Physical and Chemical Characterization of Collagen/Alginate/Poly(Vinyl Alcohol) Scaffold with the Addition of Multi-Walled Carbon Nanotube, Reduced Graphene Oxide, Titanium Dioxide, and Zinc Oxide Materials
Corresponding email: fauziyah17@ui.ac.id
Published at : 05 Feb 2024
Volume : IJtech
Vol 15, No 2 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i2.6693
Fajarani, R., Rahman, S.F., Pangesty, A.I., Katili, P.A., Park, D., Basari., 2024. Physical and Chemical Characterization of Collagen/Alginate/Poly(Vinyl Alcohol) Scaffold with the Addition of Multi-Walled Carbon Nanotube, Reduced Graphene Oxide, Titanium Dioxide, and Zinc Oxide Materials. International Journal of Technology. Volume 15(2), pp. 332-341
| Rusyda Fajarani | Department of Electrical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, West Java 16424, Indonesia |
| Siti Fauziyah Rahman | 1. Department of Electrical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, West Java 16424, Indonesia, 2. Research Center for Biomedical Engineering, Faculty of Engineeri |
| Azizah Intan Pangesty | 1. Research Center for Biomedical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, West Java 16424, Indonesia, 2. Department of Metallurgical and Materials Engineering, Fac |
| Puspita Anggraini Katili | 1. Department of Electrical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, West Java 16424, Indonesia, 2. Research Center for Biomedical Engineering, Faculty of Engineeri |
| Don-Hee Park | 1. Interdisciplinary Program of Bioenergy and Biomaterial Engineering, Chonnam National University, Gwangju 500-757, Republic of Korea, 2. Department of Biotechnology and Bioengineering, Chonnam Natio |
| Basari | 1. Department of Electrical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, West Java 16424, Indonesia, 2. Research Center for Biomedical Engineering, Faculty of Engineeri |
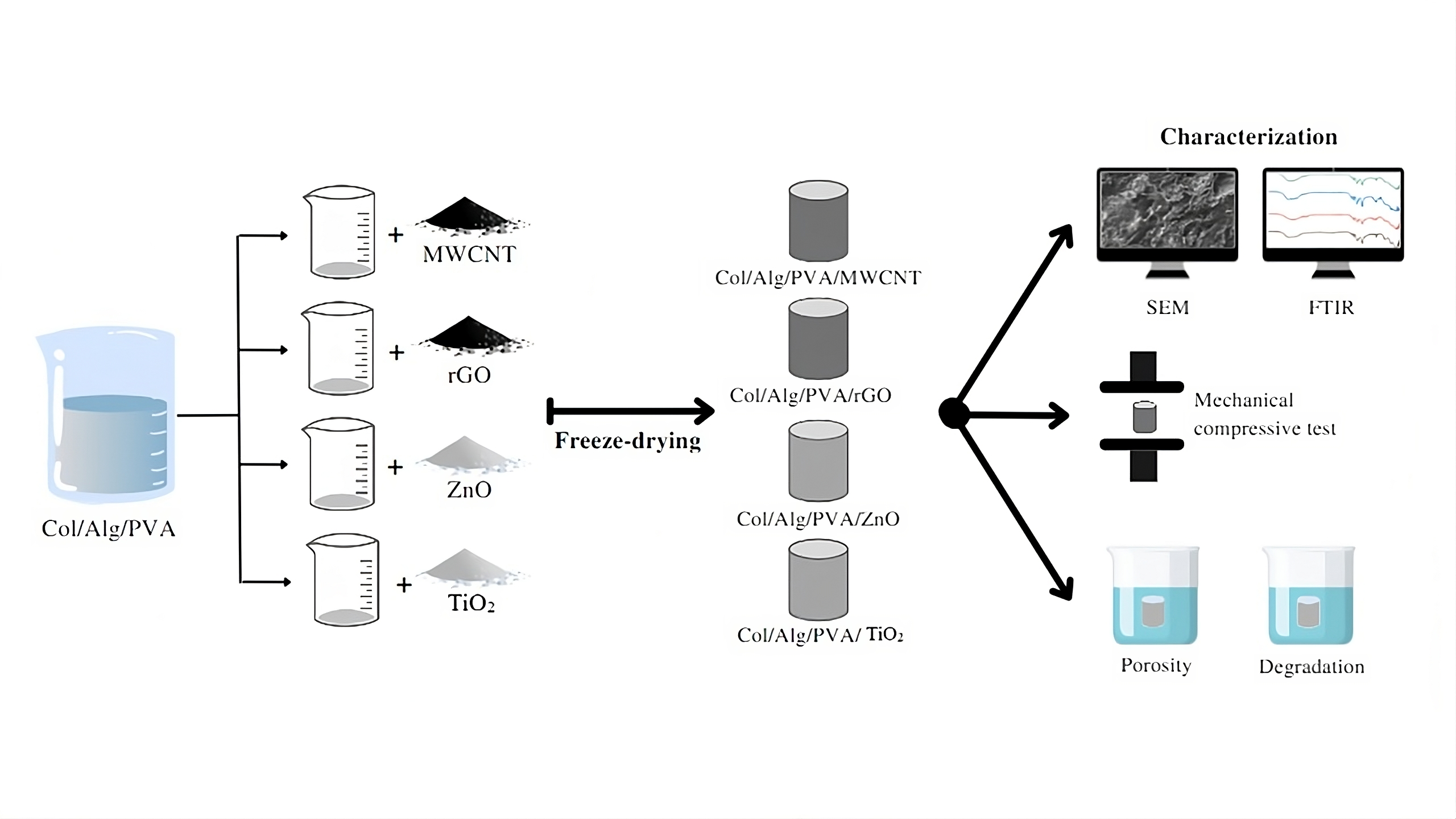
Bone damage is one of the main causes of disability in
humans, and tissue engineering technology by applying biomaterial-based
scaffold has been developed as an effective solution. This can be achieved
using various natural and synthetic polymers combined with carbon-based and
metal-oxide materials. Therefore,
this study aimed to develop bone scaffold using collagen,
alginate, and poly(vinyl alcohol), with the addition of multi-walled carbon
nanotube, reduced graphene oxide, titanium dioxide, and zinc oxide materials.
Scaffold was fabricated with the freeze-drying method and characterized
physicochemically by observing the morphology through scanning electron
microscopy (SEM), identification of functional groups by Fourier transform infrared
spectroscopy (FTIR), compressive mechanical properties, porosity, and
degradation rate. The results showed that each group of scaffold had a compact
structure, with a small pore size and less than 50% porosity. The functional
groups of each material were detected, and the compressive strength matched the
trabecular bone, approximately 6 MPa. However, the scaffold lacked appropriate
porosity and a fast degradation rate exceeding 35% in 7 days.
Biomaterials; Bone Scaffold; Carbon materials; Metal oxide materials; Physical and chemical characterization
Osteoarthritis is one of the most common public
health problems, affecting approximately 10% of the global population older
than 60 years (Yahaya et al., 2021). The number of cases worldwide increased from
247.51 million in 1990 to 527.81 million in 2019, with prevalence rising by
113.25%. Concurrently, there has been a rapid rise in age-standardized years living with disability due to
osteoarthritis by 114.5% from 1990 to 2019 (Long
et al., 2022).
Tissue engineering, a
multifaceted process aimed at replacing damaged organs, entails restoring
various biological functions (Amiryaghoubi et al.,
2022). In this context,
biomaterial-based scaffold has been developed to support cell adhesion, growth,
and determine compatibility with the body (Zhang et
al., 2023). An ideal scaffold for tissue
engineering applications should have optimal chemical and structural
characteristics along with good mechanical properties (Jafari
et al., 2017). In addition,
scaffold is expected to have good microstructure, such as high porosity with
appropriate pore size and permeability, to support cartilage regeneration (Wasyleczko, Sikorska, and Chwojnowski, 2020; Bružauskaite et al.,
2016).
Various naturally-derived
polymers have been used as bone tissue scaffold, and synthetic polymers can
also be incorporated alongside natural ones to improve hydrophilicity, cell
adhesion, and biodegradability (Jafari et al., 2017). Collagen is a major component of the extracellular
matrix (ECM) and one of the most frequently used biomaterials in protein-based
scaffold for bone tissue engineering (Zhang et al., 2018). Alginate is a natural polysaccharide with wider
availability, good biocompatibility, and ease of gelation (Hu and Lo, 2021). The
addition to the scaffold is an easy and effective
way to increase porosity and suitability for use as a matrix (Chandika et al., 2015). However, collagen and alginate have limitations
in the mechanical stability, showing the need to add other polymers with higher
mechanical capacity (Shirehjini et al., 2022). Poly(vinyl
alcohol) (PVA) is a synthetic polymer with good biocompatibility,
biodegradability, high hydrophilicity, and mechanical resistance as a scaffold
material (Rochardjo
et al., 2021).
Carbon-based
materials are advantageous by providing electrical conductivity, mechanical
reinforcement, and high surface area (Massoumi et al.,
2021).
Previous studies showed that multi-walled carbon nanotube (MWCNT) scaffold had
good characteristics for adhesion, proliferation, and osteogenesis
differentiation (Xu et al., 2019). Meanwhile, graphene-based
materials and the derivatives, such as graphene oxide (GO) and reduced graphene
oxide (rGO), which possess high surface area and electrical conductivity (Hardi and Rahman, 2020), have also been
investigated to enhance cell attachment and differentiation (Sanati et al., 2022).
2.1. Materials
King cobia collagen was extracted using the deep
eutectic solvent method adapted from Batista et al. (2022). MWCNT 95% [Sigma Aldrich] was functionalized by
adapting Shrestha et al. (2017), and reduced graphene oxide was reduced using graphene oxide (50% of
carbon) [Sigma Aldrich] based on Habte and Ayele (2019). TiO2
was synthesized with titanium trichloride 15% [Sigma Aldrich] according
to Fayyadh et al. (2019), and zinc oxide was prepared using zinc acetate dihydrate 99.5% [Sigma
Aldrich] in line with Haque et al. (2020). Sodium hydroxide 99% to neutralize pH and ethanol
99% were purchased from Merck. Meanwhile, sodium alginate powder, poly(vinyl
alcohol) 98% hydrolyzed, acetic acid (glacial) 100%, and phosphate-buffered
saline were all purchased from Sigma Aldrich.
2.2. Fabrication of Scaffold
The development of scaffold commenced with the
preparation of collagen, sodium alginate, and PVA solutions. Collagen material
was dissolved in 0.5 M acetic acid to obtain a 2 wt% solution, which was
further added with 2 M NaOH to adjust the pH to neutral. Alginate material was
dissolved in distilled water with stirring at 40oC for 2 hours to
obtain 1 wt% solution. Meanwhile, PVA material was dissolved in distilled water
with stirring at 80oC for 2 hours to obtain a 5 wt% solution. The
collagen solution was mixed with the alginate and stirred for 2 hours to
produce a homogeneous solution. Subsequently, PVA solution was mixed with
Col/Alg mixture and stirred for 2 hours.
Each Col/Alg/PVA container was added with 0.5 wt%
MWCNT, 0.1 wt% rGO, 1 wt% ZnO, and 1 wt% TiO2 separately according
to the container. Stirring was carried out for 2 hours with sonication for 1
hour. Each container was then transferred to a 48-well tissue culture plate.
The four scaffold groups were frozen in a freezer at -80oC for 24
hours and freeze-dried for 48 hours.
2.3. Scaffold Characterization
2.3.1. Scanning Electron Microscopy (SEM) Characterization
The morphology of the scaffold was observed through
SEM (Zeiss, EVO-MA10) with an acceleration voltage of 15 kV. Specimens were
coated before observing scaffold surface.
2.3.2. Fourier
Transform Infrared Spectroscopy (FTIR) Characterization
The functional groups in the sample material were
characterized using FTIR (Thermo Scientific Nicolet iS10).
2.3.3. Mechanical
Compressive Test
A compressive test was conducted to determine the
durability parameters of the scaffold. This was conducted through Instron's
Universal Testing Machine (UTM) using the ASTM D143 standard with a compressive
force rate of 10 mm/minute until it reached a change in the shape of the
scaffolding to be destroyed. Scaffold used for the compressive test had a diameter
of 10 mm and a height of 5 mm in each group. It was then placed in a horizontal
position in the middle between the pressure plate and the compressive force was
applied in a downward pressure direction to determine the strength limit of
scaffold.
2.3.4. Porosity
The porosity test, aimed at
observing the porosity of scaffold surface, was conducted by the liquid
displacement method. Scaffold was cut into pieces, with the volume determined
first by measuring the diameter (D) and height (H). The dry sample was weighed
(Wd) and immersed in 10 mL ethanol at room temperature for 5 minutes.
Filtration was conducted with filter paper to remove excess ethanol, then the
wet weight (Wp) was immediately weighed. The porosity was calculated using
Equation (1), where is the density of ethanol (
= 0.789 g/cm3)
(Jing et
al., 2017).
2.3.5. Degradation
Rate
The degradation test was conducted to determine
scaffold ability when dissolved in the body. The test was conducted by
dissolving the weighed dry scaffold in 10 mL PBS (pH 7.4), which was designed
to mimic the body environment when cells would be grown, with intervals of H+1,
H+5, and H+7. The dissolved product was incubated at 37oC and
filtered using filter paper to remove excess PBS. Scaffold was oven-dried at
70°C for 40 minutes until the water content disappeared, and the final weight
(Wt) was weighed. The percentage degradation rate was calculated using Equation
(2) (Gholizadeh et al., 2017).
2.4. Statistical and Graphical Analysis
Statistical analysis was performed to compare test
values for each physicochemical characteristic. All quantitative experimental
data were represented as mean ± standard deviation with 3 repetitions for each
test.
3.1. Scaffold Characterization
SEM characterization was performed on each group of
Col/Alg/PVA scaffold with the addition of MWCNT, rGO, TiO2, and ZnO
to determine and compare the morphology as well as topography.
This was conducted to provide valuable information regarding the potential for
cell interactions with scaffold structure.
Figure 1(a) shows SEM image morphology of Col/Alg/PVA/MWCNT scaffold to have a rough surface with fairly wide gaps (marked with red arrows). Thickening of the pore walls could reduce porosity, lowering the area available for cell growth. As shown in Figure 1(b), SEM image morphology of Col/Alg/PVA/rGO scaffold had a dense and interconnected structure but possessed tiny pores. This was in accordance with Kavya et al. (2013), stating that the high density caused a reduction in porosity but contributed to high mechanical strength (Kavya et al., 2013). Figure 1(c) shows that SEM image morphology of Col/Alg/PVA/TiO2 scaffold has a rough but interconnected structure. There were fibers from collagen fused with other materials, including TiO2 particles (marked with red arrows). Figure 1(d) shows SEM image morphology of Col/Alg/PVA/ZnO scaffold to have a dense but interconnected structure. Scaffold also had fairly wide gaps (marked with red arrows) but did not show interconnected pores.
Figure 1 SEM
results of Col/Alg/PVA scaffold with the addition of (a) MWCNT, (b) rGO, (c)
TiO2, and (d) ZnO
Based on SEM characterization results,
the pore sizes of the four scaffold groups did not meet the required minimum
pore size. Although the pore size was generally small, it could indirectly
benefit cell growth, by increasing their retention. This was proven in the
study by Kosowska
et al. (2020), showing that smaller pores could increase
cell proliferation and cellular interactions. According to Morejón et al.
(2019), a micropore size of approximately <10 µm
created a larger surface area that stimulated greater ion exchange and bone
protein adsorption.
3.1.2. FTIR Characterization of Scaffold
FTIR
characterization was performed on each group of Col/Alg/PVA scaffold with the
addition of MWCNT, rGO, TiO2, and ZnO to determine the content of
functional groups resulting from the mixture of materials. The collagen
absorption peaks comprised 3306 cm-1 (N-H group stretching
vibrations), 1632 cm-1 (amide I bond), 1546 cm-1 (amide
II bond), and 1236 cm-1 (amide III bond). Sodium alginate peaks
included 3402 cm-1 (hydroxyl (O-H) bonds), 2926 cm-1 (CH2
groups), 1607 cm-1 and 1410 cm-1 (asymmetric and
symmetric –COO stretches), 1607 cm-1 (C=O carboxyl bonds), and 1031
cm-1 (antisymmetric C-O-C stretches) (Sobhanian et al.,
2019). Meanwhile, the peak spectrum of PVA showed a broad
absorption band at 3000–3600 cm-1 attributed to hydroxyl group
symmetrical stretching, and 1090 cm-1 representing the carboxyl
vibration (–CO–) of PVA (Cao et al., 2018).
Figure 2
shows FTIR results on all scaffold groups with each having carboxyl, hydroxyl,
and amide groups. The presence of hydroxyl (-OH) and carboxyl (-COOH) groups
enhanced the formation of many hydrogen bonds with water molecules (Dibazar et al.,
2022). Furthermore, the acquisition of amide groups on
scaffold played a significant role in organic chemical activity and cell
biology associated with the structure of proteins, enzymes, polypeptides, and
other biological molecules (Jia et al., 2013).
3.1.3. Mechanical Compressive Test of Scaffold
Mechanical testing of scaffold was conducted to determine the compressive strength and compare with the mechanical characteristics of bone, as shown in Figure 3.
Figure 3 Mean
and standard deviation of compressive test results of Col/Alg/PVA scaffold with
the addition of MWCNT, rGO, TiO2, and ZnO materials
One of
the characteristics of an ideal scaffold for tissue engineering is having a
mechanical strength similar to native bone tissue. The compressive strength for
each scaffold group was about 6 MPa, meeting the mechanical criteria for
trabecular bone (0,1–16 MPa) but not for cortical bone (130–200 MPa) (Gerhardt and
Boccaccini, 2010). The mechanical properties tended to decrease
exponentially with increasing porosity (Abbasi et al., 2020).
The compressive test results were relatively large with small porosity. This
implied that the addition of MWCNT, rGO, TiO2, and ZnO materials did
not affect mechanical strength of Col/Alg/PVA scaffold.
3.1.4. Scaffold Porosity
The porosity test was conducted to determine the nature of scaffold to support cell proliferation and migration. The results in Figure 4 showed that the addition of MWCNT, rGO, TiO2, and ZnO materials did not significantly affect the porosity of Col/Alg/PVA scaffold. The porosity of the four scaffold groups was relatively small and fell below the desired specifications. Porosity results with a 50–90% percentage range are considered optimal (Mishra et al., 2019). However, several studies found an important role of low porosity. In Liu et al. (2018), it was found that lower pore size was associated with the formation of osteoid or fibrous tissue.
Figure 4 Mean and standard deviation of porosity test
results of Col/Alg/PVA scaffold with added MWCNT, rGO, TiO2, and ZnO
materials
3.1.5. Scaffold Degradation Rate
The degradation rate of good scaffold for tissue engineering should match the rate of new tissue formation to meet the necessary conditions (Alizadeh et al., 2013). The results (Figure 5) showed a fairly stable weight loss as the duration of the test increased. Scaffold with a favorable degradation rate should be compatible with the maturation and regeneration time of new tissue after in vivo transplantation (Wissing et al., 2017). Several factors, including pore homogeneity, morphology, and pore size, can cause high degradation rates. Furthermore, scaffold with better mechanical properties also have slower degradation rates (Diogo et al., 2018). Based on the results, the addition of rGO material reduced the degradation rate with fairly good strength. All four groups had a high degradation rate for 7 days, showing that the scaffold did not meet the desired specifications in the degradation test parameters.
Figure 5 Mean and standard deviation of degradation
rate test results of Col/Alg/PVA scaffold with the addition of MWCNT, rGO, TiO2,
and ZnO materials
In conclusion, SEM results showed
that scaffold had a dense and bonded structure but lacked the appropriate pore
size and porosity. All four groups had a high swelling percentage with a high
degradation rate. There was no significant difference between each group in
terms of mechanical characterization and porosity test. This implied that the
addition of MWCNT, rGO, TiO2, and ZnO materials did not affect the
physicochemical characteristics of Col/Alg/PVA scaffold. Therefore, fabricated
scaffold cannot be used as a candidate for bone tissue engineering, and further
development is needed regarding the composition and concentration of the
materials added.
The author is grateful for the funding from Ministry of Research,
Technology and Higher Education Indonesia through Penelitian Dasar Unggulan
Perguruan Tinggi (PDUPT) 2022 No. NKB-846/UN2.RST/HKP.05.00/2022.
Abbasi, N., Hamlet, S., Love,
R.M., Nguyen, N.-T., 2020. Porous Scaffolds for Bone Regeneration. Journal of Science: Advanced Materials and
Devices, Volume 5, pp. 1–9
Alizadeh, M., Abbasi, F.,
Khoshfetrat, A.B., Ghaleh, H., 2013. Microstructure and Characteristic
Properties of Gelatin/Chitosan Scaffold Prepared by A Combined
Freeze-Drying/Leaching Method. Materials Science and Engineering C,
Volume 33, pp. 3958–3967
Amiryaghoubi, N., Fathi, M.,
Barar, J., Omidi, Y., 2022. Hydrogel-Based Scaffolds for Bone and Cartilage Tissue Engineering
and Regeneration. Reactive and Functional Polymers, Volume 177, p.
105313
Batista, M.P., Fernández, N.,
Gaspar, F.B., Bronze, M. do R., Duarte, A.R.C., 2022. Extraction of
Biocompatible Collagen from Blue Shark Skins Through the Conventional
Extraction Process Intensification Using Natural Deep Eutectic Solvents. Frontiers
in Chemistry, Volume 10, p. 937036
Bružauskaite, I., Bironaite,
D., Bagdonas, E., Bernotiene, E., 2016. Scaffolds and Cells for Tissue Regeneration: Different
Scaffold Pore Sizes—Different Cell Effects. Cytotechnology, Volume 68,
pp. 355–369
Cao, L., Wu, X., Wang, Q.,
Wang, J., 2018. Biocompatible Nanocomposite of TiO2 Incorporated Bi-Polymer for
Articular Cartilage Tissue Regeneration: A Facile Material. Journal of
Photochemistry and Photobiology B: Biology, Volume 178, pp. 440–446
Chandika, P., Ko, S.-C., Oh,
G.-W., Heo, S.-Y., Nguyen, V.-T., Jeon, Y.-J., Lee, B., Jang, C.H., Kim, G.,
Park, W.S., Chang, W., Choi, I.-W., Jung, W.-K., 2015. Fish Collagen/Alginate/Chitooligosaccharides
Integrated Scaffold for Skin Tissue Regeneration Application. International
Journal of Biological Macromolecules, Volume 81, pp. 504–513
Christy, P.N., Basha, S.K.,
Kumari, V.S., 2022. Nano Zinc Oxide And Nano Bioactive Glass Reinforced
Chitosan/Poly(Vinyl Alcohol) Scaffolds For Bone Tissue Engineering Application. Materials Today
Communications, Volume 31, p. 103429
Dibazar, Z.E., Mohammadpour,
M., Samadian, H., Zare, S., Azizi, M., Hamidi, M., Elboutachfaiti, R., Petit,
E., Delattre, C., 2022. Bacterial Polyglucuronic Acid/Alginate/Carbon
Nanofibers Hydrogel Nanocomposite as a Potential Scaffold for Bone Tissue
Engineering. Materials, Volume 15, p. 2494
Diogo, G., López-Senra, E.,
Pirraco, R., Canadas, R., Fernandes, E., Serra, J., Pérez-Martín, R., Sotelo,
C., Marques, A., González, P., Moreira-Silva, J., Silva, T., Reis, R., 2018.
Marine Collagen/Apatite Composite Scaffolds Envisaging Hard Tissue Applications. Marine Drugs, Volume 16, p.
269
Fayyadh, A.A., Essa, A.F.,
Batros, S.S., Shallal, Z.S., 2019. Studying the Crystal Structure, Topography,
and Antibacterial of a Novel Titania (TiO2 NPs) Prepared by a
Sol-gel Manner. Baghdad Science Journal, Volume 16, p. 0910
Gerhardt, L.C., Boccaccini,
A.R., 2010. Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering. Materials,
Volume 3, pp. 3867–3910
Gholizadeh, S., Moztarzadeh,
F., Haghighipour, N., Ghazizadeh, L., Baghbani, F., Shokrgozar, M.A.,
Allahyari, Z., 2017. Preparation And Characterization of Novel Functionalized
Multi-Walled Carbon Nanotubes/Chitosan/?-Glycerophosphate Scaffolds for Bone
Tissue Engineering. International Journal of Biological Macromolecules,
Volume 97, pp. 365–372
Habte, A.T., Ayele, D.W.,
2019. Synthesis and Characterization of Reduced Graphene Oxide (rGO) Started
from Graphene Oxide (GO) Using the Tour Method with Different Parameters. Advances
in Materials Science and Engineering, Volume 2019, pp. 1–9
Haque, M.J., Bellah, M.M.,
Hassan, M.R., Rahman, S., 2020. Synthesis of ZnO Nanoparticles by Two Different
Methods & Comparison of Their Structural, Antibacterial, Photocatalytic and
Optical Properties. Nano Express, Volume 1(1), p. 010007
Hardi, G.W., Rahman, S.F., 2020.
Amperometric Detection of Dopamine based on a Graphene Oxide/PEDOT:PSS
Composite Electrode. International Journal of Technology, Volume 11(5),
pp. 974–983
Hu, T., Lo, A.C.Y., 2021.
Collagen–Alginate Composite Hydrogel: Application in Tissue Engineering and
Biomedical Sciences. Polymers (Basel), Volume 13, p. 1852
Jafari, M., Paknejad, Z., Rad,
M.R., Motamedian, S.R., Eghbal, M.J., Nadjmi, N., Khojasteh, A., 2017.
Polymeric Scaffolds in
Tissue Engineering: A Literature Review. Journal of Biomedical Materials
Research Part B: Applied Biomaterials, Volume105, pp. 431–459
Jia, L., Duan, Z., Fan, D.,
Mi, Y., Hui, J., Chang, L., 2013. Human-Like Collagen/Nano-Hydroxyapatite Scaffolds for The Culture of Chondrocytes. Materials
Science and Engineering C, Volume 33(2), pp. 727–734
Jing, Z., Wu, Y., Su, W.,
Tian, M., Jiang, W., Cao, L., Zhao, L., Zhao, Z., 2017. Carbon Nanotube
Reinforced Collagen/Hydroxyapatite Scaffolds Improve Bone Tissue Formation In Vitro and In Vivo. Annals of
Biomedical Engineering, Volume 45, pp. 2075–2087
Kavya, K.C., Jayakumar, R.,
Nair, S., Chennazhi, K.P., 2013. Fabrication And Characterization of
Chitosan/Gelatin/nSiO2 Composite Scaffold for Bone Tissue Engineering. International
Journal of Biological Macromolecules, Volume 59, pp. 255–263
Khalil, M., Rahmaningsih, G.,
Gunlazuardi, J., Umar, A., 2019. The Influence of Plasmonic Au Nanoparticle
Integration on the Optical Bandgap of Anatase TiO2 Nanoparticles. International
Journal of Technology, Volume 10(4), pp. 808–817
Kosowska, K., Domalik-Pyzik,
P., Sekula-Stryjewska, M., Noga, S., Jagiello, J., Baran, M., Lipinska, L.,
Zuba-Surma, E., Chlopek, J., 2020. Gradient Chitosan Hydrogels Modified with
Graphene Derivatives and Hydroxyapatite: Physiochemical Properties and Initial
Cytocompatibility Evaluation. International Journal of Molecular Sciences,
Volume 21, p. 4888
Liu, J., Chen, G., Xu, H., Hu,
K., Sun, J., Liu, M., Zhang, F., Gu, N., 2018. Pre-Vascularization in Fibrin
Gel/PLGA Microsphere Scaffolds Designed for Bone Regeneration. NPG Asia Materials, Volume 10,
pp. 827–839
Long, H., Liu, Q., Yin, H.,
Wang, K., Diao, N., Zhang, Y., Lin, J., Guo, A., 2022. Prevalence Trends of
Site?Specific Osteoarthritis From 1990 to 2019: Findings from the Global Burden
of Disease Study 2019. Arthritis & Rheumatology, Volume 74, pp.
1172–1183
Massoumi, B., Abbasian, M.,
Khalilzadeh, B., Jahanban-Esfahlan, R., Rezaei, A., Samadian, H.,
Derakhshankhah, H., Jaymand, M., 2021. Gelatin-Based Nanofibrous Electrically
Conductive Scaffolds for
Tissue Engineering Applications. International Journal of Polymeric
Materials and Polymeric Biomaterials, Volume 70, pp. 693–702
Mishra, R., Varshney, R., Das,
N., Sircar, D., Roy, P., 2019. Synthesis and characterization of gelatin-PVP
polymer composite scaffold for potential application in bone tissue
engineering. Eur Polym J 119, 155–168
Morejón, L., Delgado, J.A.,
Antunes Ribeiro, A., Varella de Oliveira, M., Mendizábal, E., García, I.,
Alfonso, A., Poh, P., van Griensven, M., Balmayor, E.R., 2019. Development,
Characterization and In Vitro Biological Properties of Scaffolds Fabricated from Calcium Phosphate
Nanoparticles. International Journal of Molecular Sciences, Volume 20,
p. 1790
Rochardjo, H.S.,
Fatkhurrohman, F., Kusumaatmaja, A., Yudhanto, F., 2021. Fabrication of
Nanofiltration Membrane based on Polyvinyl Alcohol Nanofibers Reinforced with
Cellulose Nanocrystal using Electrospinning Techniques. International
Journal of Technology, Volume 12(2), pp. 329–338
Sanati, A., Kefayat, A.,
Rafienia, M., Raeissi, K., Siavash Moakhar, R., Salamat, M.R., Sheibani, S.,
Presley, J.F., Vali, H., 2022. A Novel Flexible, Conductive, and
Three-Dimensional Reduced Graphene Oxide/Polyurethane Scaffold for Cell
Attachment and Bone Regeneration. Materials & Design, Volume 221, p.
110955
Shalaby, M.A., Anwar, M.M.,
Saeed, H., 2022. Nanomaterials for Application in Wound Healing: Current
State-Of-The-Art and Future Perspectives. Journal of Polymer Research,
Volume 29(3), p. 91
Shirehjini, L.M., Sharifi, F.,
Shojaei, S., Irani, S., 2022. Poly-Caprolactone Nanofibrous Coated with Sol-Gel
Alginate/ Mesenchymal Stem Cells for Cartilage Tissue Engineering. Journal
of Drug Delivery Science and Technology, Volume 74, p. 103488
Shrestha, B.K., Shrestha, S.,
Tiwari, A.P., Kim, J.-I., Ko, S.W., Kim, H.-J., Park, C.H., Kim, C.S., 2017.
Bio-Inspired Hybrid Scaffold of Zinc Oxide-Functionalized Multi-Wall Carbon
Nanotubes Reinforced Polyurethane Nanofibers for Bone Tissue Engineering. Materials
& Design, Volume 133, pp. 69–81
Sobhanian, P., Khorram, M.,
Hashemi, S.-S., Mohammadi, A., 2019. Development of Nanofibrous Collagen-Grafted
Poly (Vinyl Alcohol)/Gelatin/Alginate Scaffolds as Potential Skin Substitute. International
Journal of Biological Macromolecules, Volume 130, pp. 977–987
Wasyleczko, M., Sikorska, W.,
Chwojnowski, A., 2020. Review of Synthetic and Hybrid Scaffolds in Cartilage Tissue Engineering. Membranes
(Basel), Volume 10, p. 348
Wissing, T.B., Bonito, V.,
Bouten, C.V.C., Smits, A.I.P.M., 2017. Biomaterial-Driven in Situ
Cardiovascular Tissue Engineering—A Multi-Disciplinary Perspective. NPJ
Regenerative Medicine, Volume 2, p. 18
Xu, J., Hu, X., Jiang, S.,
Wang, Y., Parungao, R., Zheng, S., Nie, Y., Liu, T., Song, K., 2019. The
Application of Multi-walled Carbon Nanotubes in Bone Tissue Repair Hybrid Scaffolds and the Effect on Cell Growth In Vitro. Polymers
(Basel), Volume 11, p. 230
Yahaya, I., Wright, T.,
Babatunde, O.O., Corp, N., Helliwell, T., Dikomitis, L., Mallen, C.D., 2021.
Prevalence of Osteoarthritis in Lower Middle- and Low-Income Countries: A
Systematic Review and Meta-Analysis. Rheumatology International, Volume
41, pp. 1221–1231
Zhang, D., Wu, X., Chen, J.,
Lin, K., 2018. The Development of Collagen Based Composite Scaffolds for Bone Regeneration. Bioactive
Materials, Volume 3, pp. 129–138.
Zhang, W., Hou, X., Wang, H., Kong, D., Zhou, Y., 2023. Preparation of
Chitosan-Sodium Alginate/Bioactive Glass Composite Cartilage Scaffolds with High Cell Activity and Bioactivity. Ceramics International,
Volume 49(2), pp. 1987–1996
