The Study of Methylene Blue Loading into Chitosan-graft-Maleic Sponges
Corresponding email: yuni_kusumastuti@ugm.ac.id
Published at : 30 Dec 2022
Volume : IJtech
Vol 13, No 8 (2022)
DOI : https://doi.org/10.14716/ijtech.v13i8.6133
Timotius, D., Kusumastuti, Y., Omar, R., Kamal, S.M.M., Jenie, S.N.A., Petrus, H.T.B.M., 2022. The Study of Methylene Blue Loading into Chitosan-graft-Maleic Sponges. International Journal of Technology. Volume 13(8), pp. 1796-1805
| Daniel Timotius | Department of Chemical Engineering, Faculty of Industrial Engineering, Universitas Pembangunan Nasional “Veteran” Yogyakarta, Jl. SWK 104, Yogyakarta 55283, Indonesia |
| Yuni Kusumastuti | Department of Chemical Engineering, Faculty of Engineering, Universitas Gadjah Mada, Jalan Grafika 2, Yogyakarta 55281, Indonesia |
| Rozita Omar | Department of Chemical and Environmental Engineering, Faculty of Engineering, Universiti Putra Malaysia, Serdang 43400, Selangor, Malaysia |
| Razif Harun | Department of Chemical and Environmental Engineering, Faculty of Engineering, Universiti Putra Malaysia, Serdang 43400, Selangor, Malaysia |
| Siti Mazlina Mustapa Kamal | Department of Food and Process Engineering, Faculty of Engineering, Universiti Putra Malaysia, Serdang 43400, Selangor, Malaysia |
| Siti Nurul Aisyiyah Jenie | Research Centre for Chemistry, National Research and Innovation Agency (BRIN), Kawasan Puspiptek Building 452, Serpong, Tangerang Selatan 15314, Indonesia |
| Himawan Tri Bayu Murti Petrus | Department of Chemical Engineering, Faculty of Engineering, Universitas Gadjah Mada, Jalan Grafika 2, Yogyakarta 55281, Indonesia |
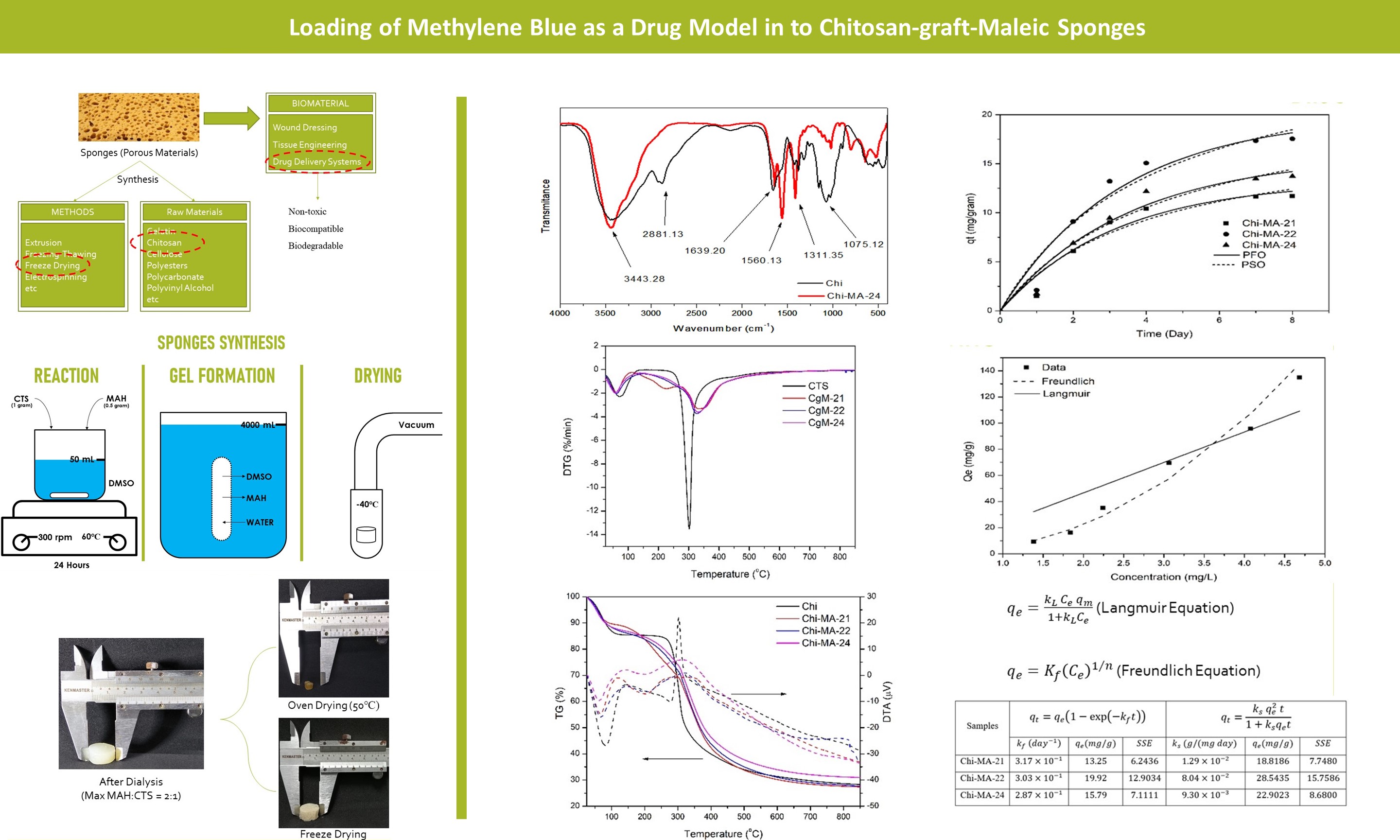
This work succeeded in synthesizing sponges
from chitosan-graft-maleic anhydride. Chitosan and maleic anhydride at a
certain mass ratio (1:2, 1:1; and 2:1) were reacted in dimethyl sulfoxide
(DMSO) at 60? for 24 hours. While the gel formation was carried out in distilled
water using a dialysis tube. The removal of DMSO from the gel was carried out
by soaking the gel in distilled water for two days. After that, the obtained
gel was frozen at -25oC overnight before being lyophilized under a
vacuum at -40oC for 24 hours. The dry sponge weight obtained after lyophilization is
about 5.61% – 6.77% (dry sponge/wet gel). FTIR and TG/DTA then characterized
the sponges. The intensity of the new strong FTIR band appeared in the
chitosan-graft-maleic sponge at 1560.13 cm-1, which corresponds to
the C=C strain. The results of TG/DTA showed that pure chitosan and sponge
chitosan-maleate underwent two stages of degradation, namely evaporation of
water and pyrolysis of organic compounds. The shift in the peak rate of
degradation in the second stage occurred at 300? for pure chitosan and at 340? for a
graft-chitosan-maleate sponge. Both results indicate that the reaction was
successful. The drug loading capability was investigated using methylene blue
as the drug model. The drug loading kinetics corresponded to a
pseudo-first-order model with kf ranging from 0.287 to 0.317 day-1.
According to the Freundlich model, the adsorption equilibrium with Kf
and 1/n values was 4.923 mg/gram and 2.192, respectively.
Chitosan; Drug delivery system; Maleic anhydride; Methylene blue; Sponges
Sponges are porous materials
synthesized from various materials and methods (Deb
et al, 2018). It has many advantages in the field of biomaterials, such
as wound dressings (Horio et al., 2010),
tissue engineering (Nikolova & Chavali, 2019),
and drug delivery systems (Cai et al., 2018).
A large surface area is required to attach the active molecule or drug in drug delivery systems.
Not only surface area but also materials for drug delivery systems require
several properties, such as non-toxicity, biocompatibility, and
biodegradability (Patel et al., 2018). These
properties are found in many natural polymers, one of which is chitosan (Peers et al., 2020).
Chitosan
(1-4)-2-amino-2-deoxy-?-D-glucan is the second most abundant polysaccharide (Ali & Ahmed, 2018). And it is usually
synthesized from the deacetylation of chitin (Sinha
et al., 2004) which extracted from the exoskeleton of crustaceans (Liu et al., 2016). Chitosan and chitin can be
distinguished by deacetylation (DD) degree. If the DD greater than 50%, it will
consider as chitosan (Knidri et al.,
2018). Chitosan
has been extensively used as a drug delivery system due to its properties,
namely non-toxic, biodegradable, and mucoadhesive (Krisanti
et al., 2020). Chitosan has two active functional groups, amine and hydroxyl,
and is usually further modified by other chemicals. Sponges from chitosan are
made by lyophilization of gel or chitosan solution. Lyophilization or
freeze-drying is a process in which water is frozen and removed through
sublimation (primary drying) and desorption (secondary drying). In sponges,
pore size is an important parameter (Deb et al.,
2018). The pore size of the sponge can be controlled by varying several
parameters, such as the geometry of the thermal gradient during freezing, ice
crystal size, freezing temperature, and freezing rate (Pottathara
et al., 2021).
Chitosan is
usually reacted with a crosslinking agent to form a gel. There are many
crosslinking agents with different interactions, namely chemical and physical
crosslinkers (Hamedi et al., 2018). Chemical
crosslinks are formed by irreversible covalent bonds between polymers and
crosslinkers (Liu et al., 2014), whereas
physical crosslinks are reversible interactions between polymers and
crosslinkers (Croisier & Jérôme, 2013).
In physical crosslinking, crosslinking can be found in the form of ions or
ionic molecules. Chitosan is polycationic with a protonated amine group.
Therefore, to physically crosslink chitosan, anions or anionic molecules are
needed (Kono et al., 2013). Maleic anhydride becomes an
attractive anionic molecule to use if it is to be further modified. Chitosan
reacts with maleic anhydride through the amidation and esterification
processes. After the reaction, chitosan has a cationic functional group from
the protonated amine group and anionic functional group from the deprotonated
carboxyl group, therefore it becomes a polyampholyte (Timotius
et al., 2022).
The drug model used in this study is methylene blue. Methylene blue has a long journey as a drug that is applied on malaria diseases (Ashley & Phyo, 2018). Now, this cationic drug has been widely used, not only on malaria, but also on other diseases, namely methemoglobinemia, ifosfamide-induced neurotoxicity, paraplegic adrenaline-resistant shock, and Alzheimer's (Schirmer et al., 2011). Another study uses methylene blue to enhance the apoptosis of cells in lung cancer by photodynamic therapy (Lim et al, 2013). Another study reviews its potency as anti-aging drug (Xue et al., 2021).
This study is aiming the potency of chitosan-graft-maleic sponges as a drug carrier. In this study, the lyophilization method successfully synthesized a novel chitosan base sponge from chitosan and maleic anhydride. The modified chitosan solution is prepared by reacting low molecular weight chitosan with maleic anhydride in dimethyl sulfoxide (DMSO). Furthermore, the gel formation is carried out by dialysis of the modified chitosan solution under aquadest. Then before lyophilization, the gels were frozen at –25?. The obtained sponges are then further characterized.
2.1. Materials
Low molecular weight chitosan (50
– 190 kDa, 75 – 85% DD) was obtained from Sigma Aldrich, USA. Acetic acid
(100%) was supplied from Merck, Germany. Maleic anhydride (>99% purity) was
purchased from Nacalai Tesque Inc., Japan. Methylene blue was obtained from
Merck, India. Merck, Japan, supplied dimethyl sulfoxide (DMSO). All reagents
were used without further purification or treatment.
2.2. Methods
2.1.1. Synthesis
of Chitosan-graft-Maleic Sponges
The chitosan-graft-maleic (Chi-MA) synthesis followed previous studies with slight modifications (Zhou et al., 2017). The scheme of sponges synthesis is provided in Figure 1. About 1 gram of chitosan is dissolved in 50 mL of dimethyl sulfoxide. After the chitosan was dissolved entirely, maleic anhydride was added to the solution, followed by stirring at 300 rpm, 60oC for 24 hours. Gel formation was carried out using a dialysis tube. After the reaction, the solution is placed in cellulose dialysis tubing (MWCO 15 kDa) and dialyzed under deionized water to form a gel. The removal of DMSO from the gel was carried out by soaking the gel in distilled water for two days. Afterward, the obtained gel was frozen at -25oC overnight before being lyophilized under a vacuum at -40oC for 24 hours. This method was performed for several MA masses (0.5 grams, 1 gram, and 2 grams) labeled Chi-MA-21, Chi-MA-22, and Chi-MA-24.
Figure 1 Preparation of Chitosan-graft-Maleic
Sponges
2.1.2. Characterization
FTIR and
thermogravimetric analysis were carried out for the characterization of
chitosan sponges. The FTIR study was performed by SHIMADZU IR Pestige-21. The sponges
were scanned with wavenumber ranging from 400 – 4000 cm-1. The
thermogravimetric analysis (TGA) study was conducted by NEXTA STA (Hitachi
STA200RV) with actual view sample observations, and 15 mg of the sample was
heated to a temperature of 900oC with a heating rate of 10oC /min.
2.1.3. Drug
Loading
Drug loading
was carried out using the Chi-MA gel state. Methylene blue was dissolved in
deionized water and analyzed using a Vis Spectrophotometer (Genesys 20) as the
initial concentration (). Chi-MA gel was weighed and immersed in methylene blue solution.
A decrease in concentration (
) was observed for several increments. Observations are completed
when equilibrium has been reached. The adsorbed methylene blue (
) was calculated using Equation 1, where the V and
Kinetic
studies were evaluated using two simple models, namely pseudo-first-order (PFO,
Equation 2) and pseudo-second-order (PSO, Equation 3) (Syafiuddin
et al., 2018). Where and
The
adsorption model evaluation was conducted by two different models: Langmuir
adsorption isotherm and Freundlich adsorption isotherm. The Langmuir model was
presented using Equation 5, while the Freundlich model was shown in Equation 6 (Ayawei et al., 2017). The
adsorption isotherm was conducted by measuring the equilibrium state of drug
loading.
3.1. Gel Preparation
The gel synthesis is successfully obtained up to 2:1 of MA to chitosan mass ratio. According to previous work (Zhou et al., 2017), the MA to chitosan ratio of about 3.5:1 makes the modified chitosan dissolve in the water instead of gel formation. It might happen because most of the amine groups are grafted by MA. Therefore, carboxyl groups instead of amine groups will dominate the chitosan. In the present work, 2:1 is being a maximum MA to chitosan ratio. The appearance of the gel is presented in Figure 1.
Figure 2 Gel State
of Chitosan-graft-Maleic
The drying process of the gel is performed
with two different methods, namely oven and freeze-drying. The oven-drying process shows an extreme shrinkage in the gel volume, as shown in
Figure 2a. This might have happened because the polymers cannot hold their
structure. Unlike the oven-drying
process, the freeze-drying or lyophilization process creates a sponge with low
shrinkage, and the polymer can maintain its structure, as presented in Figure
2b. The shrinkage on oven drying is about 92.97% in volume, while freeze-dried
sponge only shrank about 12.11%. This is occurred due to the sublimation
process when the ice crystal is directly converted into a gas state (Kassem et al., 2015). The sponge's sample varies
from 5.61 – 6.77%, while the rest is water.
|
|
Figure 3 Result of Oven Drying (a) and
Freeze Drying (b) of Chi-MA Gels
3.2. Component Analysis
FTIR study on chitosan and
Chi-MA-24 is conducted to observe each sample's functional group. Both results
are shown in Figure 3. The FTIR spectrum of pure chitosan shows a broad peak at
around 3443.28
|
|
|
Figure 4 FTIR Spectra of Pure
Chitosan (a) and Chitosan-graft-Maleic (b)
3.3. Thermogravimetric
Analysis
The study is
conducted to analyze the thermal stability of the Chi-MA samples. The result of
TG/DTA from Chi and Chi-MA samples is presented in Figure 5. The result shows
that the first endothermic peak in DTA result at 82.56? for pure chitosan
and around 63-67? for Chi-MA are due to the evaporation of
water and volatile organic compound (Kusumastuti et
al., 2020), which in line with the TG
result. At the same time, the second endothermic flow corresponds to the glass
transition temperature. The rate of degradation of chitosan and Chi-MA samples
consists of 2 steps, as shown in Figure 5b. The first step is due to the
samples' drying process, water evaporation, and volatile organic matter. In
contrast, the second step occurs due to the degradation of organic compounds to
form char. In the first step of pure chitosan, water evaporation occurs up to
100? with a weight loss of about 15%. Meanwhile, the primary degradation
occurs from about 250 to 450?, with a minimal degradation
rate. It is consistent with another study (Kumar
& Koh, 2012). The degradation pattern of Chi-MA samples is similar
to pure chitosan. However, there is a slight shift in the degradation rate
between chitosan and Chi-MA samples, as shown in Figure 5a. The chitosan has a
steeper degradation rate, while the Chi-MA samples are thermally more stable.
Figure 5 TG/DTA of Chi and
Chi-MA Samples at 10?/min Heating Rate
3.4. Drug Loading
The result of
methylene blue adsorption into the hydrogel’s matrices is presented in Figure
6. The result shows that the equilibrium state appeared after eight days of
adsorption for all samples. The optimum drug loading appears in Chi-MA-22
samples with the highest methylene blue loading. Kinetic adsorption of
methylene blue onto Chi-MA samples is determined using pseudo-first order and
pseudo-second order kinetics (Aljeboree et al,
2017). The plot for both models is shown in Figure 6, while the
parameter values are shown in Table 1. From the results, it can be concluded
that pseudo-first
order best fits all samples. Where ).
The pseudo-first-order rate model primarily models the physical adsorption
phenomenon, while the pseudo-second-order model primarily models the chemical
adsorption phenomenon. According to the result, the kinetic has the best fit on
the pseudo-first order model with higher compared to pseudo-second-order model. The
physical adsorption occurred due to the interaction between positive charges of
the methylene blue with the COO- functional groups in the Chi-MA
samples (Wirawan et al, 2022).
The equilibrium study
is carried out by measuring the equilibrium concentration of methylene blue.
The measurements are conducted at room temperature and several Chi-MA-22 doses
involving two equilibrium models: Langmuir (Equation 5) and Freundlich
(Equation 6), which are presented in Table 2. The Langmuir model consists of 2
parameters: full monolayer coverage()
and Langmuir constant (
).
While in the Freundlich model,
and
Table 1 Kinetic Parameter Values of Each
Model
Figure 6 Methylene Blue Loading into Chi-MA Samples
Table 2 Adsorption Equilibrium Parameter
Values of Each Model
Figure 7 Adsorption Isotherm of
Methylene Blue in Chi-MA-22
The
chitosan-graft-maleic sponge was synthesized using the lyophilization method.
The maximum ratio of maleic anhydride to chitosan mass ratio is 2:1. According
to the result, the reaction and lyophilization were successfully conducted,
which showed by the appearance of a peak at 1560.13cm-1,
corresponding to the C=C bond. The shift in the peak rate of degradation in the
second stage occurred at 300oC for pure chitosan and at 340oC for a graft-chitosan-maleate sponge. The methylene blue loading followed a pseudo-first-order
kinetics model with Kf values varying from 0.287 – 0.317day-1.
While the adsorption isotherm was best fitted with the Freundlich isotherm
model with Kf and 1/n values are 4.92 and 2.19,
respectively. Hence it can be concluded that a chitosan-graft-maleic sponge can
be used as a methylene blue carrier.
The authors would like to acknowledge Ministry of Education and
Culture of Indonesia for the financial support through PDUPT scheme (No.
2573/UN1-DITLIT/DIT-LIT/LT/2019) and partial support from SEAMEO for providing
financial support under the University Consortium Seed Fund for Collaborative
Research Grant. The authors also would like to extend their gratitude to the
Universitas Gadjah Mada and Universiti Putra Malaysia for the research
facilities and raw materials for completing this study.
Ali, A., Ahmed, S., 2018. A Review on Chitosan
and Its Nanocomposites in Drug Delivery. International Journal of Biological Macromolecules, Volume 109, pp. 273–286
Aljeboree,
A.M., Alshirifi, A.N., Alkaim, A.F., 2017. Kinetics and Equilibrium Study for
the Adsorption of Textile Dyes on Coconut Shell Activated Carbon. Arabian Journal of Chemistry, Volume 10, pp. S3381–S3393
Ayawei, N.,
Ebelegi, A.N., Wankasi, D., 2017. Modeling and Interpretation of Adsorption Isotherms.
Journal Chemistry, Volume 2017, p. 3039817
Ashley, E.A., Phyo. A.P., 2018.
Drugs in Development for Malaria. Drugs, Volume 78(9), pp 861-879
Barleany, D.R.,
Ananta, C.V., Maulina, F., Rochmat, A., Alwan, H., Erizal, 2020. Controlled
Release of Metformin Hydrogen Chloride from Stimuli-Responsive Hydrogel Based
on Poly(N-Isoprpylacrylamide)/Chitosan/Polyvinyl Alcohol Composite. International Journal of Technology, Volume 11(3), pp. 511–521
Cai, B., Zhong,
T., Chen, P., Fu, J., Jin, Y., Liu, Y., Huang, R., Tan, L., 2018. Preparation, Characterization
and In Vitro
Release Study of Drug-Loaded
Sodium Carboxymethyl Cellulose/Chitosan Composite Sponge. PLoS ONE, Volume 13(10), pp. e0206275
Croisier, F.,
Jérôme, C., 2013. Chitosan-Based Biomaterials for Tissue Engineering. European Polymer Journal, Volume 49, pp. 780–792
Deb, P.,
Deoghare, A.B., Borah, A., Barua, E., Lala, S.D., 2018. Scaffold Development
Using Biomaterials: A Review. Materials
Today: Proceedings, Volume 5(5),
pp. 12909–12919
Haerudin, H.,
Pramono, A.W., Kusuma, D.S., Jenie, A., Voelcker, N.H., Gibson, C., 2010.
Preparation and Characterization of Chitosan/Montmorillonite (MMT)
Nanocomposite Systems. International
Journal of Technology, Volume 1(1), pp. 65–73
Hamedi, H.,
Moradi, S., Hudson, S.M., Tonelli, A.E., 2018. Chitosan Based Hydrogels and
Their Application for Drug Delivery in Wound Dressing: A Review. Carbohydrate Polymers, Volume 199, pp. 445–460
Horio, T.,
Isihara, M., Fujita, M., Kishimoto, S., Kanatani, Y., Ishizuka, T., Nogami, Y.,
Nakamura, S., Tanaka, Y., Morimoto, Y., Maehara, T., 2010. Effect of
Photocrosslinkable Chitosan Hydrogel and Its Sponges to Stop Bleeding in a Rat
Liver Injury Model, Artificial
Organs, Volume 34(4), pp. 342–347
Kassem, M.A.A.,
Elmeshad, A.N., Fares, A.R., 2015. Lyophilized Sustained Release Mucoadhesive
Chitosan Sponges for Buccal Buspirone Hydrochloride Delivery: Formulation and In Vitro Evaluation. AAPS
PharmSciTech, Volume 16(3), pp. 537–547
Knidri, H.E.,
Belaabed, R., Addaou, A., Laajeb, A., Lahsini, A., 2018. Extraction, Chemical
Modification, and Characterization of Chitin and Chitosan. International Journal of Biological
Macromolecules, Volume 120, pp. 1181–1189
Kono, H., Oeda,
I., Nakamura, T., 2013. The Preparation, Swelling Characteristics, and Albumin
Adsorption and Release Behaviors of a Novel Chitosan-Based Polyampholyte
Hydrogel. Reactive &
Functional Polymers, Volume 73,
pp. 97–107
Krisanti, E.A.,
Lazuardi, D., Kiresya, K.K., Mulia, K., 2020. Tablet Formulation Containing
Chitosan-Alginate Microparticles: Characterization and Release Profile of
Xanthones. International Journal
of Technology, Volume 11(5), pp. 900–909
Kumar, S., Koh,
J., 2012. Physiochemical, Optical and Biological Activity of Chitosan-Chromone
Derivative for Biomedical Applications. International Journal of Molecular Sciences, Volume 13, pp. 6102–6116
Kusumastuti, Y., Shibasaki, Y., Hirohara, S.,
Kobayashi, M., Terada, K., Ando, T., Tanihara, M., 2017. Encapsulation of Rat
Bone Marrow Stromal Cells using a Poly-Ion Complex Gel of Chitosan and
Succinylated Poly (Pro-Hyp-Gly). Journal
of Tissue Engineering and Regenerative Medicine, Volume 11(3), pp. 869–876
Kusumastuti,
Y., Timotius, D., Putri, N.R.E., Syabani, M.W., Rochmadi, 2020. Rheological and
Kinetic Studies of Low-Density Polyethylene (LDPE)-Chitosan Biocomposite Film. IOP Conference Series: Materials Science
and Engineering, Volume 722, p. 012054
Lavanya, R.,
Gomathi, T., Vijayalakshmi, K., Saranya, M., Sudha, P.N., 2017. Adsorptive
Removal of Copper (II) and Lead (II) Using Chitosan-g-Maleic
Anhydride-g-Methacrylic Acid Copolymer. International Journal of Biological Macromolecules, Volume 104, pp. 1495–1508
Liu, R., Xu,
X., Zhuang, X., Cheng, B., 2014. Solution Blowing of Chitosan/PVA Hydrogels
Nanofiber Mats. Carbohydrate Polymers, Volume 101, pp. 1116–1121
Liu, Y., Shen,
X., Zhou, H., Wang, Y., Deng, L., 2016. Chemical Modification of Chitosan Film
Via Surface Grafting of Citric Acid Molecular to Promote Biomineralization. Applied Surface Science, Volume 370, pp. 270–278
Nadtoka, O.,
Virych, P., Kutsevol, N., 2020. Hydrogels Loaded with Methylene Blue:
Sorption-Desorption and Antimicrobial Photoactivation Study. International Journal of Polymer Science, Volume 2020, p. 9875290
Nikolova, M.P.,
Chavali, M.S., 2019. Recent Advances in Biomaterials for 3D Scaffolds: A
Review. Bioactive Materials, Volume 4, pp.
271–292
Patel, S.,
Srivastava, S., Singh, M.R., Singh, D., 2018. Preparation and Optimization of
Chitosan-Gelatin Films for Sustained Delivery of Lupeol for Wound Healing. International journal of Biological Macromolecules, Volume 107(Part B), pp. 1888–1897
Peers, S.,
Montembault, A., Ladavière, C., 2020. Chitosan Hydrogels for Sustained Drug
Delivery. Journal of Controlled
Release, Volume 326, pp. 150–163
Pottathara,
Y.B., Vuhere, T., Maver, U., Kokol, V., 2021. Morphological, Mechanical, and In-Vitro Bioactivity of Gelatine/Collagen/Hydroxyapatite Based Scaffolds Prepared by Unidirectional Freeze-Casting. Polymer Testing. Volume 102, p. 107308
Schirmer, R.H., Adler, H., Pickhardt., Mandelkow,
E., 2011. Lest We Forget You – Methylene Blue. Neurobiology of Aging, Volume
32(12), pp. e7–e16
Sinha, V.R.,
Singla, S.K., Wardhawan, S., Kaushik, R., Kumria, R., Bansal, K., Dhawan, S.,
2004. Chitosan Microspheres as a Potential Carrier for Drugs. International Journal of Pharmaceutics, Volume 274, pp. 1–33
Syafiuddin, A.,
Salmiati, S., Jonbi, J., Fulazzaky, M.A., 2018. Application of the Kinetic and
Isotherm Models for Better for Better Understanding of the Behaviors of Silver
Nanoparticles Adsorption onto Different Adsorbents. Journal of Environmental Management, Volume 218, pp. 59–70
Timotius, D.,
Kusumastuti, Y., Rochmadi., 2022. Characterization and Equilibrium Study of
Drug Release of pH-Responsive Chitosan-graft-Maleic Film. International Journal of Technology, Volume 13(2), pp. 398–409
Wirawan, S.K.,
Timotius, D., Nugraha, I.M., Restana, A., Anggara, A.L., Hidayatullah, S.,
2022. Kinetics and Adsorption Equilibrium Study of Free Fatty Acid (FFA) from
Crude Palm Oil (CPO) on Anionic Resin. ASEAN Journal of Chemical Engineering, Volume 22(1), pp. 33–41
Xue, H.,
Thaivalappil, A., Cao, K., 2021. The Potentials of Methylene Blue as an
Anti-Aging Drug. Cells. Volume 10(12), p. 3379
Zhou, Y., Dong,
Q., Yang, H., Liu, X., Yin, X., Tao Y., Bai, Z., Xu, W., 2017. Photocrosslinked
Maleilated Chitosan/Methacrylated Poly (Vinyl Alcohol) Bicomponent Nanofibrous Scaffolds
for Use as Potential Wound Dressings. Carbohydrate Polymers, Volume 168,
pp. 220–226
