Diffusivity Coefficient of Shogaol Degradation into Paradol in the Reactive Extraction of Ginger Dregs through Subcritical Water
Published at : 28 May 2025
Volume : IJtech
Vol 16, No 3 (2025)
DOI : https://doi.org/10.14716/ijtech.v16i3.6512
Yulianto, ME, Jos, B & Budiyono 2025, ‘Diffusivity coefficient of shogaol degradation into paradol in the reactive extraction of ginger dregs through subcritical water’, International Journal of Technology, vol. 16, no. 3, pp. 960-971
| Mohamad Endy Yulianto | 1. Department of Industrial Chemical Engineering Technology, Vocational School of Diponegoro University, Universitas Diponegoro, Semarang, 50239, Indonesia. 2. Department of Chemical Engineering, Facu |
| Bakti Jos | Department of Chemical Engineering, Faculty of Engineering, Universitas Diponegoro, Semarang, 50239, Indonesia |
| Budiyono | Department of Chemical Engineering, Faculty of Engineering, Universitas Diponegoro, Semarang, 50239, Indonesia |
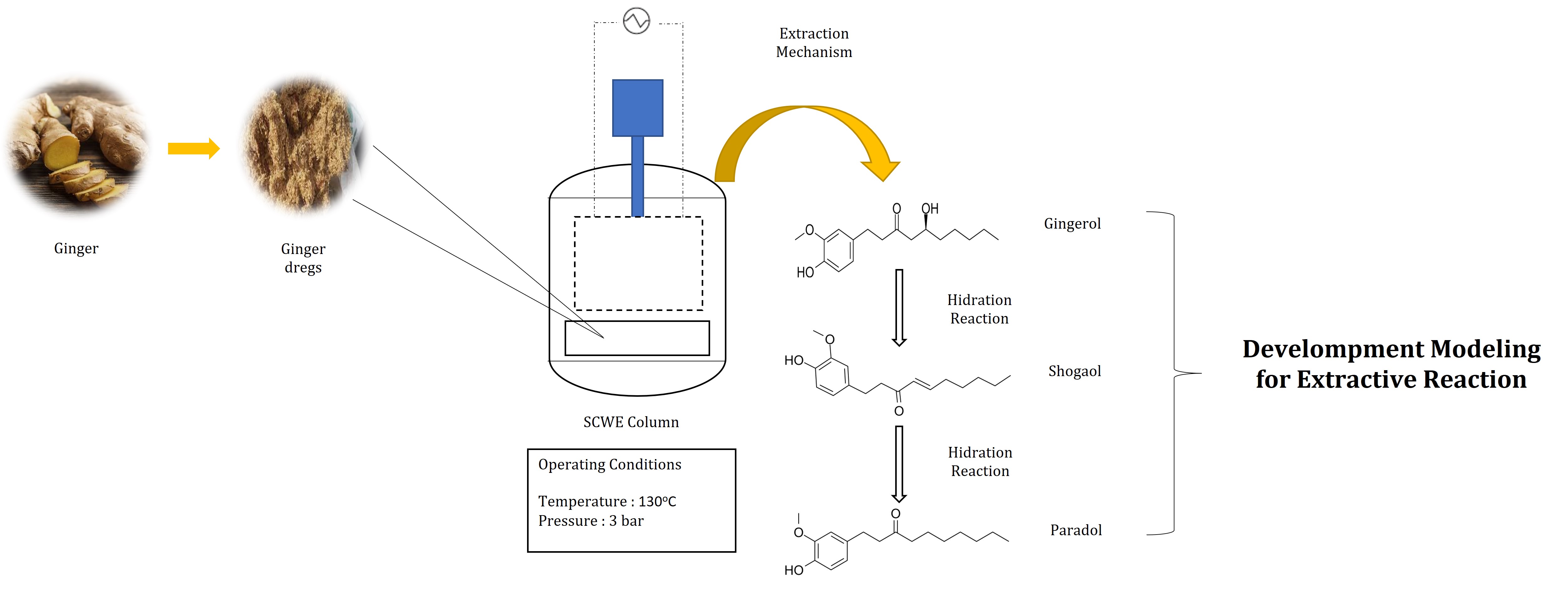
The reactive extraction of shogaol and gingerol bioactive compounds using subcritical water is believed to increase diffusivity and selectivity of extraction process. However, a significant problem in extracting shogaol is the thermal degradation of shogaol into paradol under certain process conditions, leading to a reduction in shogaol yield. Therefore, this research aimed to determine diffusivity coefficient of the thermal degradation of shogaol into paradol to explain the reaction mechanism and direct formation process. The investigation was conducted in two work stages, namely experimentation and modeling. A mathematical model was developed based on the mass transfer to determine the value of diffusivity (De) in the bulk solution and the micro size of ginger dregs, as well as the reaction speed (k) for the formation of shogaol and paradol. The experiment was conducted at a temperature of 130oC and a pressure of 3 bars using subcritical water reactive extractor. Sampling was performed every 10 minutes and the extracts were analyzed using the HPLC. The results showed that diffusivity of gingerol (DeA), subcritical water (Dew), shogaol (DeS), and paradol (DeP) was 0.000324 cm2/minute, 0.0012 cm2/minute, 0.000345 cm2/minute, 0.3875 cm2/minute, respectively, with a reaction rate constant (k''') of 3.7918 cm3/mol/minute.
Diffusivity; Extraction; Modeling; Paradol; Subcritical water
The use of subcritical water is associated with immense potential during extraction of shogaol reactive and gingerol from ginger dregs (Razak et al., 2023; Yulianto et al., 2022). This subcritical water solvent has polarity equivalent to organic solvents, low viscosity, and surface tension (Cheng et al., 2021). Furthermore, it increases diffusivity by approximately 10 times, reduces hydrogen bonds in water, and improves the rate of mass transfer, particle absorption in the matrix, and the high selectivity of extraction process (Md Sarip et al., 2014; Teo et al. 2010). Despite the numerous advantages, shogaol tends to experience thermal degradation under certain conditions, thereby becoming paradol (Utama-ang et al., 2021; Johnson et al., 2020), with a significant decrease in yield (Handayani et al. 2018; Yulianto et al. 2017).
Paradol, gingerol, and shogaol are compounds that imbue ginger with a spicy and hot taste (Claudya et al., 2023; Ramakrishnan 2013). Similar to shogaol, paradol occurs naturally in ginger but is produced from gingerol transformation due to heating on gingerol (Kamaruddin et al., 2023; Riyadhi et al., 2022; Mao et al., 2019; Rahmani et al., 2014). Bager (2012) stated that under acidic conditions and high temperatures, gingerol changed to shogaol, which was further converted to paradol. In the reaction mechanism at a high temperature and microwave power, 6-gingerol was hydrated in structure to 6-shogaol, and reduction occurred (CH2)2, thereby producing paradol (Utama-ang et al., 2021). The attainment of the maximum paradol content had also been observed under the influence of microwave power ranging from 400 to 600 W. Moreover, the optimal conditions for achieving the highest concentrations of 6-gingerol and paradol were determined to be 400 W for 1 minute, producing values of 73.4±1.3 mg/g and 25.5±7.8 mg/g, respectively.
The hydration of shogaol to paradol is a continuation of the reactive extraction process of gingerol from ginger dregs. The mechanism includes the hydration of gingerol to produce shogaol, followed by shogaol to generate paradol at operating temperatures above 130oC (Gonzalez-Gonzalez et al. 2023; Dalsasso et al., 2022; Bhattarai, 2007). Although data regarding the mechanism in the hydration reaction of shogaol to paradol are available, there are still limited reports on diffusivity degradation of the process. Therefore, this modeling research is expected to be carried out using mathematical software without investigating several data because the mathematical parameter values have already been found to minimize paradol formation (Porgo et al., 2019).
Based on the description, this research aimed to determine diffusivity coefficient data of the thermal degradation of shogaol to paradol using subcritical water necessary to direct the reaction that produces shogaol. These data can be used to design process equipment to increase selectivity, improve shogaol yields, and minimize paradol byproducts. Currently, there is no mathematical model regarding the mechanistic reaction of shogaol hydration to paradol. Existing mathematical model is limited to diffusion liquefaction without including simultaneous reaction mechanisms. Therefore, this research discusses the submission of a mechanistic mathematical model from shogaol hydration to mass balance-based paradol.
The novelty of this research is to develop a mathematical model needed to determine the coefficient diffusion during extraction process of paradol, gingerol, and shogaol from ginger. The development is based on mechanistic reaction phenomena which has not been carried out in relation to reactive extraction of ginger dregs. Therefore, the data obtained could be used to confirm relativity with the predicted value.
Materials
Emprit ginger was purchased from local farmer with a further cleaning process and distilled water was used throughout the entire experiment. Subsequently, formic acid 95% and acetonitrile 99% were purchased from Merck (Bogor, Indonesia). All the chemicals used were of reagent grade unless otherwise showed in this research.
Methods
Subcritical water extraction process in this research with the additional extraction column. Ginger cultivar known as emprit ginger was purified to remove impurities before being subjected to a grated-squeezing machine to obtain a powdered form. Subsequently, ginger powder was dried, ground using a grinder, and filtered through a 50-mesh sieve as raw material. For extraction process, 100 g of ginger dregs powder was combined with 4,000 ml of pure distilled water in a stainless tube equipped with a lid, which served as extraction cell. To ensure the elimination of air and dissolved oxygen, extraction cell was sealed with a stainless lid, and a stream of N2 gas was introduced for a duration of 2 minutes. Excess pressure was released through a valve and the extractor temperature was gradually increased until it reached 130oC, which was the predetermined condition for extraction process initiation (t = 0). All experiments were conducted under a pressure of 3 bars.
After the completion of extraction process, the resulting extract was quickly transferred to a cooling cell maintained at a temperature of 25oC and a pressure of 1 MPa, facilitating rapid cooling within a duration of 1 minute. Throughout extraction process, multiple samples were collected at regular intervals of 10 minutes. The extracted compounds, specifically shogaol and paradol, present in the continuous phase (water) were subjected to chemical composition analysis using HPLC-MS. The HPLC-MS system used was a UPLC-QToFMS/MS System manufactured by Water and the LC-MS column used in the analysis was Acquity UPLC BEH C18 1.7um, with dimensions of 2.1x50 mm.
The eluent consisted of two components, namely (A) H2O with 0.1% formic acid (Merck) and (B) acetonitrile (Merck) with 0.1% formic acid. The flow rate of the eluent was set at 0.3 mL/min, and the injection was performed at 40 dC with a volume of 5 uL. The MS instrument used was the XEVO-G2QTOF (Water), operating in the resolution mode with electrospray ionization (ESI) in the positive polarity. Subsequently, the data obtained were analyzed using MassLynk software, version 4.1.
Submission of a Mathematical Model
In this research, several assumptions were provided before the submission of a mathematical model. These assumptions include the shape of ginger dregs being a slab, resulting in the distribution of concentration being in the axial position. Furthermore, the state of ginger dregs is unsteady, without influence of temperature and pressure changes (Beverly et al., 2020; Hartati et al., 2020; Porgo et al., 2019; Corrochano et al., 2015), the system is considered fixed, which includes the stationary phase, namely components in ginger dregs and subcritical water as mobile phase. The solvent flow rate, density, and viscosity are assumed to be constant throughout the process, the pressure and temperature gradients are ignored (Ghoreishi and Shahrestani, 2009). Gingerol and shogaol compounds are hydrophobic, thereby the system operates in a heterogeneous phase (Maghraby et al., 2023; Mao et al., 2019).
The reactive extraction mechanism of gingerol with subcritical water in zone one is diffusion. In this zone, subcritical water transport occurs towards the surface of the solid phase (ginger dregs) at a certain speed. In zone two, there is a reaction between subcritical water and gingerol, followed by the reduction of shogaol to paradol reversibly (Ko et al., 2019; Amiri et al., 2018; Jolad et al., 2004). Zone three is the diffusion zone, where gingerol, shogaol, and paradol from the surface of ginger dregs are transported to the liquid phase (Prihutami et al., 2022; Hartati et al., 2020; Yulianto et al., 2020).
The bulk solution model is influenced by several factors, such as the initial concentration of the solute that is uniform throughout ginger dregs (Zhou et al., 2015). Other factors include resistance to mass transfer, the presence of a reaction, the geometry of the solute on the solid surface, the volume of the solution, and the experiment duration (Asl and Khajenoori, 2013). Mathematical models for bulk solutions, such as the diffusion of A from the surface to the core, can be constructed using a mass balance (Lyu et al., 2005).
Input mass rate – Output mass rate + Generational mass rate = Accumulated mass rate (see equation 1)
Equation 1 is transformed into equation 2.
With the same method and assumptions, the diffusion of paradol from the core to the surface can be expressed using equation 3
Initial condition, at
In zone two, the reaction occurs between subcritical water and gingerol, while the reduction of shogaol to paradol is reversible, as shown in Figure 1.
Figure 1 The hydration mechanism of gingerols includes shogaol and paradol which were reported by Utama-ang et al. (2021) and Johnson et al. (2020) with some modification
The proposed mathematical model is based on the phenomenon of the reaction mechanism, as presented with the following denotation: A is gingerol, W is water, E is shogaol, and P is paradol. The reaction can be simplified as follows (equations 4-8):
The reaction mechanism in equations 4–8 is arranged to produce the total reaction rate, as presented in equation 9.
Equation (9) is simplified to obtain equation 10.
Mass Balance of A in the Volume Element
Input mass rate – Output mass rate + Generation mass rate = Accumulation mass rate. The mass balance of A in the volume element is presented (see equation 11-15).
the form of equation 11 is solved using limitations to obtain the final result presented in equation 12.
The values of W, S, and P can be compiled simultaneously through a mass balance. The results of the compilation are presented in Equations 13–15.
The mathematical model to describe shogaol reactive extraction phenomenon uses Equation 14.
In this case, paradol acts as the end product of the reactive extraction process. The phenomenon of paradol formation is generally described in a mathematical model, as presented in Equation 15.
The boundary conditions are Equation 20 shows the initial condition of shogaol. The data used is the fitting result of shogaol extraction with subcritical water at 20 minutes. Boundary conditions are at time
To solve the above equation, the calculated parameters used are shown in Table 1
Table 1 Calculated parameters
Figure 2 depicts a representative curve showing subcritical water reactive extraction process conducted at a temperature of 130°C and with a pressure of 3 bars. This curve shows the concentrations of gingerol, shogaol, and paradol in the extract as a function of extraction time. Based on the results, it is observed that as extraction time is prolonged, the yield of gingerol, shogaol, and paradol progressively increases. This phenomenon can be attributed to the enhanced contact between the dispersed and the continuous phase (subcritical water), leading to an increased penetration and a greater transfer. However, after the 20-minute mark, the yield of gingerol, shogaol, and paradol shows a reduction due to the degradation of gingerol into shogaol, shogaol into paradol, and paradol into zingerone.
Figure 2 Extraction results compounds of gingerol, shogaol, and paradol
The recovery of gingerol, shogaol, and paradol was observed to increase significantly at a temperature of 130°C. Subcritical water acting as a non-polar solvent also served as a catalyst by producing a greater number of ions through the process of water ionization. At 130°C, the strength of hydrogen bonds in water significantly reduced, leading to the auto-ionization of water into hydronium ions (H3O+), which showed acid catalytic properties. Consequently, with a longer extraction time, the dehydration conversion of gingerol to shogaol was enhanced. Ruiz et al. (2013) reported that temperatures above 150°C led to weakened hydrogen bonds, causing the auto-ionization of water into hydronium ions (H3O+) and hydroxide ions (OH-), functioning as acid and alkaline catalysts, respectively. Subcritical water also possessed the ability to reduce the activation energy required for extraction process (Peterson et al., 2008).
According to (Mao et al., 2019), shogaol formation occurs under high temperatures for a specific duration. (Wei et al., 2017) proposed that shogaol could potentially revert back to gingerol or experience hydration to form other compounds, such as paradol. This shows the possibility of reducing the concentration of paradol by facilitating the conversion into other derivative compounds, such as zingeron (Sediawan et al., 2023; Mandal et al., 2016; Rahath Kubra et al., 2013; Zhang et al., 2011).
Figures 3 and 4 show the research data and models that refer to equations 3-20 regarding the phenomenon of increasing shogaol and paradol. The results showed that the concentration of shogaol increased as the time was extended. This was because the longer phase contact opportunities and subcritical water conditions led to a reduction in the dielectric constant of water and polarity. Moreover, decreasing the polarity of water can dissolve fewer polar compounds, resulting in a high concentration of solutes (Yulianto et al., 2023).
The results for the proposed model showed that equilibrium occurred at the 20th minute, but there was a decrease in shogaol concentration. This showed that the hydration of shogaol could become paradol compound. According to previous research, significant gingerol dehydration occurred at temperatures above 60°, with 50% of the degraded sample obtained when the operation period reached 24 hours. Dehydration reactions that run at temperatures above 100° will have a faster reaction rate, reaching the equilibrium in a period of 2 hours (Bhattarai et al., 2007).
Figure 3 Comparison of shogaol data and calculations
Figure 4 Comparison of paradol data and calculations
Table 2 shows the values of diffusivity coefficient and the reaction rate constant for the reactive extraction of ginger dregs through subcritical water, validated through a mathematical model. Based on the results, diffusivity of shogaol is higher due to greater molar volume compared to gingerol (Bhattarai et al., 2007). A higher shogaol diffusivity coefficient shows a greater solute transfer rate into the solvent compared to the other components (Teng et al., 2019; Guo et al., 2015; Ghoreishi and Shahrestani, 2009). However, previous research obtained smaller diffusivity values of gingerol and ginger shogaol at extraction temperature of 130°, which were 8.562 x 10-11 m2/s and 3.886 x 10-11 m2/s, respectively (Anisa et al., 2014).
Table 2 Diffusivity coefficient and reaction rate constant
Subcritical water diffusivity values in Table 2 show that 0.0012 cm2/minute was higher compared to the values obtained by Ghoreishi and Shahrestani (2009). This shows that diffusivity of water in olive leaves is equal to 5.2 x10-5 m2/s. The difference in results is also shown by the dielectric constant (), which is 49.03, in line with reference value
at a temperature of 130°. Based on the dielectric constant, the movement of gingerol and shogaol is minimal compared to the reference (Ghoreishi and Shahrestani, 2009).
Table 3 Distribution of constant value to measure the limiting step
The limiting step in the reactive extraction mechanism is determined by comparing diffusivity coefficient (D) value with the reaction rate constant (k). The mechanism for constructing the graph includes varying the value of diffusivity coefficient and doubling the reaction rate constant to show the most influential domination. The D and k values for the trial graph are presented in Tables 3 and 4, respectively.
Figure 5 The distribution of the data is dominated by the reaction rate constant (k)
Figure 5 shows the data distribution indicating the dominance of the reaction rate constant (k). Moreover, a decrease in the value of the reaction rate constant influences the resulting calculated data. A smaller value of the reaction rate constant (k) leads to a decrease in the distribution of calculated data and vice versa. When the value of the reaction rate constant is increased, paradol formation value will simultaneously result in a greater concentration.
Table 4 Distribution of diffusivity value for measuring the limiting step
Table 3 shows the distribution of paradol diffusivity data at a certain scale, as presented in Figure 5. The results show that there is a change in the distribution of concentration data at various diffusivity coefficient values. Specifically, a greater value of diffusivity coefficient affects the increase in the concentration of extraction results. A significant disparity is also observed between Figures 5 and 6, where the effect of diffusivity on extraction process is dominant compared to extraction mechanism.
The calculation of the overall mass transfer coefficient (De) is based on the mass balance equation for subcritical water extraction process. Moreover, it is assumed that the amount transferred is proportional to the concentration difference and the interface area, leading to the proposal of two different model expressions. The first model expression accounts for the particle size of ginger dregs, the total surface area, and the overall absolute mass transfer coefficient (Mandal et al., 2016).
Figure 6 Distribution of diffusivity coefficient (D)
In conclusion, this research showed that critical water-assisted extraction carried out at a temperature of 130oC, caused the hydration of the active gingerol compound to become shogaol and paradol. This phenomenon was characterized by a decrease in levels at a certain point in time. The results suggested that diffusivity of gingerol (D_eA), subcritical water (D_ew), shogaol (D_eS), and paradol (D_eP) in ginger dregs were 0.000324 cm2/minute, 0.0012 cm2/minute, 0.0003435 cm2/minute, and 0.03875 cm2/minute, respectively, with reaction rate constant (k’’’) of 0.37918 cm3/mol.minute. Based on the data distribution test, diffusivity coefficient in the reactive extraction process showed greater dominancy compared to the reaction rate constant. Furthermore, SSE value showed the potential of the proposed mathematical model to describe the phenomena occurring in the reactive extraction process of ginger dregs.
Amiri, Z,
Nourbakhsh, G, Najafpour, G D, Mohammadi, M & Moghadamnia, A A 2018,
'Subcritical water extraction of bioactive compounds from ginger (Zingiber
officinale Roscoe)', International Journal of Engineering, vol. 31, no.
12, pp. 1991–2000, https://doi.org/10.5829/ije.2018.31.12c.01
Anisa, NI, Noor
Azian, M S & Iwai, Y 2014, 'Temperature effects on diffusion coefficient
for 6-gingerol and 6-shogaol in subcritical water extraction', Journal of
Physics: Conference Series, vol. 495, no. 1, https://doi.org/10.1088/1742-6596/495/1/012009
Asl, AH & Khajenoori,
M 2013, ' Subcritical Water Extraction', Intech, pp. 460-487, https://dx.doi.org/10.5772/54993
Bager, S 2012,
'Assessment report on Zingiber officinale Roscoe, rhizoma', Assessment
Report, vol. 44, pp. 1-49
Beverly, D,
Lopez-Quiroga, E, Farr, R, Melrose, J, Henson, S, Bakalis, S & Fryer, P J
2020, 'Modeling mass and heat transfer in multiphase coffee aroma extraction', Industrial
and Engineering Chemistry Research, vol. 59, no. 24, pp. 11099–11112, https://doi.org/10.1021/acs.iecr.0c01153
Bhattarai, S,
Tran, VH & Duke, CC 2007, 'Stability of [6]-gingerol and [6]-shogaol in
simulated gastric and intestinal fluids', Journal of Pharmaceutical and
Biomedical Analysis, vol. 45, no. 4, pp. 648–653, https://doi.org/10.1016/j.jpba.2007.07.006
Cheng, Y, Xue,
F, Yu, S, Du, S & Yang, Y 2021, 'Subcritical water extraction of natural
products', Molecules, vol. 26, no. 13, https://doi.org/10.3390/molecules26134004
Claudya, RP,
Sugiaman, HA, Labiba, SI, Utari, MP & Gunawan, D 2023, 'The therapeutic
effects of ginger extract on gastrointestinal disorders to adults', Science
Midwifery, vol. 11, no. 1, pp. 2721–9453
Corrochano, BR,
Melrose, JR, Bentley, AC, Fryer, PJ & Bakalis, S 2015, 'A new methodology
to estimate the steady-state permeability of roast and ground coffee in packed
beds', Journal of Food Engineering, vol. 150, pp. 106–116, https://doi.org/10.1016/j.jfoodeng.2014.11.006
Dalsasso, RR,
Valencia, GA & Monteiro, AR 2022, 'Impact of drying and extractions
processes on the recovery of gingerols and shogaols, the main bioactive
compounds of ginger', Food Research International, vol. 154, https://doi.org/10.1016/j.foodres.2022.111043
Ghoreishi, SM
& Shahrestani, RG 2009, 'Subcritical water extraction of mannitol from
olive leaves', Journal of Food Engineering, vol. 93, no. 4, pp. 474–481,
https://doi.org/10.1016/j.jfoodeng.2009.02.015
Gonzalez-Gonzalez,
M, Yerena-Prieto, BJ, Carrera, C, Vázquez-Espinosa, M, González-de-Peredo, AV,
García-Alvarado, MÁ, Palma, M, Rodríguez-Jimenes, GC & Fernández Barbero, G
2023, 'Determination of gingerols and shogaols content from ginger (Zingiber
officinale Rosc.) through microwave-assisted extraction', Agronomy, vol.
13, no. 9, https://doi.org/10.3390/agronomy13092288
Guo, JB, Zhang,
WJ, Wu, H & Du, LM 2015, 'Microwave-assisted decomposition coupled with
acidic food condiment as an efficient technology for ginger (Zingiber
officinale Roscoe) processing', Separation and Purification Technology,
vol. 146, pp. 219–226, https://doi.org/10.1016/j.seppur.2015.03.049
Handayani, D,
Amalia, R, Yulianto, ME, & Murni 2018, 'Nanoemulsion production of ginger
oil from enzymatic extraction of isolated cow rumen enzyme', AIP Conference
Proceedings, vol. 1977, https://doi.org/10.1063/1.5042893
Hartati, I,
Sulistyo, H, Sediawan, WB, Azis, MM, & Fahrurrozi, M 2020, 'Mathematical
modeling of reactive extraction of solute from slab solid material', Indonesian
Journal of Chemistry, vol. 20, no. 2, pp. 458–467, https://doi.org/10.22146/ijc.47181
Johnson, J,
Mani, J & Naiker, M 2020, 'Gingerol, shogaol and paradol: The chemistry of
pungent ginger constituents', Education and Organic Chemistry, Nov, pp.
89–90, https://doi.org/10.13140/RG.2.2.36210.63681
Jolad, SD,
Lantz, RC, Solyom, AM, Chen, GJ, Bates, RB & Timmermann, BN 2004, 'Fresh
organically grown ginger (Zingiber officinale): Composition and effects on
LPS-induced PGE2 production', Phytochemistry, vol. 65, no. 13, pp.
1937–1954, https://doi.org/10.1016/j.phytochem.2004.06.008
Kamaruddin, MSH,
Chong, GH, Umanan, F & Suleiman, N 2023, 'Enhancement of 6-gingerol
extraction from Bentong ginger using supercritical carbon dioxide', Journal
of CO2 Utilization, vol. 72, pp. 102505, https://doi.org/10.1016/j.jcou.2023.102505
Ko, MJ, Nam, HH
& Chung, MS 2019, 'Conversion of 6-gingerol to 6-shogaol in ginger
(Zingiber officinale) pulp and peel during subcritical water extraction', Food
Chemistry, vol. 270, pp. 149–155,
https://doi.org/10.1016/j.foodchem.2018.07.078
Lyu, S, Sparer R
& Untereker, D 2005,'ANalytical solution to mathematical models of the
surface and bulk erosion of solid polymers', Journal of Polymer Science Part
B Polymer Physics, vol. 43, no. 4, pp. 383-397, https://doi.org/10.1002/polb/20340
Maghraby, YR,
Labib, RM, Sobeh, M & Farag, MA 2023, 'Gingerols and shogaols: A
multi-faceted review of their extraction, formulation, and analysis in drugs
and biofluids to maximize their nutraceutical and pharmaceutical applications',
Food Chemistry: X, https://doi.org/10.1016/j.fochx.2023.100947
Mandal, V,
Mohan, Y & Hemalatha, S 2016, 'Microwave assisted extraction - An
innovative and promising extraction tool for medicinal plant research', Phcog
Rev, Jan
Mao, QQ, Xu, XY,
Cao, SY, Gan, RY, Corke, H, Beta, T & Li, HB 2019, 'Bioactive compounds and
bioactivities of ginger (Zingiber officinale Roscoe)', Foods, vol. 8,
no. 6, pp. 1–21, https://doi.org/10.3390/foods8060185
Md Sarip, MS,
Morad, NA, Mohamad Ali, NA, Mohd Yusof, YA & Che Yunus, MA 2014, 'The
kinetics of extraction of the medicinal ginger bioactive compounds using hot
compressed water', Separation and Purification Technology, vol. 124, pp.
141–147, https://doi.org/10.1016/j.seppur.2014.01.008
Peterson, AA,
Vogel, F, Lachance, RP, Fröling, M, Antal, MJ & Tester, JW 2008,
'Thermochemical biofuel production in hydrothermal media: A review of sub- and
supercritical water technologies', Energy and Environmental Science,
vol. 1, no. 1, pp. 32–65, https://doi.org/10.1039/b810100k
Porgo, TV,
Norris, SL, Salanti, G, Johnson, LF, Simpson, JA, Low, N, Egger, M &
Althaus, CL 2019, 'The use of mathematical modeling studies for evidence
synthesis and guideline development: A glossary', Research Synthesis Methods,
vol. 10, no. 1, pp. 125–133, https://doi.org/10.1002/jrsm.1333
Prihutami, P,
Sediawan, WB, Prasetya, A & Petrus, HTB M 2022, 'A product diffusion model
for the extraction of cerium and yttrium from magnetic coal fly ash using
citric acid solution', International Journal of Technology, vol. 13, no.
4, pp. 921–930, https://doi.org/10.14716/ijtech.v13i4.4826
Rahath Kubra, I,
Kumar, D & Rao, JM 2013, 'Effect of
microwave-assisted extraction on the release of polyphenols from ginger
(Zingiber officinale)', International Journal of Food Science and Technology,
vol. 48, no. 9, pp. 1828–1833, https://doi.org/10.1111/ijfs.12157
Rahmani, AH, Al
Shabrmi, FM & Aly, SM 2014, 'Active ingredients of ginger as potential
candidates in the prevention and treatment of diseases via modulation of
biological activities', International Journal of Physiology, Pathophysiology
and Pharmacology, vol. 6, no. 2, pp. 125–136
Ramakrishnan, R
2013, 'Anticancer properties of Zingiber officinale-ginger: A review', International
Journal of Medicine and Pharmaceutical Sciences (IJMPS), vol. 3, no. 5, pp.
11–20
Razak, AM,
Zakaria, SNA, Abdul Sani, NF, Ab Rani, N, Hakimi, NH, Mohd Said, M, Tan, JK,
Gan, HK, Mad Nordin, MF & Makpol, S 2023, 'A subcritical water extract of
soil grown Zingiber officinale Roscoe: Comparative analysis of antioxidant and
anti-inflammatory effects and evaluation of bioactive metabolites', Frontiers
in Pharmacology, vol. 14, https://doi.org/10.3389/fphar.2023.1006265
Rice, RG &
Do, DD 1995, Applied mathematics and modeling for chemical engineers,
vol. 50
Riyadhi, A,
Yulizar, Y, & Susanto, BH 2022, 'Catalytic conversion of beef tallow to
biofuels using MgO nanoparticles green synthesized by Zingiber officinale
Roscoe rhizome extract', International Journal of Technology, vol. 13,
no. 4, pp. 900–911, https://doi.org/10.14716/ijtech.v13i4.4821
Ruiz, HA,
Rodríguez-Jasso, RM, Fernandes, BD, Vicente, AA & Teixeira, JA 2013,
'Hydrothermal processing, as an alternative for upgrading agriculture residues
and marine biomass according to the biorefinery concept: A review', Renewable
and Sustainable Energy Reviews, vol. 21, pp. 35–51, https://doi.org/10.1016/j.rser.2012.11.069
Sediawan, WB,
Hartati, I, Sulistyo, H, Azis, MM & Al Rahma, U 2023, 'Microwave assisted
hydrotropic distillation of myrcene-rich essential oil of Cymbopogon citratus',
International Journal of Technology, vol. 14, no. 2, pp. 310–319, https://doi.org/10.14716/ijtech.v14i2.5381
Teng, H, Seuseu,
KT, Lee, WY & Chen, L 2019, 'Comparing the effects of microwave radiation
on 6-gingerol and 6-shogaol from ginger rhizomes (Zingiber officinale Rosc)', PLoS
ONE, vol. 14, no. 6, pp. 1–16, https://doi.org/10.1371/journal.pone.0214893
Teo, CC, Tan,
SN, Yong, JWH, Hew, CS & Ong, ES 2010, 'Pressurized hot water extraction
(PHWE)', Journal of Chromatography A, vol. 1217, no. 16, pp. 2484–2494, https://doi.org/10.1016/j.chroma.2009.12.050
Utama-ang, N,
Sida, S, Wanachantararak, P & Kawee-ai, A 2021, 'Development of edible Thai
rice film fortified with ginger extract using microwave-assisted extraction for
oral antimicrobial properties', Scientific Reports, vol. 11, no. 1, pp.
1–10, https://doi.org/10.1038/s41598-021-94430-y
Wei, CK, Tsai,
YH, Korinek, M, Hung, PH, El-Shazly, M, Cheng, YB, Wu, YC, Hsieh, TJ &
Chang, FR 2017, '6-Paradol and 6-shogaol, the pungent compounds of ginger,
promote glucose utilization in adipocytes and myotubes, and 6-paradol reduces
blood glucose in high-fat diet-fed mice', International Journal of Molecular
Sciences, vol. 18, no. 1, pp. 1–18, https://doi.org/10.3390/ijms18010168
Yulianto, ME,
Jos, B & Budiyono, B 2023, 'Kinetic modelling of liquid-solid extraction of
bioactive compounds from ginger waste using subcritical water', Sainteknol:
Jurnal Sains dan Teknologi, vol. 21, no. 1, pp. 28–35, https://doi.org/10.15294/sainteknol.v21i1.44589
Yulianto, ME,
Kusumo, P, Hartati, I & Wahyuningsih, A 2017, 'Esubcritical water
extraction of gingerol from Zingiber officinale', Rasayan Journal of
Chemistry, vol. 10, no. 3, pp. 738–743, https://doi.org/10.7324/RJC.2017.1031619
Zhang, HF, Yang,
XH & Wang, Y 2011, 'Microwave assisted extraction of secondary metabolites
from plants: Current status and future directions', Trends in Food Science
and Technology, vol. 22, no. 12, pp. 672–688, https://doi.org/10.1016/j.tifs.2011.07.003
Zhou, X,
Hetrick, S E, Cuikipers, P, Qin, B, Barth, J, Whittington, C J, Cohen, D,
Giovane, C D, Liu, Y, Michael, K D, Zhang, Y, Weisz, J R & Xie, P 2015
'Comparative efficacy and acceptability of psychotherapies for depression In
children and adolescents : A systematic review and network meta-analysis', World
Psychiatrym, vol.14, no 2, pp. 207-222, https://doi.org/10.1002/wps.202187
