel Tester (ASTM D522)
(ASTM, 2001) was used
to assess the resistance of a coated product to cracking and detachment from a
metal substrate when subjected to bending under standard conditions. The
tubular impact (ASTM D2794) (ASTM, 2019) and the economic cross-hatch testers (ASTM D3359) (ASTM, 2001a) were used
to evaluate the film resistance to impact and adhesion of applied coatings,
respectively. Additionally, the adhesion strength measurements of epoxy and
diluted mixture were conducted using pull-out tests according to EN 1542
standard (Krzywinski and Sadowski, 2019).
3.1. Copolymerization
An overview of
copolymerization process of Poly)St-co-GMA( is shown in
Figure 2. This reaction mechanism comprised three distinct steps, initiation
(I), propagation (II), and termination (III), conducted at a temperature of 80oC
for 1 hour.
Several factors, such as monomer reactivity, initiator selection, reaction conditions, and kinetics, must be considered to successfully copolymerize St and GMA. The reactivity ratio of the monomers determined the composition and structure of copolymer. The initiator, typically a free radical, must be compatible with the monomers, desired reaction and decomposition temperatures, solubility, and reactivity. Copolymerization process relied on specific reaction conditions, namely temperature, solvent, and time. The temperature, solvent and reaction time must be suitable for reactivity, compatible, and adequate to complete the process. According to Tzoumani et al. (2022), reaction kinetics played a crucial role in determining the composition and molecular weight distribution of copolymer (Tzoumani et al., 2022).
3.2. FTIR spectral
analysis of Poly(St-co-GMA)
Figure 3 shows FTIR absorption spectra of
GMA, where peaks in 942.18 cm-1 to 814.57 cm-1 were
attributed to the oxirane ring in the structure (Zhao et al., 2018). The intensive peak at 1716.38 cm-1 represents the stretching
vibration of C=O in carbonyl groups. In addition, the peak at 1154.49
Figure 3 FTIR spectra for poly GMA, M1 and M2. Copolymerization process included combining St and GMA with different feed monomer compositions, namely M1= 70/30, and M2= 50/50
3.3. Thermal
properties of Poly(St-co-GMA)
TGA
was used to examine the thermal stabilities, including the impact of various St
and GMA ratios on weight loss in copolymer. TGA thermograms of Poly(St-co-GMA)
with various ratios are in Supplementary 1 (S1). Meanwhile, Table 3 shows the
initial decomposition and maximum polymer degradation temperatures (PDTmax), as
well as weight loss (%) after thermal decomposition.
TGA
curves showed that copolymers experienced three-step degradation processes for
M1, M3 and two steps for M2. The temperature range in the first stage was
between 0°C and 170°C, while the weight loss (%) increased with higher GMA
content, attributed to bound water or impurities. M3 and M1 showed the highest
and lowest weight loss of 29% and 9%, respectively. In the second stage, weight
loss for M1 and M3 started at 170°C and continued to 340°C, while for M2, it
extended from 210oC to 900oC, corresponding to the ester
decomposition and loss of CO2 (Negim et al., 2014; Vitaliy et al., 2003). The analysis led to a
higher weight loss for M2 than M3 and M1. The weight loss for the third stage
started at 340°C and continued to 900°C for M1 and M3. However, an increase in
the GMA ratio caused a decrease and an increase in the weight loss for M3 (19%)
and M1 (29%) due to GMA decomposition. Copolymer, with increased GMA content,
showed exceptional thermal stability and versatility, making it suitable for
various applications. The maximum polymer degradation (PDTmax) was
related to the temperature where the highest rate of weight loss occurred, and it
increased as GMA content in copolymer decreased. Meanwhile, M1 and M3 showed the highest and lowest PDTmax of 400oC,
and 180oC, respectively.
High thermal degradation resistance in materials offered several
advantages, namely structural integrity and performance stability, which made
it suitable for high-temperature applications such as automotive components,
electronic devices, and aerospace, ensuring longevity and reliability.
According to Ramezani
et al. (2023), thermal degradation is a process where the mechanical properties of a
material, such as strength and toughness, deteriorate.
High thermal degradation resistance enabled a material to maintain the
properties even under increased temperatures, improving durability and safety.
This extends the shelf life of the material, reducing the need for frequent
repairs, thereby leading to cost savings and increased efficiency. High thermal
degradation resistance enhanced stability, mechanical properties, extended shelf
life, and thermal cycling resistance. The degradation process is important in
demanding environments under elevated temperature conditions (El-Gamal et al., 2023).
Table 3 Thermal properties of Poly)St-co-GMA( at different feed ratios
|
Sample (%) |
Temperature (°C) |
Weight loss (%) |
PDTmax (°C) |
|
M1 |
0 -170 |
9 |
400 |
|
170 -340 |
26 | ||
|
340 -900 |
29 | ||
|
M2 |
0 -210 |
12 |
320 |
|
210 -900 |
55 | ||
|
M3 |
0-180 |
21 |
180 |
|
180 -330 |
40 | ||
|
330 -900 |
16 |
3.4. Applications with epoxy
3.4.1. Viscosity
Viscosity of epoxy resin
(E0) mixed with 10% reactive diluents of varying composition ratios was
measured at different speeds (5 and 50 rpm) and 25oC, as shown in
Table 4. The results depicted that as the viscosity of E0 decreased, the
spindle speed increased, a phenomenon attributed to the orientation of polymers
in the flow direction and chain deformation (Devrani et al., 2017). Viscosity of epoxy resin decreased when mixed with reactive diluents,
from 8592 mPa-s to 430 mPa-s, 680 mPa-s, and 1656 mPa-s for EM1, EM2, and EM3,
respectively. However, viscosity of epoxy resin increased with a higher ratio
of GMA in reactive diluents. The increase was attributed to the dilution effect
of reactive diluents on epoxy resin and the subsequent increase in weight per
epoxide (g/eq) between cross-linking points (Malburet et al., 2023; Rudawska and Frigione, 2022; Jagtap and More,
2021; Negim et al., 2021; Ozeren and Ozkul, 2018). Viscosity and thixotropic
index are important factors when applying epoxy resins spatially to metal and
concrete substrates. The thixotropic index is a ratio of viscosities at low and
high speeds by a factor of ten. The effect of reactive diluents with different
compositions on Thixotropic index (TI) of epoxy resin is shown in Table 5. TI
of epoxy resin (E0) was 3.58, decreasing to less than 1 when epoxy resin was
diluted with reactive diluents. Furthermore, TI of diluted epoxy resin
increased with a higher ratio of GMA in reactive diluents.
Table
4 Viscosity and
thixotropic index of epoxy resin mixed with reactive diluents.
|
Sample code |
E0 |
EM1 |
EM2 |
EM3 |
|
Viscosity at 5 rpm (mPa-s) |
8592 |
430 |
680 |
1656 |
|
Viscosity at 50 rpm (mPa-s) |
2400 |
1400 |
1150 |
1700 |
|
Thixotropic index (TI) |
3.58 |
0.31 |
0.59 |
0.97 |
3.4.2. Adhesion
The adhesion strength of epoxy resin to
concrete and metal substrates is an important parameter in surface bonding (Naderi, 2008). The effect of reactive diluents with
different compositions on the adhesion strength of epoxy on these surfaces is
shown in Table 5. It was reported that as hardener ratio increased from 0.3 %
to 1.0%, the adhesion strength of epoxy resins mixed with reactive diluents
decreased. The decrease was attributed to the type and ratios of hardener (Vidil et al., 2016; Negim et
al., 2011). The mixed epoxy resin with reactive
diluents increased the adhesion strength compared to the reference epoxy resin
(E0). The rise was due to the presence of epoxy ring in GMA and
increased cross-linking resulting from the interaction between the rings from
epoxy resin and reactive diluents with hardener (Thakor et al., 2021). However, the adhesion strength of epoxy mixture on the concrete and
metal substrates increased with the content of GMA in reactive diluents due to
the increasing epoxy ring in the mixture. EM3 containing 70% GMA had the
highest adhesion strength of 3.34 MPa and 5.12 MPa for metal and concrete mixed
with 0.3% hardener. Meanwhile, EM1 mixed with reactive diluents containing 30%
GMA had the lowest adhesion strength of 2.6 MPa and 3.6 MPa for metal and
concrete mixed with 0.3% hardener.
Table 5
The effect of reactive diluents composition on the adhesion of concrete and
metal substrates.
|
Sample code |
E0 |
EM1 |
EM2 |
EM3 | ||||||||
|
|
Adhesion,
MPa | |||||||||||
|
Epoxy: hardener ratios |
1:0.3 |
1:0.5 |
1:1 |
1:0.3 |
1:0.5 |
1:1 |
1:0.3 |
1:0.5 |
1:1 |
1:0.3 |
1:0.5 |
1:1 |
|
Concrete |
3.33 |
2.82 |
2.45 |
3.6 |
3.12 |
2.58 |
3.91 |
3.4 |
3.1 |
5.12 |
4.58 |
4.35 |
|
Metal |
2.19 |
1.51 |
1.93 |
2.6 |
2.3 |
2.1 |
2.72 |
2.53 |
2.4 |
3.34 |
2.86 |
2.7 |
3.4.3. Tensile strength and elongation at break
Tensile strength of both
pure and modified epoxy resin (E0) with reactive diluents containing different
amounts of GMA is shown in Figure 4. An increase in hardener ratio from 0.3 to
1.0 % correlates to higher tensile strength. Vidil et al. (2016) stated that this was
attributed to the cross-linking between epoxy and hardener. Comparing epoxy E0
mixed with a hardener in the ratio of 1:0.3 had lower tensile strength than
1:1. For modified epoxy, namely EM1 – EM3, reactive diluents affected the
tensile strength of the films. EM3 had the highest GMA content and maximum
tensile strength of 65.3 MPa when mixed with the hardener ratio of 1:1, while
EM1, with the lowest GMA content, had a minimum tensile strength of 57.2 MPa
under the same condition. This disparity was due to the higher cross-linking
observed in sample EM3 compared to EM1 (Huang et al., 2017). Figure 4 shows that tensile
strength increased with increasing viscosity due to the rise in molecular
weight as well as the cross-link of epoxy mixture and hardener (Jie et al., 2022).
Figure 4 Tensile strength of pure
epoxy (E0) and modified epoxies (EM1-EM3) at different hardener ratios
Figure 5 shows that the elongation at the
break of pure and modified epoxy decreased with increasing hardener ratios and
GMA content in reactive diluents due to side effects (Rahman et al., 2012).
Figure
5 Elongation at break
of pure epoxy (E0) and modified epoxies (EM1-EM3) at different hardener ratios.
3.4.4. Hardness
The effect of hardener
ratio and GMA content in reactive diluents on the hardness of epoxy films is
shown in Table 6. The hardness of epoxy films increased with increasing
hardener ratios and GMA content in reactive diluents attributed to the rising
cross-link between epoxy ring (epoxy resin & GMA) and hardener (Szewczak and Maciej, 2020). EM1 with 30% GMA content
and a hardener ratio of 1:0.3 had a lower hardness (78) than EM3 containing 70%
GMA and a hardener of 1:1. Generally, the hardness of epoxy films depended on
different factors, including hardener ratios, diluent type, solvent, and
concentrations (Ozeren and
Ozkul, 2018; Syrmanova et al., 2016; Villanueva et al., 2009). Table 6 shows that
increasing hardener ratios and GMA concentrations in reactive diluents enhanced
the impact resistance and flexibility of cured epoxy-coated films.
Table 6 Mechanical properties
of epoxy resin mixed with different reactive diluents.
|
Sample code |
E0 |
EM1 |
EM2 |
EM3 | ||||||||||
|
Mechanical
properties | ||||||||||||||
|
Epoxy: hardener |
1:0.3 |
1:0.5 |
1:1 |
1:0.3 |
1:0.5 |
1:1 |
1:0.3 |
1:0.5 |
1:1 |
1:0.3 |
1:0.5 |
1:1 | ||
|
Impact test |
P |
P |
F |
F |
P |
P |
F |
P |
P |
P |
P |
P | ||
|
Cylindrical Mandrel |
P |
F |
F |
P |
P |
F |
P |
P |
P |
P |
P |
P | ||
|
Cross Hatch |
p |
F |
F |
P |
P |
F |
P |
P |
P |
P |
P |
P | ||
|
Hardness |
75 |
79 |
80.5 |
78 |
79.5 |
81.9 |
84 |
86.9 |
88.7 |
85 |
86.5 |
89.6 | ||
In conclusion, Poly(St-co-GMA) copolymer, one of reactive diluents for
epoxy resin, was successfully synthesized with various ratios of monomers
(70:30, 50:50, and 30:70 w/w) using free the radical solution polymerization
method to improve the physical and mechanical properties of epoxy resin in the
presence of an amine-based hardener at ratios of 1:1, 1:0.5 and 1:0.3. The incorporation of GMA epoxy groups improved the adhesion of anticorrosion coatings,
barrier properties, thermal stability, and service life. Meanwhile,
mixed epoxy resins enhanced adhesion and tensile strength. Reactive diluents
and hardener with ratios of 30:70 and 1:0.3 had the highest mechanical
properties. The hardness of epoxy films, impact resistance, and flexibility
increased with higher hardener ratios and GMA concentration in reactive
diluents, enhancing mechanical and physical properties. The results provided
insights into optimizing the solvents used in the coating industries,
potentially changing it to solvent-free coatings with exceptional mechanical
properties and reduced environmental pollution. The knowledge and use of
Poly(St-Co-GMA) for various epoxy resin applications improved further by these
initiatives.
This research is funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. AP 19676595 Development of high electrically conductive paint for corrosion prevention in concrete structures). Authors also thank to Assoc. Prof. Dr. Anwar Usman from Universiti Brunei Darussalam for his contribution to analyze, finalize and fine tuning this article.
| Filename | Description |
|---|---|
| R1-CE-6920-20240628103021.docx | --- |
Ainakulova, D., Muradova, S., Khaldun, M.A.A., Bekbayeva, L.,
Megat-Yusoff, P., Mukatayeva, Z., Ganjian, E., Negim, E.-S., 2023. Analytical
Review of Conductive Coatings, Cathodic Protection, and Concrete. Kompleksnoe
Ispolzovanie Mineralnogo Syra = Complex Use of Mineral Resources, Volume
329(2), pp. 92–102
American Society for Testing and Materials (ASTM), 2001a. ASTM D3359. Standard Test Methods for Rating Adhesion by Tap Test
American Society for Testing and Materials
(ASTM), 2001b. ASTM D522. Standard Test Methods for Mandrel Bend Test of
Attached Organic Coatings
American Society for
Testing and Materials (ASTM), 2019. ASTM D2794. Standard Test Methods for Resistance of Organic
Coatings to The Effects of Rapid Deformation (Impact)
American Society for Testing and Materials (ASTM), 2019. ASTM D1652. Standard Test Method for
Epoxy Content of Epoxy Resins
Asha, A.B., Srinivas, S., Hao, X., Narain, R., 2019. Enzyme-Responsive
Polymers: Classifications, Properties, Synthesis Strategies, and Applications. Smart
Polymers and their Applications, Volume 2019, pp. 155–189
Azzahari, A.D., Yahya, R., Hassan, A., Sheikh, Md.R.K., 2012. Synthesis
and Characterization of New Copolymers from Glycidyl Methacrylate and
Tetrahydrofurfuryl Acrylate: Determination of Reactivity Ratios. Fibers and
Polymers, Volume 13(5), pp. 555–563
Dagdag, O., Hsissou, R., El-Harfi, A., Berisha, A., Safi, Z., Verma, C.,
Ebenso, E. E., Ebn-Touhami, M., El-Gouri, M., 2020. Fabrication of Polymer
Based Epoxy Resin As Effective Anti-Corrosive Coating For Steel: Computational
Modeling Reinforced Experimental Studies. Surfaces and Interfaces,
Volume 18, p. 100454
Devianto, H., Nurdin, I., Widiatmoko, P., Silvia, D.,
Prakarsa, C., 2023. Tobacco Extract for
Inhibition of Carbon Steel Corrosion in H2S-contained NaCl Solution. International
Journal of Technology, Volume 14(5), pp. 1167-1176
Devrani, S., Sharma, R., Rajesh, M., Kapoor, A., 2017. Exploring Viscoelastic Characteristics of Polymer-Water
Solutions by Viscometric Analysis. Asian Journal of Chemistry, Volume
29(9), pp. 1953–1958
El-Gamal, R., Song, C., Rayan, A.M., Liu, C., Al-Rejaie, S., ElMasry, G.,
2023. Thermal Degradation of Bioactive Compounds During Drying Process of
Horticultural and Agronomic Products: A Comprehensive Overview. Agronomy,
Volume 13(6), p. 1580
Fu, A.Q., Cheng, Y.F., 2011. Characterization of The Permeability of a
High Performance Composite Coating to Cathodic Protection and its Implications
on Pipeline Integrity. Progress in Organic Coatings, Volume 72(3), pp.
423–428
Hermán, V., Takacs, H., Duclairoir, F., Renault, O.,
Tortai, J. H., Viala, B., 2015. Core
Double–Shell Cobalt/Graphene/Polystyrene Magnetic Nanocomposites Synthesized By
In Situ Sonochemical Polymerization. RSC Advances, Volume 5(63), pp.
51371–51381
Huang, Y., Tian, Y., Li, Y., Tan, X., Li, Q., Cheng, J., Zhang, J., 2017.
High Mechanical Properties of Epoxy Networks with Dangling Chains and Tunable
Microphase Separation Structure. RSC Advances, Volume 7(77), pp.
49074–49082
International Organization for Standardization (ISO), 2018. ISO 12058-1. International Standard: Determination of
Viscosity Using a Falling-Ball Viscometer
Jagtap, A.R., More, A., 2021. Developments in Reactive Diluents: A Review.
Polymer Bulletin, Volume 79(8), pp. 5667–5708
Jain, R., Kukrejia, P., Naryla, A.K., Chaudhary, V.K., 2006. Studies of
The Curing Kinetics and Thermal Stability of Epoxy Resins Using a Mixture of
Amines and Anhydrides. Journal of Applied Polymer Science, Volume 100,
pp. 3919–3925
Jie, J., Qingwen, S., Ran, Z., Zhi, S., Jiani, W.,
2022. Viscosity, Mechanical Properties, and
Phase-Separated Morphology of Waterborne Epoxy Asphalt. Construction and
Building Materials, Volume 334, p. 127074
Jingyu, L., Haichao, Z., Guoxin, S., 2022. Renewable Green Reactive
Diluent for Bisphenol a Epoxy Resin System: Curing Kinetics And Properties. RSC
Advances, Volume 12, pp. 31699–31710
Krzywinski, K., Sadowski, L., 2019. The Effect of Texturing of the Surface of Concrete Substrate on the
Pull-Off Strength of Epoxy Resin Coating. Coatings, Volume 9,
p. 143.
Makhlouf, A.S.H., 2014. Protective Coatings for Automotive, Aerospace and
Military Applications: Current Prospects And Future Trends. In Handbook of
Smart Coatings for Materials Protection, pp. 121–131
Malburet, S., Bertrand, H., Richard, C., Lacabanne, C., Dantras, E.,
Graillot, A., 2023. Biobased Epoxy Reactive Diluents Prepared from Monophenol
Derivatives: Effect on Viscosity and Glass Transition Temperature of Epoxy
Resins. RSC Advances, Volume 13(22), pp. 15099–15106
Maruyama, T., 2001. FT-IR Analysis of BSA Fouled on Ultrafiltration and
Microfiltration Membranes. Journal of Membrane Science, Volume 192(1-2),
pp. 201–207
Mehmet, D., 2000. Styrene Polymers and Copolymers. Applied Polymer
Science: 21st Century, pp. 93–106
Msekh, M.A., Cuong, N.H., Zi, G., Areias, P., Zhuang, X., Rabczuk, T.,
2018. Fracture Properties Prediction of Clay/Epoxy Nanocomposites With
Interphase Zones Using A Phase Field Model. Engineering Fracture Mechanics,
Volume 188, pp. 287–299
Naderi, M., 2008. Adhesion of Different Concrete Repair Systems Exposed to
Different Environments. The Journal of Adhesion, Volume 84(1), pp.
78–104
Najuma, A.R., Lity, A.V., 2014. The Effect of Various Hardeners on The
Mechanical and Thermal Properties of Epoxy Resin. International Journal of
Engineering Research & Technology, Volume 3(1), pp. 2662–2665
Negim, E.-S., Bekbayeva, L., Irmukhametova, G.,
Kalugin, S., Alfergani, A., 2021. The Effect of
Poly (Propylene Glycol – G – Styrene) on The Physicomechanical Properties of
Unsaturated Polyester Resin. Egyptian Journal of Chemistry, Volume
65(4), pp. 715–722
Negim, E.S.M., Nurpeissova, Zh.A., Mangazbayeva, R.A., Khatib, J.M.,
Williams, C., Mun, G.A., 2014. Effect of pH on the Physic-Mechanical Properties
and Miscibility of Methyl Cellulose/Poly(Acrylic Acid) Blends. Carbohydrate
Polymers, Volume 101, pp. 415-422
Negim, S. M., Bahruddin, S., Mahyuddin, R., Idiris, M.S., 2011. Effects of
TDI and FA-703 on Physico-Mechanical Properties of Polyurethane Dispersion. Journal of Applied
Polymer Science, Volume121(1), pp. 8–13
Ningrum, E.O., Khoiroh, I., Nastiti, H.I., Affan, R.A., Karisma, A.D.,
Agustiani, E., Surono, A., Suroto, H., Suprapto, S., Taji, L.S., Widiyanto, S.,
2023. Surface Coating Effect on Corrosion Resistance of Titanium Alloy Bone
Implants by Anodizing Method. International Journal of Technology.
Volume 14(4), pp. 749-760
Ozeren, E.E., Ozkul, M.H., 2018. Effects of Epoxy, Hardener, and Diluent
Types on The Hardened State Properties of Epoxy Mortars. Construction and
Building Materials, Volume 187, pp. 360–370
Pineda, A.F.E., Garcia, F.G., Simoes, A.Z., Silva, E.L.D., 2016.
Mechanical Properties, Water Absorption And Adhesive Properties of Diepoxy
Aliphatic Diluent-Modified DGEBA/Cycloaliphatic Amine Networks on 316 L
Stainless Steel. International Journal of Adhesion and Adhesives, Volume
68, pp. 205–211
Pramanik, M., Mendon, S.K., Rawlins, J.W., 2012. Determination of Epoxy
Equivalent Weight of Glycidyl Ether Based Epoxides via Near Infrared
Spectroscopy. Polymer Testing, Volume 31(5), pp. 716–721
Rahman, M.M., Hosur, M., Zainuddin, S., Jajam, K.C., Tippur, H.V.,
Jeelani, S., 2012. Mechanical Characterization of Epoxy Composites Modified
with Reactive Polyol Diluent And Randomly-Oriented Amino-Functionalized MWCNTs.
Polymer Testing, Volume 31(8), pp. 1083–1093
Rajiv, P., Deepa, A., Vanathi, P., Vidhya, D., 2017. Screening for Phytochemicals and FTIR Analysis of Myristica
Dactyloids Fruit Extracts. International Journal of Pharmacy and
Pharmaceutical Sciences, Volume 9(1), pp. 315–318
Ramezani, M., Mohd-Ripin, Z., Pasang, T., Jiang, C-P.,
2023. Surface Engineering of Metals:
Techniques, Characterizations and Applications. Metals, Volume 13(7), p.
1299
Riyanto, Jazuli, M.M., Sahroni, I., Musawwa, M.M., Cahyandaru, N.,
Wahyuni, E.T., 2023. A Simple Technique for the Corrosion Inhibition of
Underwater Cannonball from a Shipwreck. International Journal of Technology,
Volume 14(4), pp. 843–853
Rudawska, A., Frigione, M.. 2022. Effect of Diluents on Mechanical
Characteristics of Epoxy Compounds. Polymers, Volume 14(11), p. 2277
Srikanth, A.P., Sunitha, T.G., Raman, V., Nanjundan,
S., Rajendran, N., 2007. Synthesis,
Characterization and Corrosion Protection Properties of
Poly(N-(acryloyloxymethyl) benzotriazole-co-glycidyl methacrylate) Coatings on
Mild Steel. Materials Chemistry and Physics, Volume103(2–3), pp. 241–247
Syrmanova, K., Negim, E., Kaldybekova, J., Tuleuov,
A.M., 2016. Epoxylitane Compositions Modification
with Using Thermoplastic Polyurethane. Oriental Journal of Chemistry,
Volume 32(1), pp. 1–7
Szewczak, A., Maciej S., 2020. Physico-Mechanical and Rheological
Properties of Epoxy Adhesives Modified by Microsilica and Sonication Process.
Materials, Volume 13(23), p. 5310
Teh, P.L., Mariatti, M., Akil, H.M., Yeoh, C.K., Seetharamu, K.N.,
Wagiman, A.N.R., Beh, K.S. 2007. The Properties of Epoxy Resin Coated Silica
Fillers Composites. Materials Letters, Volume 61(11-12), pp. 2156–2158
Thakor, S.G., Rana, V.A., Vankar, H.P., Pandit, T.R., 2021. Dielectric
Spectroscopy and Structural Characterization of Nano-Filler-Loaded Epoxy Resin.
Journal of Advanced Dielectrics, Volume 11(2), pp. 2150011
Tran, A.D., Koch, T., Knaack, P., Liska, R., 2020. Radical Induced
Cationic Frontal Polymerization For Preparation of Epoxy Composites. Composites Part A: Applied
Science and Manufacturing, Volume 132, p.
105855
Tzoumani, I., Soto-Beobide, A., Iatridi, Z., Voyiatzis, G.A., Bokias, G.,
Kallitsis, J.K., 2022. Glycidyl Methacrylate-Based Copolymers as Healing Agents
of Waterborne Polyurethanes. International Journal of Molecular Sciences,
Volume 23(15), pp. 8118–8139
Vidil, T., Tournilhac, F., Musso, S., Robisson, A., Leibler, L., 2016.
Control of Reactions and Network Structures of Epoxy Thermosets. Progress in
Polymer Science, Volume 62, pp. 126–179
Villanueva, M., Fraga, I., Rodríguez-Añón, J.A., Proupín-Castiñeiras, J., 2009.
Study of the Influence of a Reactive Diluent on the Rheological Properties of
an Epoxy-Diamine System. Journal of Thermal Analysis and Calorimetry,
Volume 98(2), pp. 521–525
Vitaliy, V.K., Maria, G.C., Luigi, L., Zauresh, S.N., Grigoriy, A.M.,
Rauash, A.M., 2003. Phase Behaviour of Methylcellulose-Poly(Acrylic Acid)
Blends and Preparation of Related Hydrophilic Films. Polymer International,
Volume 52(1), pp. 62–67
Wang, H., Han, W., Tian, H., Wang, Y., 2005. The Preparation and
Properties of Glass Powder Reinforced Epoxy Resin. Materials Letters, Volume
(1), pp. 94–99
Zhao, J., Wang, S., Zhang, L.,
Wang, C., Zhang, B., 2018. Kinetic, Isotherm, and Thermodynamic Studies for
Ag(I) Adsorption Using Carboxymethyl Functionalized Poly(Glycidyl
Methacrylate). Polymers, Volume 10(10), pp. 1090–1110
