Novel Multiphase CO2 Photocatalysis System Using N-TiO2/CNCs and CO2 Nanobubble
Corresponding email: y.wibisono@itb.ac.id
Published at : 05 Feb 2024
Volume : IJtech
Vol 15, No 2 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i2.6694
Madani, H., Wibowo, A., Sasongko, D., Miyamoto, M., Uemiya, S., Budhi, Y.W., 2024. Novel Multiphase CO2 Photocatalysis System Using N-TiO2/CNCs and CO2 Nanobubble. International Journal of Technology. Volume 15(2), pp. 432-441
| Haroki Madani | Department of Chemical Engineering, Institut Teknologi Bandung, Jl. Ganesha 10, Bandung, 40132, Indonesia |
| Arie Wibowo | 1. Material Science and Engineering Research Group, Faculty of Mechanical and Aerospace Engineering, Institut Teknologi Bandung, Jl. Ganesha 10, Bandung, 40132 Indonesia, 2. Research Center for Nanosc |
| Dwiwahju Sasongko | Department of Chemical Engineering, Institut Teknologi Bandung, Jl. Ganesha 10, Bandung, 40132, Indonesia |
| Manabu Miyamoto | Department of Chemistry and Biomolecular Science, Gifu University, 1-1 Yanagido, 501-1193 Gifu, Japan |
| Shigeyuki Uemiya | Department of Chemistry and Biomolecular Science, Gifu University, 1-1 Yanagido, 501-1193 Gifu, Japan |
| Yogi Wibisono Budhi | 1. Department of Chemical Engineering, Institut Teknologi Bandung, Jl. Ganesha 10, Bandung, 40132, Indonesia, 2. Research Center for Nanoscience and Nanotechnology, Institut Teknologi Bandung, Jl. Gan |
In this study, a novel CO2 photocatalysis system was developed
by modifying TiO2 as a photocatalyst and introducing CO2
nanobubble to the system. TiO2 photocatalyst is modified by adding
CNCs as support to increase the surface area and adding nitrogen doping to
reduce the band gap and minimize electron–hole recombination. CO2
nanobubbles are introduced to increase the surface area between the CO2
gas and liquid phases to reduce mass transfer limitations. Thus, the amount of
CO2 in the liquid phase increases. Nanobubbles have been successfully
generated by the hydrodynamic cavitation method, which produces bubbles with
two size clusters, namely 200–400 nm, which belong to nanobubbles, and 2–10 um,
which belong to microbubbles. The TiO2 has an anatase phase and
crystallite size of 20.90 nm for TiO2/CNCs and 19.20 nm for N-TiO2/CNCs.
The activity test without nitrogen doping produced a methanol product of 0.77
mmol/g catalyst, which shows that this multiphase CO2 photocatalytic
system is feasible for CO2 photocatalytic reactions. The addition of
nitrogen doping succeeded in reducing the band gap from 3.20 eV to 3.10 eV and
increasing the methanol yield. The photocatalysis activity test with N-doped
TiO2/CNCs resulted in a higher methanol yield, which is 1.13 mmol/g
catalyst under UV-C irradiation for 6 hours.
CO2 photocatalysis; Multiphase reaction; Nanobubble; Nitrogen doping; TiO2
CO2
photocatalysis is an appealing solution to reduce CO2 on the earth's
surface because this method not only can reduce CO2 but also can
provide solar fuels (CH4, CH3OH, HCHO, HCOOH) as an
alternative to fossil energy (Shao et al.,
2022). TiO2 is the most used semiconductor for CO2
photocatalysis due to its non-toxic characteristics, high energy potential, and
low cost. However, TiO2 has a low photoconversion rate due to its
high band gap, which results in a high energy requirement for electron
excitation, electron-hole recombination, a low ability to absorb sunlight, and
a low ability to adsorb CO2 (Low, Cheng,
and Yu, 2017). Thus, TiO2 needs to be modified further to improve
its performance
TiO2
can be modified in various ways, such as the addition of co-catalysts and
doping. The effects of co-catalysts and doping can vary based on the type of
atom used. Some examples of co-catalysts
are Ag, Co, and Cu. The addition of a plasmonic Ag metal core can increase the
photon flux on TiO2, which can increase the CO2
photocatalysis conversion (Hong et al.,
2019). A co-based co-catalyst can increase CO2 adsorption
capacity and provide more CO2 in the reaction (Zhang et al., 2020). Cu co-catalyst is
more beneficial for CO2 adsorption and a good co-catalyst for
improving catalytic selectivity to CH3OH (Xi
et al., 2022). Doping is another type of TiO2
modification that can use metal or non-metal atoms as dopants. The addition of
dopant didn’t lead to a structural defect that occurs in co-catalyst
modification, which is an advantage of doping modification if compared with
co-catalyst. An example of TiO2 modification with doping is using N
and Mo. N-doping can reduce the band gap and minimize electron-hole
recombination. In addition to lowering the band gap, nitrogen doping can
minimize electron-hole recombination by filling the oxygen defect in TiO2,
which is the key recombination site (Kumar, Das,
and Deepa, 2020). Mo-doping can increase the selectivity toward CH4
products and increase electron-hole separation and proton supply (Feng et al., 2020). A smaller band gap
allows electrons to be excited by lower energy light so that photocatalysts can
be used in visible light irradiation. For example, as Kumar,
Das, and Deepa (2020) reported,
nitrogen doping can lower the band gap of TiO2/CdS from 3.17 eV to
2.91 eV.
Besides using
co-catalyst and doping, TiO2 can also be modified by creating a
mesoporous structure, which can increase the active site of the photocatalyst.
For example, Yudha et al. (2020) use
dammar-gum as a soft template for mesoporous TiO2 synthesis. Another
interesting material that can be used for mesoporous TiO2 templates
is cellulose nanocrystals (CNCs) (Chen et al.,
2016). CNCs are cellulose-based nanomaterial with high crystallinity,
between 54–88% (Moon et al., 2011).
CNCs are renewable materials that can be made from various sources containing
cellulose, such as wood (Leung et al., 2011),
flax (Leung et al., 2011), empty palm
oil bunch (Harahap et al., 2023;
Marpongahtun et al., 2023; Budhi et
al., 2018; Wibowo et al., 2018),
and other lignocellulosic materials (Restiawaty et
al., 2022). Even CNCs can be produced from waste materials
containing cellulose, such as paper and denim waste (Culsum
et al., 2021). CNCs have several superior properties, such as
high mechanical properties, high surface area, high biocompatibility, and low
toxicity (Brinchi et al., 2013). With
renewable sources of raw materials and superior properties, CNCs can be a
long-term alternative green material for various purposes. CNCs can be used as
a material to support various catalysts, one of which is TiO2. Maimaiti et al. (2019) conducted a study
related to TiO2 photocatalysts dispersed on the surface of CNCs with
the addition of EDA treatment. The photocatalyst produced 4.5 times more
products than pure TiO2 (P25) photocatalyst. The small size of CNCs
causes a large surface area that increases the surface area of the TiO2
photocatalyst active center. With many active centers, the number of products
produced is also greater.
Besides
photocatalyst modification, CO2 photocatalytic conversion can also
be increased by improving the photocatalytic process. In multiphase system, the
mass transfer limitation become a hindrance for CO2 conversion. To
overcome the issue of CO2 mass transfer limitation in a multiphase
system, reducing the bubble size to the smallest possible can increase the
contact surface area between the gas and liquid phases. In addition, the
smaller bubble size can produce a larger mass transfer coefficient (kLa)
so that the mass transfer of CO2 from the gas phase to the liquid
phase becomes faster. Nanobubbles are defined as gas bubbles that have a
diameter smaller than 1000 nm. The use of nanobubbles over regular bubbles in a
process has various advantages, including high mass transfer efficiency, high
bubble stability, and low rising velocity (Ulatowski
et al., 2019). The utilization of nanobubble in chemical
reactions remains relatively limited. Nevertheless, its application exhibits
considerable potential. For example, Mase et al.
(2013) reported a significant enhancement in the conversion rate from 2%
to 99% in the hydrogenation of alkenes through the usage of nanobubble.
In this study, a CO2
photocatalysis reaction is carried out using CO2 nanobubble as the
source of CO2. This is expected to increase the contact surface area
of the gas and liquid phases and increase the CO2 mass transfer
coefficient. Besides, TiO2 is modified by dispersing it on the CNC
surface and adding nitrogen doping. Nitrogen doping is expected to lower the
band gap and minimize the electron-hole recombination so that more electrons
are utilized for CO2 reduction, leading to higher CO2
conversion. The aim of this study is to study the feasibility of CO2
nanobubble usage in multiphase CO2 photocatalysis reaction in a
batch reactor without the in and out flow of CO2 gas. We believe
that the result of this study can open new possibilities in the development of CO2
photocatalysis reactions.
2.1. Materials
Titanium tetra isopropoxide (TTIP, 97.0%),
ethanol absolute (99.99%), nitric acid (69%), triethylamine, and sodium
hydroxide (50%) were purchased from Merck kGaA, Darmstadt, Germany. Cellulose
nanocrystals (CNCs) were purchased from Celluforce, Quebec, Canada. Deionized
water was used in all experiments.
2.2. Photocatalyst Synthesis
2.3. Photocatalyst Characterization
2.4. Nanobubble Generation and Photocatalytic Activity Test
CO2 nanobubbles were
generated by the hydrodynamic cavitation method using a nanobubble generator
from Nanobubble.id with the type of NB S1. The according to the system depicted
in Figure 2a. During the generating process, the temperature was kept below
28°C. Water was pumped into the main container to create a circulation from the
main container to the nanobubble generator and vice versa. The CO2
gas with 0.1 LPM flow rate was injected into the nanobubble generator via the
water that passed through it. After 1 hour of nanobubble generation, the water
in the main container was either stored for characterization or utilized in CO2
photocatalysis. The characterization of nanobubble is carried out by dynamic
light scattering (DLS) to analyze nanobubble size and its distribution.
The photocatalytic activity test was carried out in a batch reactor, as illustrated in Figure 2b. The reactor was filled with 200 mL of water containing generated nanobubble and 0.2 g of photocatalyst. The mixture in the reactor was stirred at 300 rpm during the reaction process. The photocatalysis was carried out by irradiating the reactor using a UV lamp (UV-C, 15 W, 280 nm) or visible lamp (15 W, 580–750 nm) on both sides, as illustrated in Figure 2b. The reaction was carried out for 6 hours, and the sample of liquid product was taken to be analyzed using Gas Chromatography with Porapack Q column. All the CO2 used in this system is sourced exclusively from pre-generated CO2 nanobubbles. However, there is a drawback to this setup as the product yield is limited due to the finite supply of available CO2. Nevertheless, this system was intentionally designed to showcase the significance of CO2 nanobubbles in the photocatalytic conversion of CO2 into solar fuel.
Figure 2 (a) Nanobubble generation system for CO2 nanobubble generation and (b) CO2
photocatalysis batch reactor using nanobubble
3.1. Visual Observation of Photocatalyst
Synthesis
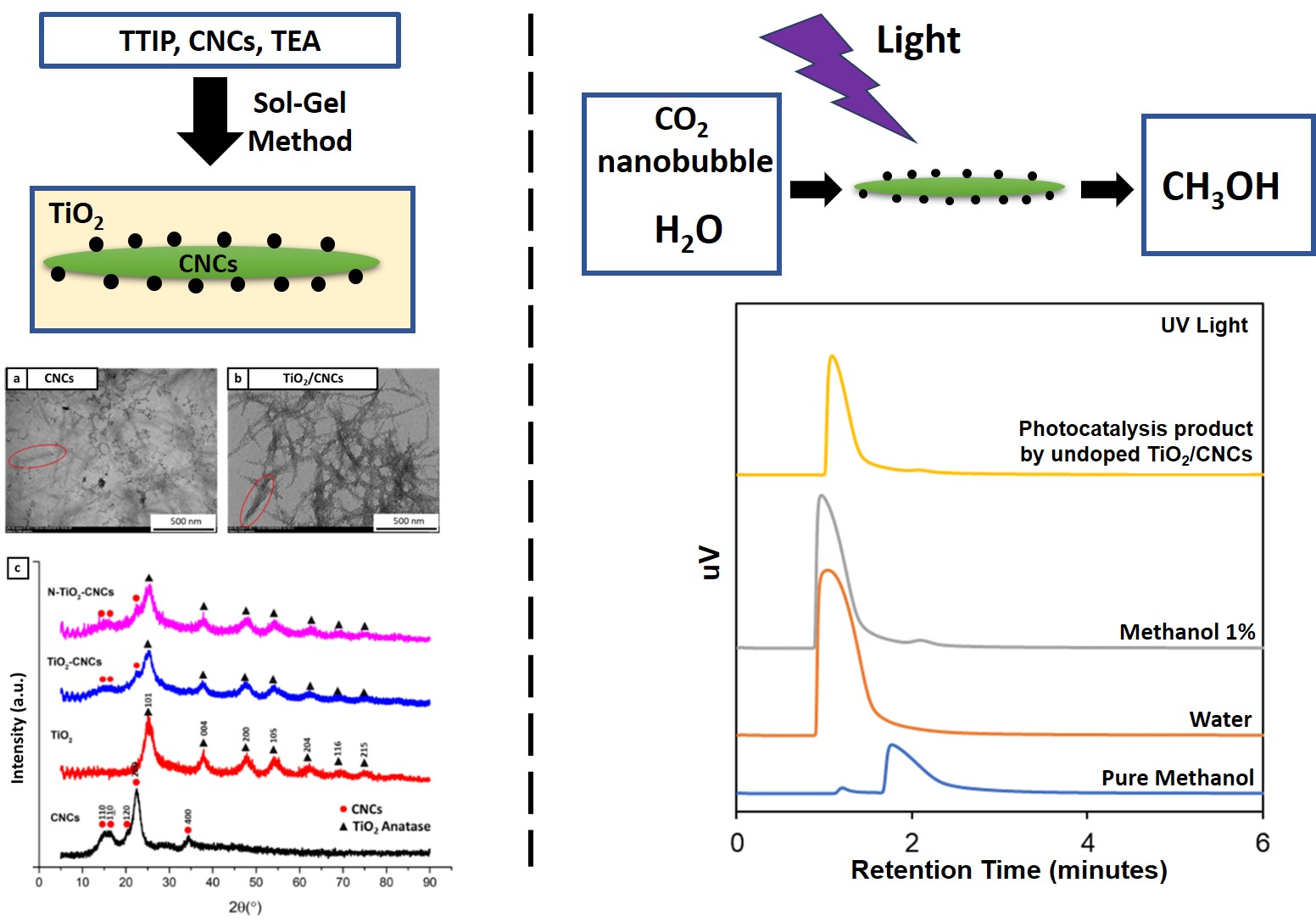
TiO2/CNCs synthesis processes can be separated into four steps: 1) TTIP mixing with solvent, 2) HNO3 to lower the pH, 3) NaOH addition, and 4) aging. The visual observation of each step is presented in Figure 3.
Figure
3 Visual
observation of Photocatalyst Synthesis through the sol-gel method
In
the first step, TTIP was mixed into absolute ethanol with a purity of 99.99%
and followed by HNO3 1 M addition to avoid uncontrolled formation of
Ti(OH)4 due to the hygroscopic nature of TTIP. Ti(OH)4
particles were formed during the hydrolysis of TTIP, which was triggered by the
gradual addition of NaOH (Mustapha
et al., 2020). The hydrolysis process was then
followed by a condensation process that occurred during the aging stage, which
took 24 hours. In this stage, the Ti(OH)4 was converted to TiO2
while producing water. As reported by Martakov et
al. (2018), during the aging stage, not only does the formation of TiO2
occur, but also the deposition of TiO2 on the surface of the CNCs.
The deposition of TiO2 on the surface of TiO2 occurs
through electrostatic and chemical interaction. The electrostatic interaction
is triggered by the positive surface charge on TiO2 particles and
the negative surface charge on the CNCs surface due to the existence of
hydroxyl groups. The chemical interaction between TiO2 and CNCs occurs
in the form of hydrogen bonds between TiO2 and hydroxyl groups on
the surface of CNCs.
3.2. Photocatalyst
Characterization
Figure 4 (a,b) TEM observations and (c) XRD curve of CNCs and TiO2/CNCs
TEM characterization results show that
CNCs and TiO2/CNCs have needle-like shapes. The average diameter and length of the
CNCs particles are 30.30 ± 7.90 nm and 299 ± 67 nm, respectively, as determined
from the analysis results using ImageJ software. Although the CNCs and TiO2/CNCs particles have a common shape, the two
have differences when viewed from the edges. On TiO2/CNCs, black spots are suspected to be TiO2 particles attached to the surface of the
CNCs. Further confirmation of the presence of TiO2 can be seen from the results of the XRD
characterization, as presented in Figure 4c.
The
CNCs sample showed peaks of 2 at 15°, 17°, 23°, and 34°, which indicated that
the cellulose contained in the CNCs was type I cellulose. In detail, each of
these peaks successively represents the crystallographic plane of (1 0 1), (1 0
-1), (0 0 2), and (0 0 4) (Jiang et al.,
2017). Meanwhile, the TiO2 sample showed 2
peaks at 24.9°, 37.6°, 47.8°, 53.7°, 62.5°, 68.5°,
70.1° and 74.88° which represents the crystallographic plane of (1 0 1), (0 0
4), (2 0 0), (1 0 5), (2 0 4), (1 1 6), (2 2 0) and (2 1 5). These peaks are in
good agreement with anatase TiO2 peaks based on JCPDS no. 21-1272.
As depicted in Figure 5, CNCs exhibit a
relatively straight reflectance curve, suggesting that there is no dominant
light absorption activity at a specific wavelength. This is expected since CNCs
are not semiconductor materials capable of exciting electrons when exposed to
light at a particular wavelength. In contrast, TiO2 and TiO2/CNCs have a lower reflectance at a 200–400 nm
wavelength, meaning that more light is absorbed in that area. In other words, TiO2 and TiO2/CNCs can excite electrons in irradiating
light with a wavelength below 400 nm. This wavelength is equivalent to a band
gap energy of ~3.2 eV, which is the band gap of TiO2 (Mustapha et al., 2020). From Kubelka-Munk
Plot, the TiO2 and TiO2/CNCs samples have a band gap of 3.21 and 3.19 eV, respectively. The N-TiO2/CNCs photocatalyst has a band gap of 3.1 eV,
which is lower than TiO2/CNCs. This result confirms that nitrogen doping can lower the band gap
energy and enable the photocatalyst to be used under visible light irradiation.
3.3. Nanobubble
Characterization
Nanobubble has high
stability because it has a very long rising time. In comparison, the rising
time of nanobubble particles can be days or even months (Ulatowski et al., 2019), while microbubbles and
macrobubbles, respectively, have rising times in the range of minutes and
seconds (Temesgen et al., 2017).
Figure 6a shows that CO2 nanobubble still remained in the water
after 4 days, which is an important justification for the usage of CO2
nanobubble in batch reactors after its generation.According to the DLS results
in Figure 6b, the generated CO2 nanobubbles exhibit two size
clusters, categorized as nanobubbles with a size of 200–400 nm and microbubbles
with a size of 2–10 µm. Based on the plot of undersize (y-axis on the right) vs
particle size, 35% of the total generated nanobubbles are below 400 nm in size.
3.4. Photocatalyst Activity Test of TiO2/CNCs
Figure 7 GC curve of liquid
product produced from CO2 photocatalysis by TiO2/CNCs photocatalyst
at (a) UV-C light and (b) visible light
Based
on Figure 7a, the liquid product of CO2 photocatalysis has two peaks
at 1.1 and 2 minutes, which are the retention times for water and methanol,
respectively. In detail, the resulting methanol product was 0.77 mmol/g
catalyst for TiO2/CNCs and 1.13 mmol/g catalyst for N-TiO2/CNCs.
The existence of methanol in CO2 photocatalysis products at least proves two things.
First, TiO2/CNCs and N-TiO2/CNCs photocatalyst has
photocatalytic activity under UV-C light, so the photocatalysis reaction can
occur. Second, the proposed system using CO2 nanobubble is feasible for CO2
photocatalysis in multiphase reactions. An important point to highlight from
this result is that there was no CO2 gas flow during the
photocatalysis reaction. All CO2 utilized during the reaction solely originated from
CO2
nanobubbles pre-existing in the system before the reaction.
Figure
7b shows the comparison of TiO2/CNCs (undoped) and N-TiO2/CNCs
(N-doped) photocatalysts under visible light irradiation. It is shown that the
doped photocatalyst exhibits visible light photocatalytic activity while the
undoped does not. This is due to the lower band gap energy of N-TiO2/CNCs,
so visible light with lower energy can excite the electron in the doped
photocatalyst. From the Kubelka-Munk Plot that has been discussed earlier, N-TiO2/CNCs
have a band gap of 3.1 eV. Based on the Planck equation (E (eV) = 3.1 eV is equivalent to
the wavelength of 400 nm, which belongs to visible light. In comparison, Kumar, Das, and Deepa (2020) reported that
nitrogen doping addition to TiO2/CdS can reduce the band gap from
3.17 eV to 2.91 eV (Kumar,
Das, and Deepa, 2020).
In this study, the
photocatalysis reaction of CO2 was carried out in a batch reactor,
where CO2 gas in the form of nanobubbles in water was directly
irradiated with light. There is no flow of CO2 gas in and out. All CO2
used for the reaction was provided from nanobubbles in the water. The presence
of methanol in the photocatalysis product with this system proves the
feasibility of multiphase systems with CO2 nanobubbles for CO2
photocatalytic reactions. Nitrogen doping to the photocatalyst reduced the band
gap from 3.20 eV to 3.10 eV, which was later proven by the photocatalytic
activity of N-TiO2/CNCs under visible light irradiation.
The authors
acknowledge the Ministry of Education, Culture, Research, and Technology of the
Republic of Indonesia for providing financial support through the National
Competitive Research-Doctoral Dissertation Research Scheme (contract number:
110/E5/PG.02.00.PL/2023; 318/IT1.B07.1/SPP-LPPM/VI/2023), the integrated master
to doctor scholarship program (PMDSU), and ITB Research 2023.
| Filename | Description |
|---|---|
| R2-CE-6694-20230930210306.docx | Supplementary material |
Brinchi, L., Cotana, F., Fortunati, E., Kenny,
J.M., 2013. Production of Nanocrystalline Cellulose from Lignocellulosic Biomass:
Technology and applications. Carbohydrate polymers, Volume 94(1), pp.
154–169
Budhi, Y.W., Fakhrudin, M., Culsum, N.T.U.,
Suendo, V., Iskandar, F., 2018. Preparation of Cellulose Nanocrystals from Empty
Fruit Bunch of Palm Oil by using Phosphotungstic Acid Preparation of Cellulose Nanocrystals
from Empty Fruit Bunch of Palm Oil by using Phosphotungstic Acid. In:
IOP Conference Series: Earth and Environmental Science, Volume 105, p. 012063
Chen, X., Kuo, D.H., Lu, D., 2016. N-doped Mesoporous
TiO2 Nanoparticles Synthesized by using Biological Renewable Nanocrystalline
Cellulose as Template for the Degradation of Pollutants under Visible and Sun Light.
Chemical Engineering Journal, Volume 295, pp. 192–200
Culsum, N.T.U., Melinda, C., Leman, I., Wibowo,
A., Budhi, Y.W., 2021. Isolation and Characterization of Cellulose Nanocrystals
(CNCs) from Industrial Denim Waste using Ammonium Persulfate. Materials
Today Communications, Volume 26, pp. 101817
Feng, S., Zhao, J., Bai, Y., Liang, X., Wang, T.,
Wang, C., 2020. Facile Synthesis of Mo-doped TiO2 for Selective Photocatalytic
CO2 Reduction to Methane: Promoted H2O Dissociation by Mo
Doping. Journal of CO2 Utilization. Volume 38, pp.
1–9
Harahap, M., Daulay, N., Zebua, D., Gea, S.,
2023. Nanofiber Cellulose/Lignin from Oil Palm Empty Fruit Bunches and the
Potential for Carbon Fiber Precursor Prepared by Wet-spinning. Journal of CO2
Utilization, Volume 14, pp. 152–161
Hong, D., Lyu, L.M., Koga, K., Shimoyama, Y.,
Kon, Y., 2019. Plasmonic Ag@TiO2 Core-Shell Nanoparticles for
Enhanced CO2 Photoconversion to CH4. ACS Sustainable
Chemistry and Engineering, Volume 7(23),
pp. 18955–18964
Jiang, H., Wu, Y., Han, B., Zhang, Y., 2017.
Effect of Oxidation Time on the Properties of Cellulose Nanocrystals from Hybrid
Poplar Residues using the Ammonium Persulfate. Carbohydrate Polymers,
Volume 174, pp. 291–298
Kumar, P.N., Das, A., Deepa, M., 2020. Nitrogen Doping
of TiO2 and Annealing Treatment of Photoanode for Enhanced Solar Cell
Performance. Journal of Alloys and Compounds, Volume 832, p. 154880
Leung, A.C.W., Hrapovic, S., Lam, E., Liu, Y.,
Male, K.B., Mahmoud, K.A., Luong, J.H.T., 2011. Characteristics and Properties
of Carboxylated Cellulose Nanocrystals Prepared from a Novel One-step Procedure.
Small, Volume 7, pp. 302–305
Low, J., Cheng, B., Yu, J., 2017. Surface Modification
and Enhanced Photocatalytic CO2 Reduction Performance of TiO2:
a Review. Applied Surface Science, Volume 392, pp. 658–686
Maimaiti, H., Awati, A., Yisilamu, G., Zhang, D.,
Wang, S., 2019. Synthesis and Visible-light Photocatalytic CO2/H2O
Reduction to Methyl Formate of TiO2 Nanoparticles Coated by Aminated
Cellulose. Applied Surface Science, Volume 466, pp. 535–544
Marpongahtun, Andriayani, Muis, Y., Gea, S.,
Amaturrahim, S.A., Attaurrazaq, B., Daulay, A., 2023. Synthesis of Nitrogen-Doped
Carbon Dots from Nanocrystalline Cellulose by Pyrolysis Method as Hg2+
Detector. International Journal of Technology, Volume 14(1),
pp. 219–231
Martakov, I.S., Torlopov, M.A., Mikhaylov, V.I.,
Krivoshapkina, E.F., Silant’ev, V.E., Krivoshapkin, P. V, 2018. Interaction of Cellulose
Nanocrystals with Titanium Dioxide and Peculiarities of Hybrid Structures Formation.
Journal of Sol-Gel Science and Technology, Volume 88, pp. 13–21
Mase, N., Isomura, S., Toda, M., Watanabe, N.,
2013. Micro and Nanobubble Based Strategy for Gas – Liquid – Solid Multiphase
Re- actions: Palladium-Catalysed Hydrogenation of Carbon – Carbon Unsaturated Bonds.
Synlett, Volume 24(17), pp. 2225–2228
Moon, R.J., Martini, A., Nairn, J., Simonsen, J.,
Youngblood, J., 2011. Cellulose Nanomaterials Review: Structure, Properties and
Nanocomposites. Chemical Society Reviews, Volume 40(7), pp. 3941–3994
Mustapha, S., Ndamitso, M.M., Abdulkareem, A.S.,
Tijani, J.O., Shuaib, D.T., Ajala, A.O., Mohammed, A.K., 2020. Application of
TiO2 and ZnO Nanoparticles Immobilized on Clay in Wastewater Treatment:
a Review. Applied Water Science, Volume 10, pp. 1–36
Restiawaty, E., Culsum, N.T., Nishiyama, N.,
Budhi, Y.W., 2022. Preparation, Characterization, and Surface Modification of
Cellulose Nanocrystal from Lignocellulosic Biomass for Immobilized Lipase. Fibers,
Volume 10(4), p. 33
Shao, B., Zhang, Y., Sun, Z., Li, J., Gao, Z.,
Xie, Z., Hu, J., Liu, H., 2022. CO2 Capture and in-situ Conversion: Recent Progresses
and Perspectives. Green Chemical Engineering, Volume 3(3), pp. 189–198
Temesgen, T., Thuy, T., Han, M., Kim, T., Park,
H., 2017. Micro and Nanobubble Technologies as a New Horizon for Water-treatment
Techniques: A Review. Advances in Colloid and Interface Science, Volume
246, pp. 40–51
Ulatowski, K., Sobieszuk, P., Mróz, A., Ciach,
T., 2019. Chemical Engineering & Processing: Process Intensification
Stability of Nanobubbles Generated in Water using Porous Membrane System. Chemical
Engineering and Processing-Process Intensification, Volume 136, pp. 62–71
Wibowo, A., Madani, H., Judawisastra, H.,
Restiawaty, E., Lazarus, C., Budhi, Y.W., 2018. An Eco-friendly Preparation of Cellulose
Nano Crystals from Oil Palm Empty Fruit Bunches. In: IOP Conference
Series: Earth and Environmental Science, Volume 105, pp. 012059
Xi, H., Xu, Y., Zou, W., Ji, J., Cai, Y., Wan,
H., Dong, L., 2022. Enhanced Methanol Selectivity of CuxO/TiO2 Photocatalytic
CO2 Reduction: Synergistic Mechanism of Surface Hydroxyl and Low-valence
Copper Species. Journal of CO2 Utilization, Volume 55, p.
101825
Yudha, S.S., Falahudin, A., Asdim, A., Han, J.I.,
2020. Utilization of Dammar-Gum as a Soft Template in Titania Synthesis for
Photocatalyst. International Journal of Technology, Volume 11(4), pp.
842–851
Zhang, X., Yuan, Z., Chen, J., Yang, G.,
Dionysiou, D.D., Huang, B., Jiang, Z., 2020. Enhanced CO2
photoconversion activity of TiO2 via double effect of CoPi as hole
traps and high CO2 capture. Catalysis Today, Volume 340, pp.
204–208
