A Novel Kinetic Model of Enzymatic Biodiesel Production in a Recirculating Fixed Bed Reactor
Corresponding email: heri.hermansyah@ui.ac.id
Published at : 17 May 2024
Volume : IJtech
Vol 15, No 3 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i3.6612
Soetandar, F., Hidayatullah, I.M., Utami, T.S., Yohda, M., Hermansyah, H., 2024. Novel Kinetic Model of Enzymatic Biodiesel Production in a Recirculating Fixed Bed Reactor. International Journal of Technology. Volume 15(3), pp. 780-791
| Frederick Soetandar | 1. Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Depok, West Java 16424, Indonesia, 2. Bioprocess Engineering Study Program, Faculty of Engineering, Universitas In |
| Ibnu Maulana Hidayatullah | 1. Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Depok, West Java 16424, Indonesia, 2. Bioprocess Engineering Study Program, Faculty of Engineering, Universitas In |
| Tania Surya Utami | 1. Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Depok, West Java 16424, Indonesia, 2. Bioprocess Engineering Study Program, Faculty of Engineering, Universitas In |
| Masafumi Yohda | 1. Division of Biotechnology and Life Science, Institute of Engineering, Tokyo University of Agriculture and Technology, Koganei, Tokyo 184-8588, Japan, 2. Institute of Global Innovation Research, Tok |
| Heri Hermansyah | 1. Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Depok, West Java 16424, Indonesia, 2. Bioprocess Engineering Study Program, Faculty of Engineering, Universitas In |
Entrapped
lipase derived from Candida rugosa can be used as an
alternative for
commercial heterogeneous catalysts in the
biodiesel synthesis process.
The inclination towards a
recirculating reactor with lipase-containing beds stems from its capability to
simultaneously improve both yield and reproducibility in the biodiesel
synthesis process. To
industrialize biodiesel production with entrapped lipase in a recirculating
reactor, optimization is essential, and this can be estimated using a kinetics
model. In this context, a kinetics model based on the Ping-Pong Bi-Bi mechanism
was developed for enzyme transesterification. Following this, experiments on biodiesel
synthesis were carried out in a fixed-bed reactor with a recirculated substrate, and a biodiesel concentration of
2177.231 mol/m3 was achieved from 917.804 mol/m3
triglyceride. In this study, 3 models, namely Model 1, 2, and 3, were developed
based on the Ping-Pong Bi-Bi mechanism,
and each has assumptions that determine its complexity. To validate these models, two sets of secondary data were taken and fitted into the respective model. The sum relative
error is used to express the differences between model and experimental data. Model 1, predicting
each component in transesterification, exhibited the highest error of 1.64,
while Model 3, assuming excess alcohol and incorporating a pseudo-steady-state
for di- and monoglyceride, yielded the lowest error. Despite these variations,
every model demonstrated good agreement in following each component profile
accurately, providing a more precise description of the reaction elements.
Biodiesel; Candida rugosa lipase; Enzyme kinetic; Modelling; Recirculating fixed-bed reactor; Transesterification
Homogeneous catalysts are commonly used in biodiesel production due to their relatively simpler production methodology. However, alternative approaches such as non-catalytic processes, including high-pressure methodology, acid-catalyzed esterification, and heterogeneous catalyst transesterification has been explored (Kalita et al., 2022; Feng et al., 2010; Demirbas, 2008). Among these methodologies, heterogeneous catalyst transesterification is an important advancement to mitigate the well-recognized limitations associated with homogeneous catalysts and non-catalytic routes (Monika, Banga, and Pathak, 2023; Wancura et al., 2021; Kareem et al., 2020). The development of heterogeneous catalysts holds the potential for low-cost and stable catalysts (Aisyah et al., 2023).
Recent advances in
heterogeneous catalyst studies present the opportunity to cost-effectively
incorporate enzymes as catalysts (Kalita et al.,
2022; Ng et al., 2022; Budžaki et al., 2018). Lipase,
particularly sourced from Candida rugosa, stands out as a recognized and
effective biocatalyst for transesterification, playing a crucial role in the
evolution of biodiesel production. The notable activity and ready availability
of Candida rugosa lipase (CRL) further emphasize its efficacy in the
transesterification process (Iuliano et al.,
2020; Yücel, Terzio?lu, and Özçimen, 2012). The use of recirculating
fixed-bed reactors is preferred over other types due to their enhanced
efficiency (Ani et al., 2018; Ren
et al., 2012). These reactors not only
reduce size requirements but also enable the reuse of heterogeneous catalysts
compared to batch processes (Aliyah et al., 2016). Despite these
advantages, addressing challenges related to reaction rates, catalyst cost, and
enzyme inhibition is imperative for enhancing the use of immobilized enzymes in
biodiesel production (Hermansyah et al.,
2023; Hidayatullah et al., 2018). To overcome these challenges,
the application of kinetic models becomes crucial in identifying correlations
among operational parameters (Hidayatullah et
al., 2021).
Various
kinetics models have been developed to illustrate the initial rate of ester
production by incorporating stepwise transesterification. These models are
designed to predict reactant, by-product, and product concentrations concerning
both time and substrate concentration (Rahma and Hidayat,
2023; Ezzati, Ranjbar, and Soltanabadi, 2021; Calabrò et al., 2010; Pessoa,
Magalhães, and Falcão, 2009; Xu, Du, and Liu, 2005). While
biodiesel synthesis often involves transesterification reactions, many models
simplify the process by assuming a single substrate, typically triglyceride
concentration (Fedosov et al., 2013;
Al-Zuhair, Ling, and Jun, 2007; Al-Zuhair, 2005). Although this simplification
aids in determining substrate consumption and product formation rates, it tends
to overlook by-product formation. Some models use empirical equations for
kinetics, lacking practicality in handling varying substrate concentrations.
Intermediate products are important to be put into kinetics to fully elucidate
the synthesis pathways (Muharam and Soedarsono,
2020). To address this, there is a need for a model showing stepwise
biodiesel production from triglycerides, emphasizing formation sequences including
enzymes.
In
this study, CRL was immobilized within calcium alginate beads to
serve as a biocatalyst in the reactor. A novel mathematical model was developed
to predict both the reaction yield and the intermediates generated during
transesterification. To illustrate changes in concentration relative to
residence time, enzymatic kinetics was integrated with plug flow mass balance.
The proposed kinetics model is closely in line with experimental data, capable
of representing each transesterification element (alcohol, biodiesel,
triglyceride, diglyceride, monoglyceride, and glycerol), and representing
enzymatic transesterification pathway following the Ping-Pong Bi-Bi mechanism. Subsequently,
3 models with different assumptions and complexity were proposed. Model 1
includes all triglyceride derivatives in the reaction and considers alcohol as
the limiting reactant. Model 2 is the simplified version of model 1 by assuming
pseudo-steady state for diglyceride and monoglyceride concentration. Model 3
assume excess alcohol, resulting in complete reactions and therefore assumes
diglyceride and monoglyceride concentration to be zero.
2.1. Materials
In this study, cooking palm oil was used as a triglyceride substrate, and was produced by PT. Salim Ivomas Pratama, Tbk. (Jakarta, Indonesia). The lyophilized CRL Type VII, bovine serum albumin (BSA), and all additional reagents were purchased from Merck & Co (Rahway, NJ, USA).
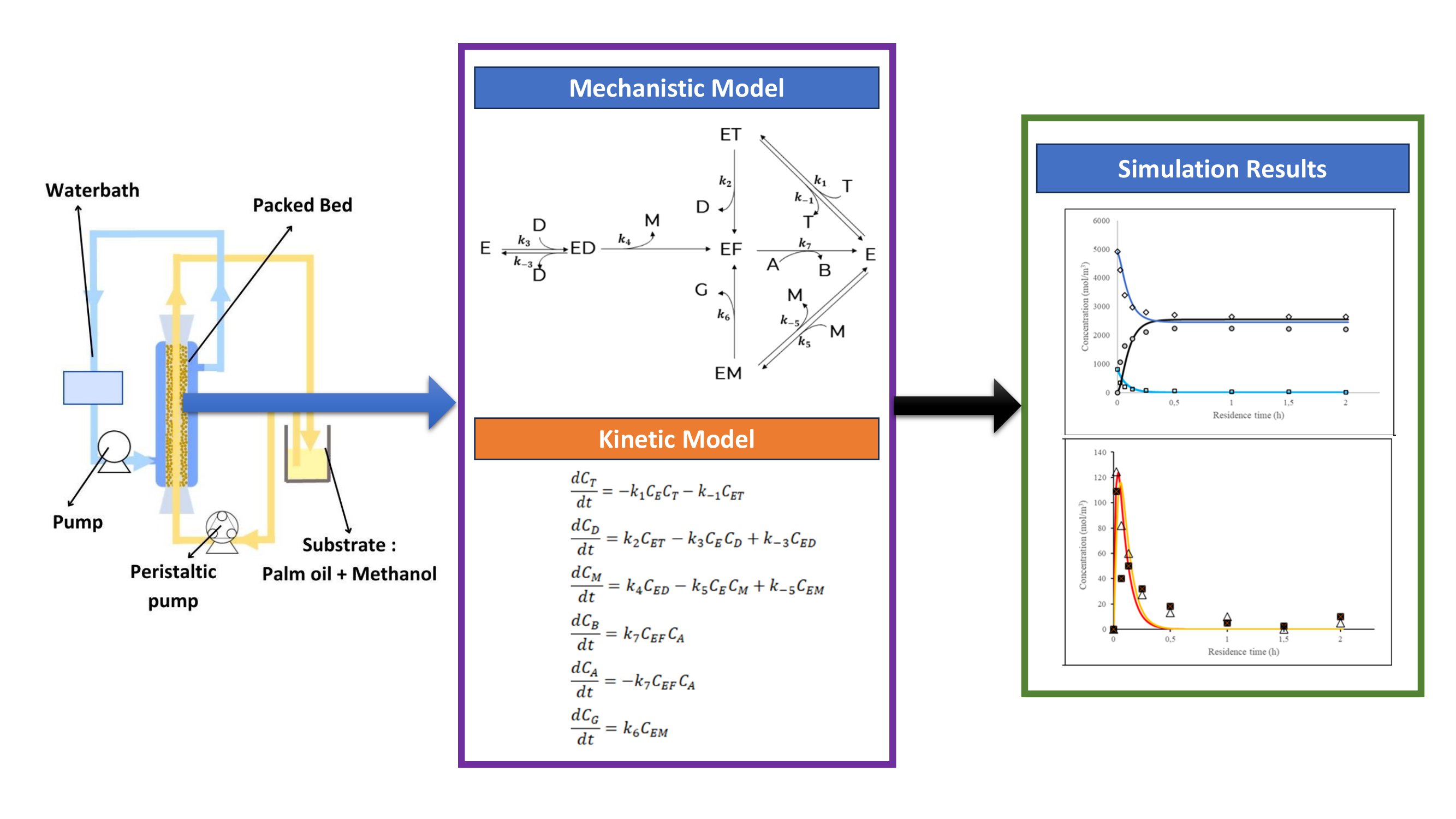
2.2. Reactor configuration and design
The reactor was configured to recirculate the substate mixture with dimensions of 15 cm in length and 1.1 cm in inner diameter. Immobilized enzymes were positioned within the reactor and secured using wire mesh. To maintain a consistent temperature of 37°C, the reactor jacket was enveloped with heated water from a water bath. This setup was linked to both a peristaltic pump and a water pump. The peristaltic pump introduced the mixed substrate into the reactor while the water pump circulated heated water to ensure the reactor temperature stability.
2.3. Enzyme Immobilization
The first step of the immobilization includes the production of sodium alginate solution. Subsequently, lipase solution was introduced to a sodium alginate solution to generate a 20 ml mixture containing 1.5% sodium alginate and 100 mg of lipase. Using a syringe, the enzyme mixture was extruded into a 2% CaCl2 solution. The resultant beads were left suspended in the CaCl2 solution for 1 hour. Then, the beads were transferred to a fresh 2% CaCl2 solution and maintained at 4°C for 24 hours.
2.4. Biodiesel Synthesis and Quantification
2.5. Model mechanism and assumptions
Biodiesel synthesis was carried out in 3 sequential elementary reaction stages. The transesterification follows Ping-Pong Bi-Bi mechanism that involves the formation of diglyceride and monoglyceride, and each reaction is reversible (Bornadel et al., 2013; Gog et al., 2012; Fjerbaek, Christensen and Norddahl., 2009). This process was carried out involving T, A, D, B, M, and G, denoting triglyceride, alcohol, diglyceride, biodiesel, monoglyceride, and glycerol respectively. Assuming there was no water-induced hydrolysis process, the only reaction in the system is transesterification.
2.6. Mathematical Model Derivation
2.7. Model Validation
The compatibility between secondary and estimation data from the developed kinetics model is shown by fitting it with transesterification data from other studies. There are 3 data sets used for model fitting, namely experimental data from this study, data from Shibasaki-Kitakawa et al. (2007), and data from Vicente (2006). Table 1 shows the experimental data and it was used to perform fittings with Models 2 and 3. Triglyceride and alcohol concentration in Table 1 were calculated from FAME concentration based on stoichiometric equilibrium. The data in Table S1 was obtained from Shibasaki-Kitakawa et al. (2007) and will be used for fitting with Model 3. The data shown in Table S2 was obtained from Vicente (2006) and will be used for fitting with Model 1. The fitting quality will be assessed using the relative error sum in Equation 38.
Using the Microsoft Excel software, differential equations were simulated through 4th-order Runge-Kutta numerical calculations, and the Solver program within Excel was used for data fitting. The alignment between experimental data and fitting results showcases the model's accuracy in estimating experimental data. Each model iteratively adjusts its kinetics parameters to minimize the sum of relative errors, aiming to achieve a value close to 0.
3.1.
Modelling result
In Figures 3-4, Model 3 presents concentration profiles with
the assumption of excess alcohol. The data fitting from Table 1 and S1 to Model
3 is shown in Figure 3a and 3b, respectively. While Model 3 is capable of
estimating the formation of both biodiesel and triglycerides, it tends to overestimate
the concentration of biodiesel. According to molar balance principles, it is
expected that additional products arise from the transformation of
triglycerides. Assuming only transesterification occurs, diglycerides and
monoglycerides are expected as secondary products. However, due to Model 3
assumption of the absence of intermediate product formation, it simulates a
thorough conversion of diglycerides and monoglycerides into biodiesel and
glycerol.
It is crucial to take into account alcohol concentration,
especially when making incremental additions, as alcohol plays the role of a
limiting reactant. Model 2 incorporates alcohol concentration into its
equations and assumes a pseudo-steady state for intermediates (D and M) to
simplify the model. The visual representation of the fitting results obtained
from the data in Table 1 can be observed in Figure 3c. Model 2 can replicate
the concentration profiles of each component by assuming a pseudo-steady state
for diglycerides and monoglycerides. The fitting results of Model 2 do not
significantly differ from those of Model 3. This shows that Model 2 is capable of simulating the data
while considering limited concentrations of methanol.
Figure 3 Fitting of a) data from this study
using Model 3, b) data from Shibasaki-Kitakawa et al. (2007) using Model 3, and c) data from this
study using Model 2
Figure 4 shows the data fitting from Table S2 into Model 1,
which considers the concentration profiles of A, D, and M. The Model examines
the formation of D and M as intermediates, recognizing their potential impact
on biodiesel yield. Diglycerides and monoglycerides can form due to the
transesterification reaction occurring in three elementary steps(Kadi et
al., 2019). When enzymes catalyze the
formation of biodiesel, one of the three fatty acid chains on triglycerides is
detached, resulting in the formation of diglycerides. Monoglycerides are
generated when one of the two fatty acids in a diglyceride reacts to form
biodiesel, leaving a single fatty acid chain on the glycerol molecule.
Model 1 shows a strong
agreement with the data. After reaching the peak, both D and M decrease and
reach very low concentrations. The reduction in the concentration of D and M is
attributed to a gradual reaction with a consumption rate higher than the formation
rate. The negligible amount of D and M at the higher reaction time has been
observed in most reports (Chen et al., 2020; Tran, Chen and Chang, 2016; Haigh et
al., 2014). Model 1 can replicate this phenomenon while
also simulating biodiesel formation. The model overestimates biodiesel and
underestimates alcohol concentration. However, the prediction of triglyceride
concentration yields accurate results. Despite triglycerides being completely
consumed, the data shows a lower concentration of biodiesel than in the
simulation. A few possibilities might explain this, including the potential
formation of other side products such as inactive enzyme-fatty acid complexes
or even the adsorption of triglycerides onto the enzyme's support matrix.
Figure 4 Fitting to Model 1 using data from Vicente et al. (2006)
Table 2 presents a
comparison of kinetics parameters, showing the highest reaction constant
derived from an experiment conducted by Vicente et
al. (2006). The experiment used 1 wt% catalyst (based on oil) for
transesterification. According to the investigation, the reactant was 240 g of B.
carinata oil (± 800 mol/m3), and for simplicity, it was assumed
to be equivalent to 300 mL (assuming a density of 800 g/L). This led to the
calculation of the concentration of catalyst used, which is 8 kg/m3.
Shibasaki-Kitakawa et al. (2007) synthesized biodiesel using 2 g of PA306s resin and
10g of reactants consisting of oil and 10 - 20 molar equivalents of methanol
(based on oil). Assuming this 10g was equal to 20 ml of reactant, the
concentration of the catalyst is estimated to be 100 kg/m3. Although
the kinetics constant for each catalyst might appear low, the overall reaction
rate can be substantial with the assistance of a significant quantity of
catalysts.
Table
2 Comparison of kinetics parameter
|
Kinetics constant |
Model 1 |
Model 3 |
Model 2 | |
|
Data from: | ||||
|
Vicente et al. (2006) |
Shibasaki-Kitakawa et al.
(2007) |
This study |
This study | |
|
K1 |
1.59 |
9.95×10-2 |
1.70×10-1 |
4.58×10-2 |
|
K2 |
6.55 |
- |
- |
- |
|
K3 |
6.27 |
- |
- |
- |
|
K4 |
0 |
2.77×10-6 |
8.00×10-5 |
6.66×10-3 |
|
K5 |
6.09 |
2.60×10-6 |
8.28×10-3 |
4.55×10-3 |
|
K6 |
8.40 |
2.70×10-6 |
8.28×10-3 |
5.47×10-2 |
|
CE,tot |
8 |
100 |
1.77 |
1.77 |
|
sum relative error |
1.64 |
3.49×10-2 |
4.13×10-2 |
5.05×10-2 |
In the
context of the stepwise transesterification
reaction, K1 signifies the
inclination to yield diglycerides. K2 represents the tendency to
generate monoglycerides from diglycerides, and K3 shows the propensity to release glycerol from
monoglycerides. These constants show
forward reactions. In Models 2 and 1, where diglycerides and monoglycerides are
assumed to react rapidly, K2 and K3 are excluded.
Conversely, K4, K5, and K6 denote hindrances
to biodiesel formation. K4-K6 are associated with k2,
k4, and k6, reflecting the rate constants of diglyceride,
monoglyceride, and glycerol release. K4 represents the dissociation
of triglyceride, leading to the enzyme complex producing biodiesel from the
fatty acid of triglyceride. The same meaning also applies to K5 and
K6, with the only difference in the source of fatty acid (such as
diglyceride for K5, and monoglyceride for K6).
In
Model 3, a total of 6 parameters need to be estimated, while Models 1 and 2
require 4. The resulting parameter data shows that ion exchange resin exhibits the highest K1 reaction
rate. Model 2, assuming a controlled alcohol
reaction, shows
significant changes in the constant K6 when compared to Model 1. The experiment carried out by Vicente
et al. (2006) had the highest value of kinetics constants compared to other
studies.
In
conclusion, 3 mechanistic models based on the Ping-Pong Bi-Bi mechanism were
developed to predict how each part of triglyceride transesterification behaves.
Each model could provide a better understanding of how both the reactants and
products change over time. Model key parameter helps to explain how operational
conditions affect the transesterification process. Data fitting of this
experiment data into Model 3 resulted in 4.13×10-2, which shows good
alignment. However, Model 1 had a high sum relative error, due to many
substrates and product concentrations that the model need to adjust, therefore
the sum relative error calculates all the misalignments, producing the highest
sum of error. This model could become a go-to reference for understanding
transesterification kinetics and further studies on the inhibition of substrate
and product are needed to improve the model.
The
study was supported by supported by the Ministry of
Education, Culture, Research, and Technology of Indonesia through the Penelitian
Dasar Unggulan Perguruan Tinggi (PDUPT) scheme, with grant numbers 021/E5/PG.02.00.PL/2023
and NKB-905/UN2.RST/HKP.05.00/2023 in the year 2023.
| Filename | Description |
|---|---|
| R3-CE-6612-20231217145727.docx | --- |
Aisyah, A.N., Ni’maturrohmah, D., Putra, R.,
Ichsan, S., Kadja, G.T.M, Lestari, W.W., 2023. Nickel Supported on MIL-96(Al)
as an Efficient Catalyst for Biodiesel and Green Diesel Production from Crude
Palm Oil. International Journal of Technology, Volume 14(2), pp. 276–289
Aliyah, A.N., Edelweiss,
E.D., Sahlan, M., Wijanarko, A., Hermansyah, H., 2016. Solid State Fermentation
Using Agroindustrial Wastes To. International Journal of Technology,
Volume 8, p.1392–1403
Al-Zuhair, S., 2005.
Production of Biodiesel By Lipase-Catalyzed Transesterification of Vegetable
Oils: A Kinetics Study. Biotechnology Progress, Volume 21(5), pp.
1442–1448
Al-Zuhair, S., Ling, F.W., Jun,
L.S., 2007. Proposed Kinetic Mechanism Of The Production Of Biodiesel From Palm
Oil Using Lipase. Process Biochemistry, Volume 42(6), pp. 951–960
Ani,
F.N., Said, N.H., Said, M.F.M., 2018. Optimization of Biodiesel Production
using a Stirred Packed-bed Reactor. International Journal of Technology,
Volume 9(2), pp. 219–228
Bornadel,
A., Åkerman, C.O., Adlercreutz, P., Hatti-Kaul, R., Borg, N., 2013. Kinetic
Modeling of Lipase-Catalyzed Esterification Reaction Between Oleic Acid and
Trimethylolpropane: A Simplified Model for Multi-Substrate Multi-Product
Ping–Pong Mechanisms. Biotechnology Progress, Volume 29(6), pp. 1422–1429
Budžaki, S. Milji?.,
G., Sundaram, S., Tišma, M., Hessel, V., 2018. Cost Analysis of Enzymatic
Biodiesel Production in Small-Scaled Packed-Bed Reactors. Applied Energy,
Volume 210(6), pp. 268–278
Calabrò,
V., Ricca, E., De Paola, M.G., Curcio, S., Iorio, G., 2010. Kinetics of
Enzymatic Trans-Esterification of Glycerides For Biodiesel Production. Bioprocess
and Biosystems Engineering, Volume 33(6), pp. 701–710
Chen,
X., Li, Z., Chun, Y., Yang, F., Xu, H., Wu, X., 2020. Effect of the Formation
of Diglyceride/Monoglycerides on the Kinetic Curve in Oil Transesterification
with Methanol Catalyzed by Calcium Oxide. ACS Omega, Volume 5(9), pp.
4646-4656
Demirbas,
A., 2008. Biodiesel: A Realistic Fuel Alternative For Diesel Engines. In: Biodiesel:
A Realistic Fuel Alternative for Diesel Engines. London: Springer
Ezzati,
R., Ranjbar, S., Soltanabadi, A., 2021. Kinetics Models Of Transesterification
Reaction For Biodiesel Production: A Theoretical Analysis. Renewable Energy,
Volume 168, pp. 280–296
Fedosov, S.N., Brask, J.,
Pedersen, A.K., Nordblad, M., Woodley, J.M., Xu, X., 2013. Kinetic Model Of
Biodiesel Production Using Immobilized Lipase Candida Antarctica Lipase B. Journal
of Molecular Catalysis B: Enzymatic, Volume 85, pp. 156–168
Feng, Y., He, B., Cao, Y.,
Li, J., Liu, M., Yan, F., Liang, X., 2010. Biodiesel Production Using
Cation-Exchange Resin as Heterogeneous Catalyst. Bioresource Technology,
Volume 101(5), pp. 1518–1521
Fjerbaek, L., Christensen,
K., Norddahl, B., 2009. A Review of The Current State of Biodiesel Production Using
Enzymatic Transesterification. Biotechnology and Bioengineering, Volume
102(5), pp. 1298–1315
Gog, A., Roman, M., To?a,
M., Paizs, C., Irimie, F., 2012. Biodiesel Production using Enzymatic
Transesterification – Current State and Perspectives. Renewable Energy, Volume
39(1), pp. 10–16
Haigh, K., Vladisavljevi?, G.T., Reynolds, J.C., Nagy, Z.,
Saha, B., 2014. Kinetics of the Pre-treatment of Used Cooking Oil using
Novozyme 435 for Biodiesel Production. Chemical Engineering Research and
Design, Volume 92(4), pp. 713–719
Hermansyah,
H., Ibnu-Maulana, H., Frederick, S., Pingkan-Vanessa, S., Patrick, C., 2023.
Ion Exchange Resin and Entrapped Candida rugosa Lipase for Biodiesel
Synthesis in the Recirculating Packed-Bed Reactor?: A Performance Comparison of
Heterogeneous Catalysts. Energies, Volume 16(12), p. 4765
Hermansyah,
H., Kubo, M., Shibasaki-Kitakawa, N., Yonemoto, T., 2006. The Mathematical
Model For Stepwise Hydrolysis Of Triolein Using Candida Rugosa Lipase In
A Biphasic Oil-Water System. Biochemical Engineering Journal, Volume
31(2), pp. 125–132
Hidayatullah,
I.M., Arbianti, R., Utami, T.S., Suci, M., Sahlan, M., Wijanarko, A., Gozan,
M., Hermansyah, H., 2018. Techno-Economic Analysis Of Lipase Enzyme Production
From Agro-Industry Waste With Solid State Fermentation Method. IOP
Conference Series: Materials Science and Engineering, Volume 316(1), p.
012064
Hidayatullah,
I.M., Makertihartha, I.G.B.N., Setiadi, T., Kresnowati, M.T.A.P., 2021.
Modeling-Based Analysis And Optimization Of Simultaneous Saccharification and Fermentation
For The Production Of Lignocellulosic-Based Xylitol. Bulletin of Chemical
Reaction Engineering & Catalysis, Volume 16(4), pp. 857–868
Iuliano, M., Sarno, M., De Pasquale, S., Ponticorvo, E.,
2020. Candida rugosa lipase for the biodiesel production from renewable
sources. Renewable Energy, Volume 162, pp. 124-133
Kadi, M., Akkouche, N., Awad, S., Loubar, K., Tazerout,
M., 2019. Kinetic Study of Transesterification Using Particle Swarm
Optimization Method. Heliyon, Volume 5(8), p. 101016
Kalita,
P., Basumatary, B., Saikia, P., Das, B., Basumatary, S., 2022. Biodiesel As Renewable Biofuel Produced Via Enzyme-Based
Catalyzed Transesterification. Energy Nexus, Voume 6, p. 100087
Kareem,
S.O., Falokun, E.I., Balogun, S.A., Akinloye, O. A., Omeike, S.O., 2020. Improved
Biodiesel From Palm Oil Using Lipase Immobilized Calcium Alginate And Irvingia Gabonensis
Matrices. Beni-Suef University Journal of Basic and Applied
Sciences, Volume 9(1), p. 101186
Monika,
Banga, S., Pathak, V.V., 2023. Biodiesel production from waste cooking oil: A
comprehensive review on the application of heterogenous catalysts. Energy
Nexus, Volume 10(5), p. 100209.
Muharam, Y., Soedarsono, J.A. 2020.
Hydrodeoxygenation of Vegetable Oil in a Trickle Bed Reactor for Renewable
Diesel Production. International Journal of Technology, Volume 11(7),
pp. 1292–1299
Ng,
W.Z., Obon, A.A., Lee, C.L., Ong, Y.H., Gourich, W., Maran, K., Tang, D.B.Y.,
Song, C.P., Chan, E.S., 2022. Techno-Economic Analysis
Of Enzymatic Biodiesel Co-Produced In Palm Oil Mills From Sludge Palm Oil For
Improving Renewable Energy Access In Rural Areas. Energy, Volume 243, p.
122745
Pessoa,
F.L.P., Magalhães, S.P., Falcão, P.W.C., 2009. Kinetic Study of Biodiesel
Production by Enzymatic Transesterification of Vegetable Oils. Computer
Aided Chemical Engineering, Volume 27, pp. 1809-1814
Rahma, F.N., Hidayat, A., 2023. Biodiesel
Production from Free Fatty Acid using ZrO2/Bagasse Fly Ash Catalyst. International
Journal of Technology, Volume 14(1), pp. 206–218
Ren, Y., He, B., Yan, F., Wang, H., Cheng,
Y., Lin, L., Feng, Y., Li, J., 2012. Continuous Biodiesel Production in a Fixed
Bed Reactor Packed with Anion-Exchange Resin as Heterogeneous Catalyst. Bioresource
Technology, Volume 113, pp. 19–22
Shibasaki-kitakawa,
N., Honda, H., Kuribayashi, H., Toda, T., 2007. Biodiesel Production Using
Anionic Ion-Exchange Resin As A Heterogeneous Catalyst. Bioresource
Technology, Volume 98(2), pp. 416–421
Tran,
D.T., Chen, C.L., Chang, J.S., 2016. Continuous Biodiesel Conversion via
Enzymatic Transesterification Catalyzed By Immobilized Burkholderia Lipase in a
Packed-Bed Bioreactor. Applied Energy, Volume 168, pp.340-350
Vicente,
G., Martínez, M., Aracil, J., 2006. Kinetics Of Brassica Carinata Oil
Methanolysis. Energy and Fuels, Volume 20(4), pp. 1722–1726
Wancura, J.H., Fantinel,
A.L., Ugalde, G.A., Donato, F.F., De Oliveira, J.V., Tres, M.V., Jahn, S.L., 2021.
Semi-Continuous Production of Biodiesel on Pilot Scale via Enzymatic
Hydroesterification of Waste Material: Process and Economics Considerations. Journal
of Cleaner Production, Volume 285, p. 124838
Xu,
Y., Du, W., Liu, D., 2005. Study On The Kinetics of Enzymatic
Interesterification Of Triglycerides For Biodiesel Production With Methyl
Acetate As The Acyl Acceptor. Journal of Molecular Catalysis B: Enzymatic,
Volume 32(5–6), pp. 241–245
Yücel,
S., Terzio?lu, P., Özçimen, D., 2012. Lipase Applications in Biodiesel
Production. In Biodiesel: Feedstocks, Production and Applications.
IntechOpen
