Wavelet Decomposition and Feedforward Neural Network for Classification of Acute Ischemic Stroke based on Electroencephalography
Corresponding email: skwijaya@sci.ui.ac.id
Published at : 30 Dec 2022
Volume : IJtech
Vol 13, No 8 (2022)
DOI : https://doi.org/10.14716/ijtech.v13i8.6132
Nurfirdausi, A.F., Apsari, R.A., Wijaya, S.K., Prajitno, P., Ibrahim, N., 2022. Wavelet Decomposition and Feedforward Neural Network for Classification of Acute Ischemic Stroke based on Electroencephalography. International Journal of Technology. Volume 13(8), pp. 1745-1754
| Annisaa Fitri Nurfirdausi | Department of Physics, Faculty of Mathematics and Natural Science, Universitas Indonesia, West Java 16424, Indonesia |
| Ratna Aditya Apsari | Program of Brain and Cognitive Engineering, Korea Advanced Institute of Science & Technology, Daejeon 3414, South Korea |
| Sastra Kusuma Wijaya | Department of Physics, Faculty of Mathematics and Natural Science, Universitas Indonesia, West Java 16424, Indonesia |
| Prawito Prajitno | Department of Physics, Faculty of Mathematics and Natural Science, Universitas Indonesia, West Java 16424, Indonesia |
| Nurhadi Ibrahim | Department of Medical Physiology, Faculty of Medicine, Universitas Indonesia, West Java 16424, Indonesia |
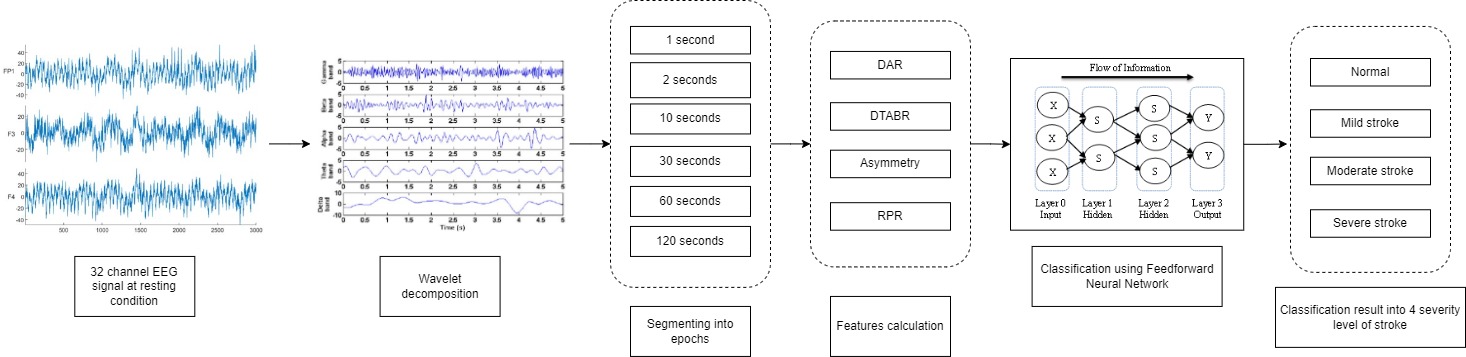
Stroke is one of the leading causes of death in Indonesia. From 2013 to
2018, the prevalence of stroke increased from 7% to 10.9%. There are two types
of strokes, namely Hemorrhagic and Acutte Ischemic Stroke (AIS) with the
majority of it being AIS. Early detection and diagnosis are essential in stroke
as it is a life-threatening disease, and the stroke treatment is based on its
type. Currently, the gold imaging standards in stroke diagnosis are Computed
Tomography (CT) scan and Magnetic Resonance Imaging (MRI). However, the
mentioned services for stroke diagnosis are primarily available in hospitals
classified as “class A” (general hospitals with extensive facilities and
medical services). Compared to CT scans
and MRI, electroencephalography (EEG) is a cost-friendly, non-invasive device
studied for various brain-related diseases. This study aimed to determine the
optimal epoch length to classify four stroke classes (healthy, minor, moderate,
and severe) during resting condition for
a machine learning-based AIS computer-aided diagnostics system. 32-channel EEG,
CT scan, and NIHSS Scores were the obtained data. The features were delta-theta
to alpha-beta ratio (DTABR), delta to alpha ratio (DAR), relative power ratio
(RPR), and asymmetry, which were extracted using wavelet decomposition
technique. The epoch length was varied by 1s, 2s, 10s, 30s, 60s, and 120s. The
severity of stroke were classified using a feedforward neural network. The best
performance was obtained at the 60-second epoch length with 89% accuracy using
15 hidden layers. This EEG-based diagnostic system would be expected to be
implemented in “class C” hospitals, where only essential medical services and
facilities are available, usually in rural areas.
Acute Ischemic Stroke (AIS); Electroencephalography (EEG); Epoch length; Feedforward Neural Network (FNN); Wavelet decomposition
Health Research conducted by the Indonesian Ministry of Health in 2013 and 2018 revealed an increase in stroke prevalence from 7% to 10.9% (Health Research and Development Department, 2018). This disease can be divided into two main types, ischemic and haemorrhagic. Acute Ischemic Stroke (AIS) constitutes the majority of stroke cases (85%), which happens when blood vessels to the brain are blocked or narrowed from fatty deposits (Rudd, 2016). The diagnosis of stroke is commonly facilitated by brain Computed Tomography (CT) scan or Magnetic Resonance Imaging (MRI). However, these neuroimaging devices are only available in “class A” hospitals, with extensive facilities and medical services. On the other hand, the hospitals in rural areas (“class C” hospitals) usually provide only the essential medical services with limited facilities and thus lack access to better and more expensive neuroimaging means. In addition, stroke rehabilitative services are only available in those large city hospitals (Kusuma et al., 2009). Meanwhile, based on a study in 2013, it was revealed that stroke was just as prevalent in rural areas as it was in the city (Health Research and Development Department, 2013). Therefore, an affordable alternative for stroke diagnostics, such as electroencephalography (EEG), is sorely needed in rural areas.
EEG is a non-invasive device that
has been used in several research, such as for depression detection (Apsari
& Wijaya, 2020), seizure detection (Srivastava
et al., 2020), and stroke detection (van
Putten, 2007).
An EEG device is more affordable to procure than a CT scan or an MRI,
and consequently less costly to operate. Ischemic stroke brain may present
abnormalities in the EEG signal (Finnigan
& van Putten, 2013). Typically, AIS EEG signals exhibit
high delta activity (1-4 Hz). The delta power has been consistently identified
as the marker for ischemic stroke. The alpha power, Brain Symmetry Index (BSI),
delta-alpha ratio (DAR) and delta-theta alpha-beta ratio (DTABR) were also
found to be good markers for stroke (Rahma et al., 2017; Finnigan et al., 2007; Müller et al.,
2002). The delta band power and alpha to
delta band ratio can distinguish patients who have sizeable ischemic stroke
from other stroke patients (Shreve
et al., 2019).
EEG comes with complex brain
signals, and thus machine learning (ML) approach has been implemented as means
to make sense of them. Extreme ML has been used to classify stroke features (Chan
et al., 2019; Rahma et al.,
2017), while XGBoost and principal
component analysis were applied to improve classification accuracy in a small
number of selected EEG channels (Fitriah
et al., 2017). Convolutional Neural Networks,
including Multi-Layered Perceptron, as well as Decision Tree and Artificial
Neural Networks, have been used for EEG-based classification (Nurfirdausi
et al., 2022; Dewi et al., 2020; Qureshi
et al., 2018; Omar et al., 2014). One of the issues with
classifying EEG signals using the ML approach is looking for the most optimal
feature settings to obtain the best performance. Thus, this study explored
variations of epoch lengths to investigate which length would yield the best results.
The current study is an extension
of previous studies (Chan
et al., 2019; Fitriah et
al., 2017; Rahma et al., 2017) using the AIS dataset. The EEG
data were obtained for healthy control and stroke patients as a 30-minute
recording. NIHSS scores were referenced to separate patients into classes based
on stroke severity, i.e., healthy, mild, moderate, and severe. EEG data were
read and analyzed in MATLAB R2020A. The 30-minute recording was segmented into
epochs of a certain length (120, 60, 30, 10, 2, and 1 second epochs) and
features—such as relative power ratio (RPR), delta-theta alpha-beta ratio
(DTABR), delta-alpha ratio (DAR), and asymmetry—were computed for each epoch.
In the future, this program is expected to be implemented into the EEG hardware
so that the most accurate prediction of stroke type can be obtained
automatically in the shortest time possible. The features were classified into
four classes based on stroke severity, and classification performance was
obtained from that. Feedforward neural network was chosen as a classification
algorithm because of its simplicity and advantage in handling nonlinear data.
2.1. Data Acquisition
Table 1 Participant’s demographic and characteristics based on stroke severity
|
Patient |
Stroke Severity |
Age |
Gender |
Onset time |
NIHSS |
EEG Device |
Frequency Sampling | |
|
1 |
stroke |
moderate |
43 |
M |
20 |
4/4 |
Xltek |
512 |
|
2 |
stroke |
moderate |
48 |
F |
24 |
10/5 |
Biologic |
256 |
|
3 |
stroke |
moderate |
43 |
M |
48 |
12/7 |
Biologic |
256 |
|
4 |
stroke |
mild |
60 |
F |
6 |
1/1 |
Biologic |
256 |
|
5 |
stroke |
mild |
56 |
F |
6 |
2/1 |
Biologic |
256 |
|
… |
… |
… |
… |
… |
… |
… |
… |
… |
|
66 |
stroke |
mild |
F |
Xltek |
512 |
The EEG devices used in this study were the Biologic Netlink System and the Xltek EEG 32U Natus, both had 32 channels and 512 Hz sampling frequency. The electrode placement was based on the international 10–20 system and was saved in European Data Format (.edf). Each AIS patient’s EEG recording was accompanied by their NIHSS score, CT scan, and EEG interpretation from the supervising physician. NIHSS score defines the level of stroke severity: 0 indicates no stroke, 1–4 indicates minor stroke, 5–15 indicates moderate stroke and above 15 indicates severe stroke (Rahma et al., 2017).
2.2. Signal
Processing and Features
After
data collection, pre-processing using Independent Component Analysis (ICA) was
done automatically in the device. The signals were then decomposed into their
respective frequency bands using wavelet transformation, and their features
were calculated before inputting into the ML algorithm. The features calculated
in this study were relative power ratio (RPR), delta-alpha ratio (DAR),
delta-theta alpha-beta ratio (DTABR), and asymmetry.
2.2.1. Segmenting into Multiple Epochs
The standard procedures for EEG recording were based on the conditions shown in Table 2. The total recording time was 30 minutes, which were divided as follows:
Table 2 EEG data timepoints and their recording conditions
|
Minute |
Description |
|
0-3 |
eyes closed |
|
3-6 |
eyes open |
|
6-9 |
photic stimulation |
|
9-12 |
hyperventilation
(rapid inhale and exhale) |
|
12-30 |
eyes closed; patients were asked to sleep if they could |
In this study, only eyes closed conditions were used. The reason for this
was to investigate AIS markers during resting condition. The total eyes closed
recording was 18 minutes. This recording was then divided into multiple epochs
of various lengths, which are 120, 60, 30, 10, 2, and 1 second epochs. Each
epoch was transformed to a frequency domain using wavelet transformation.
2.2.2. Wavelet Transformation
Wavelet transformation is a time-to-frequency domain transformation that utilizes wavelets called the “mother” and “daughter” wavelets. Wavelet is concentrated in both time and frequency, whereas standard Fourier transforms is localized only in frequency (Agarwal et al., 2017). It is known to localize signal components better than other methods (Sanei & Chambers, 2007). The wavelet is represented as:
where ??
is the angular frequency, ? is the wavenumber, and ???, ?(?)
is the wavelet function.
Wavelet transformation can be
classified into two types: Continuous Wavelet Transformation (CWT) and Discrete
Wavelet Transformation (DWT). For this study, DWT was used to decompose the EEG
signals into their respective frequency bands: delta, theta, beta, alpha, and
gamma. DWT discreetly samples the signal and acts like a filter bank that takes
the input signal and outputs the coefficients of the signal. Decomposing EEG
signals into their respective frequency components can be done using a
multi-level DWT decomposition (Tumari
et al., 2013).
To decompose a signal of EEG, the
window function and the level of decomposition must be appropriate for the
signal. The frequency sampling of the acquisition was 512 Hz, which means that
the recorded signal is 0-512 Hz. A 7-level decomposition was required to
decompose these signals. The window function used in this study was Daubechies
4 (db4) due to its small mean square error (MSE) when used for EEG signals (Tumari
et al., 2013).
The decomposed signals consisted
of approximation (A) and detail (D) signals. The first three decompositions
(D1, D2, and D3) were not used because they were considered as noise (Tumari
et al., 2013). EEG frequency bands were
obtained from the decomposition of AD3, which were D4 (gamma band, 32 – 64 Hz),
D5 (beta band, 16 – 32 Hz), D6 (alpha band, 8 – 1 6 Hz), D7 (theta band, 4 – 8
Hz), and AD7 (delta band, 0 – 4 Hz).
2.2.3. Relative Power Ratio
Relative power ratio is the ratio
between a certain frequency band's power and all bands' total power. The RPR of
a certain frequency band is computed using the equation:
where f1
and f2 are the low and high boundaries of the frequency
band, fL and fH are the low and high
boundaries of all bands. P(f1, f2) refers to the
band power of a particular frequency band, while P(fL, fH)
refers to the total power of all bands.
2.2.4. Delta-Alpha Ratio (DAR)
Delta-alpha ratio (DAR) is the ratio between the delta band's power and the alpha band's power. DAR is calculated using the equation:
DAR in AIS
patients’ EEG signals was found to be higher than healthy controls, with higher
variability as well (Finnigan
et al., 2016).
2.2.5. Delta-Theta Alpha-Beta Ratio (DTABR)
2.2.6. Asymmetry
Asymmetry is the measure of activity between the left and right brain, calculated using the band power of each EEG frequency band. A high asymmetry indicates that the brain in that frequency band is more dominant in the left or right hemispheres. When asymmetry is positive, it indicates that the right hemisphere is more dominant. When it is negative, then the left hemisphere is more dominant (Allen et al., 2004). The calculation of asymmetry is as follows:
where N is the number of electrodes on each hemisphere and Px
is the band power of frequency band x for each electrode.
This study calculated nine asymmetry
features from eight left-right electrode pairs and the total left-right
hemispheric asymmetry. These features can be further explained as follows:
prefrontal (FP2 – FP1); frontal 1 (F4 – F3); frontal 2 (F8 – F7); temporal 1
(T2 – T1); temporal 2 (T4 – T3); central (C4 – C3); parietal (P4 – P3);
occipital (O2 – O1).
2.3. Machine
Learning Classification
2.3.1. Feedforward Neural Network
Feedforward neural network or multilayer
perceptron (MLPs) is the first artificial neural network that does not have a
loop for their connections. In this network, information only flows forward from the input nodes to the hidden
layers (if any) and the output node, hence the name “feedforward”. It is also
divided into two groups depending on the number of the layers, which are
single-layer and multi-layer (SAZLI,
2006). Feedforward neural network is considered simple
compared to a recurrent neural network (RNN), in which is constructed as a
loop. This chain's length is called the network's depth.
2.3.2. K-Fold
Cross Validation
Cross-validation is a sampling method to estimate the performance of a predictive model in testing with the advantage of giving insight into performance from an independent dataset. It separates the dataset into portions and utilizes different parts of that data as either testing or training data in each of its iterations. The estimation of cross-validation accuracy is the number of correct classifications divided by the total data in the dataset. In k-fold cross-validation, the dataset will be randomly divided into equal sizes of subsets or folds.
The results of k-fold cross-validation are
averaged from the results of k number of subsets. In k-fold
cross-validation, all subsets will take turns as training and validation data,
but each subset will only be used once for validation. The standard parameter -k
on k-fold cross-validation is 10, however, it remains an
undetermined parameter (Seni
& Elder, 2010).
3.1. Features Analysis
Figure 1 Comparison
of DAR and DTABR among stroke severity level: normal, mild, moderate, and
severe
In addition to DAR and DTABR, RPR was calculated using Decomposition Wavelet Transform (DWT). Figure 2 shows RPR in different frequency bands of EEG: alpha RPR, beta RPR, delta RPR, and theta RPR based on the level of stroke severity. The slight dominance of delta RPR is shown in mild stroke patients compared to normal subjects. In moderate and severe stroke patients, delta dominated the brain signals. Severe stroke patients had an abnormally dominant delta band, especially in the prefrontal areas (FP1, FP2, and FPZ), which proves that stroke patients have unusually high power of slow waves in their brains. Figure 2 shows that the alpha RPR of moderate stroke is slightly lower compared to the alpha RPR of mild stroke.
Figure 2 Relative
Power Ratio (RPR) comparison among all stroke severity level
Figure 3 Asymmetry
scores between healthy and stroke patients
3.2. Training and
Classification Result
A feedforward neural network is
implemented to classify AIS severity levels into four categories: normal or
healthy controls, mild, moderate, and severe. The ML training was conducted
using MATLAB R2020A with Intel® Core™ i7-8809G CPU@ 3.10GHz and 32 GB RAM. Each
epoch length variation was trained and tested using the chosen ML algorithm to
determine which would yield the best performance based on accuracy,
specificity, and sensitivity. Besides epoch length, hidden layers were also
varied to find the number of hidden layers required to achieve the best
performance. RPR, DAR, DTABR, and asymmetry features were included as input in
the ML classification without any exclusions.
Table 3. shows the training
result of the dataset. In general, ML performance increased with the number of
hidden layers used—peaking at 15 hidden layers—but performance decreased at 20
hidden layers. The same was observed for epoch length, which achieved the best
performance at a 60-second length and decreased at a 120-second length.
Table 3 Performance results
|
Parameter |
120 seconds |
60 seconds |
30 seconds | |||||||
|
Acc |
Spec |
Sen |
Acc |
Spec |
Sen |
Acc |
Spec |
Sen | ||
|
Hidden layer |
5 |
75% |
75% |
90% |
63% |
51% |
96% |
65% |
48% |
97% |
|
10 |
72% |
73% |
87% |
80% |
86% |
100% |
67% |
68% |
100% | |
|
15 |
66% |
66% |
93% |
89% |
100% |
88% |
83% |
100% |
100% | |
|
20 |
65% |
65% |
86% |
71% |
58% |
97% |
76% |
54% |
99% | |
|
Parameter |
10 seconds |
2 seconds |
1 second | |||||||
|
Acc |
Spec |
Sen |
Acc |
Spec |
Sen |
Acc |
Spec |
Sen | ||
|
Hidden layer |
5 |
57% |
45% |
100% |
68% |
68% |
96% |
62% |
61% |
97% |
|
10 |
70% |
61% |
100% |
60% |
70% |
100% |
62% |
62% |
100% | |
|
15 |
72% |
69% |
100% |
80% |
80% |
88% |
63% |
64% |
100% | |
|
20 |
74% |
70% |
100% |
80% |
80% |
97% |
68% |
68% |
99% | |
The best configuration was acquired at a 60-second epoch length
with 15 hidden layers, which performed 89% accuracy, 88% sensitivity, and 100%
specificity. The 60-second epoch gave the most optimum result among others. The
shortest epoch length, a 1-second epoch, produced the worst results overall.
The lowest accuracy was obtained
using 10 hidden layers, 60% for the 2-second epoch, compared to the accuracy
obtained by 60 seconds, 80%. This occurred because longer segments contained
more signal information than shorter segments, which resulted in better feature
calculations.
This study calculated features that could identify stroke from healthy controls from resting EEG signals, which were delta theta to alpha beta ratio (DTABR), delta to alpha ratio (DAR), relative power ratio (RPR), and asymmetry. Stroke patients had higher delta RPR value as compared to healthy controls. Differences between control and stroke patients were identified. Stroke patients had higher delta RPR value as compared to healthy controls. In general, as stroke severity increases, so does the dominance of the delta band. Severe stroke showed a very dominant delta compared to the other bands, especially in the prefrontal region. Feedforward neural network and AIS EEG features were utilized to identify stroke and predict its severity from resting EEG data. The EEG signals were segmented into different epoch lengths, and the neural network's hidden layers varied. For this study, the optimum network configuration was 60-second epochs with 15 hidden layers. This simple configuration could classify stroke into four different classes with the best accuracy of 89%, specificity of 100%, and sensitivity of 88%. Further studies could implement feature-selection methods such as Principal Component Analysis (PCA) to reduce dimensionality and improve classifier performance. The result of this study shows a promising future for a more robust AIS computer-aided diagnostic system that uses EEG as an alternative neuroimaging device for stroke diagnosis.
This study was supported by the Department of Education and
Culture of the Republic of Indonesia through DRPM Universitas Indonesia by
PDUPT 2020 with contract number NKB2822/UN2.RST/HKP.05.00/2020.
Agarwal,
S., Singh, O. P., Nagaria, D., 2017. Analysis and comparison of wavelet
transforms for denoising MRI image. Biomedical and Pharmacology Journal,
Volume 10(2), pp. 831–836
Allen, J.J.B., Coan, J.A., Nazarian, M., 2004. Issues and Assumptions on
the Road From Raw Signals to Metrics of Frontal
EEG Asymmetry in
Emotion. Biological Psychology, Volume
67(1–2), pp. 183–218
Apsari, R.A., Wijaya, S.K., 2020. Electroencephalographic Spectral Analysis
to Help
Detect Depressive Disorder. In: Proceedings of the 37th International Conference on Biomedical
Engineering
Chan, J.Y., Wijaya, S.K., Rahma, O.N., 2019. ELM (Extreme Learning
Machine) Method for
Detecting Acute Ischemic Stroke Using Conventional and Specific Asymmetry BSI (Brain Symmetry
Index) Features based on EEG Signals ELM ( Extreme Learning Machine ) Method for Detecting Acute Ischemic
Stroke Us. AIP Conference Proceedings, Volume 2092(1), p. 020023
Dewi, F.Y., Faza, A., Prajitno, P., Kusuma Wijaya, S., 2020. Stroke severity
classification based on EEG signals using 1D convolutional neural network. Journal
of Physics: Conference Series, Volume
1528, p. 012006
Finnigan, S.P., Walsh, M., Rose, S.E., Chalk,
J.B., 2007.
Quantitative EEG Indices of Sub-Acute Ischaemic Stroke Correlate with Clinical Outcomes. Clinical
Neurophysiology, Volume 118(11), pp. 2525–2532
Finnigan, S., van Putten, M.J.A.M., 2013. EEG in Ischaemic Stroke:
Quantitative EEG Can Uniquely Inform (Sub-)Acute Prognoses and Clinical Management. Clinical
Neurophysiology, Volume 124(1), pp. 10–19
Finnigan, S., Wong, A., Read, S., 2016. Defining Abnormal Slow EEG
Activity in Acute
Ischaemic Stroke: Delta/Alpha Ratio as an Optimal QEEG Index. Clinical
Neurophysiology, Volume 127(2), pp.
1452–1459
Fitriah, N., Wijaya, S.K., Fanany, M.I.,
Badri, C.,Rezal, M., 2017. EEG Channels Reduction Using PCA to Increase Xgboost’s Accuracy for Stroke Detection. AIP
Conference Proceedings, Volume
1862(1), p. 030128
Health Research and Development Department, 2013. Basic Research of
Health 2013. Ministry of Health of Indonesia.
Health Research and Development Department, 2018. Basic Research of
Health 2018. In Ministry of Health of Indonesia. Available online at https://doi.org/http://repository.bkpk.kemkes.go.id/3514/1/Laporan%20Riskesdas%202018%20Nasional.pdf,
Accessed on February 19, 2022
Kusuma, Y., Venketasubramanian, N., Kiemas,
L.S., Misbach, J., 2009.
Burden of Stroke in
Indonesia. International Journal of Stroke, Volume 4(5), pp. 379–380
Müller, C., Achermann, P., Bischof, M.,
Nirkko, A.C., Roth, C., Bassetti, C.L., 2002. Visual and Spectral Analysis of Sleep EEG in Acute Hemispheric Stroke. European
Neurology, Volume 48(3), pp. 164–171
Nurfirdausi, AF., Wijaya, S.K., Prajitno, P., Ibrahim, N., 2022. Classification of Acute
Ischemic Stroke EEG Signal Using Entropy-Based Features, Wavelet Decomposition,
and
Machine Learning Algorithms. In: The 6th Biomedical Engineering’s Recent Progress in Biomaterials, Drugs
Development, and
Medical Devices
Omar, W.R.W., Mohamad, Z., Taib, M.N.,
Jailani, R., 2014. ANN
Classification
of Ischemic Stroke Severity using EEG Sub Band Relative Power Ration. In: 2014 IEEE Conference on System,
Process and Control, ICSPC 2014, December
Qureshi, A.A., Zhang, C., Zheng, R.,
Elmeligi, A., 2018.
Ischemic Stroke Detection Using EEG Signals. In: Proceedings of the 28th
Annual International Conference on Computer Science and Software Engineering
Rahma, ON., Wijaya, S.K., Prawito, Badri, C., 2017. Electroencephalogram Analysis with Extreme Learning
Machine as a Supporting Tool for Classifying Acute Ischemic Stroke Severity. In: International Seminar on Sensor,
Instrumentation, Measurement and Metrology: Innovation for the Advancement and
Competitiveness of the Nation
Rudd, A., 2016. National Clinical Guideline for Stroke. Royal College of
Physicians, UK. Available online at: https://www.hse.ie/eng/about/who/cspd/ncps/stroke/ resources/2016-national-clinical-guideline-for-stroke-5th-edition.pdf
Sanei, S., Chambers, J., 2007. EEG Signal Processing. John Wiley & Sons
SAZLI, M. H., 2006. A brief review of
feed-forward neural networks. Communications, Faculty Of Science, University
of Ankara, January 2006, pp. 11–17. https://doi.org/10.1501/0003168
Seni, G. Elder, J.F., 2010. Ensemble Methods in Data Mining:
Improving Accuracy Through Combining Predictions. In Synthesis Lectures on
Data Mining and Knowledge Discovery. Springer
Cham: Switzerland
Shreve, L., Kaur, A., Vo, C., Wu, J.,
Cassidy, J.M., Nguyen, A., Zhou, R.J., Thuong, B., Yang, D.Z., Medizade, A.I., Chakravarthy, B.,
Hoonpongsimanont, W., Barton, E., Yu, W., Cramer, S. C., 2019. HHS Public Access, Volume 28(8), pp. 2280–2286
Srivastava, G., Tripathi, A., Maurya, P.K., 2020. Fuzzy Entropy Based Seizure
Detection Algorithms for EEG Data Analysis. In Smart Healthcare for Disease Diagnosis and
Prevention. Academic Press
Tumari, S.Z.M., Sudirman, R.,Ahmad, A.H., 2013. Selection of a Suitable
Wavelet for Cognitive Memory Using Electroencephalograph Signal. Engineering,
Volume 05(05), pp. 15–19
van Putten, M.J.A.M., 2007. The Revised Brain Symmetry Index. Clinical
Neurophysiology, Volume 118(11), pp. 2362–2367
