Comparison of Phase Change Materials of Modified Soy Wax using Graphene and MAXene for Thermal Energy Storage Materials in Buildings
Corresponding email: nandyputra@eng.ui.ac.id
Published at : 09 May 2023
Volume : IJtech
Vol 14, No 3 (2023)
DOI : https://doi.org/10.14716/ijtech.v14i3.6092
Trisnadewi, T., Kusrini, E., Nurjaya, D.M., Paul, B., Thierry, M., Putra, N., 2023. Comparison of Phase Change Materials of Modified Soy Wax using Graphene and MAXene for Thermal Energy Storage Materials in Buildings. International Journal of Technology. Volume 14(3), pp. 596-605
| Titin Trisnadewi | Applied Heat Transfer Research Group, Department of Mechanical Engineering, Universitas Indonesia, Depok, West Java 16424, Indonesia |
| Eny Kusrini | Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Depok, West Java 16424, Indonesia |
| Dwi Marta Nurjaya | Department of Metallurgical and Material, Faculty of Engineering, Universitas Indonesia, 16424 Depok, West Java, Indonesia |
| Byrne Paul | Civil and Mechanical Engineering Laboratory LGCGM, University of Rennes, Rennes, France, 35704 |
| Maré Thierry | Civil and Mechanical Engineering Laboratory LGCGM, University of Rennes, Rennes, France, 35704 |
| Nandy Putra | Applied Heat Transfer Research Group, Department of Mechanical Engineering, Universitas Indonesia, Depok, West Java 16424, Indonesia |
This
study aimed to characterize Phase Change Materials (PCM) by improving their
properties using shape stabilization; this was achieved by adding nanoparticles
as a support material. PCM soy wax was modified using two nanoparticles,
graphene, and MAXene Ti3AlC2. The synthesis process
comprised stirring using a magnetic stirrer and ultrasonication using an
ultrasonic processor with various percentages of 0.1, 0.5, and 1 wt.% of soy
wax with nanoparticles. Based on the results, the morphologies of graphene and
MAXene Ti3AlC2 were found to be in the form of sheets.
These sheets had a large surface area, so soy wax could adsorb more
nanoparticles to increase the stability of the material. The thermal
conductivity increased with increasing percentage addition of nanoparticles. The
highest values from the synthesis with graphene and MAXene Ti3AlC2 were 0.89 W/mK and 0.85 W/mK, respectively. The thermal conductivity of soy wax
increased with the ratio of pure soy wax and nano-soy wax; the thermal
conductivity was 6.01 for soy wax+graphene and 5.71 for soy wax+Ti3AlC2.
Differential scanning calorimetry (DSC) results showed an increase in the
melting and solidifying points of pure soy wax. The modified soy wax with 0.1
wt.% graphenes experienced a reduction in the melting and solidification points
up to 15% and 14%, respectively. Similar results were obtained for 0.1 wt.%
MAXene Ti3AlC2. In this case, there was a reduction in
the melting and solidifying points by 16% and 13%, respectively. Finally, the
addition of MAXene improved the material stability and thermal conductivity of
soy wax and has the potential to be used as a thermal energy storage material
for building applications.
Graphene; MAXene; Phase change material; Soy wax; Thermal energy storage
The ever-increasing world population, combined with the considerable increase in energy demand, has resulted in an environmental crisis (Vennapusa et al., 2020). One sector that consumes a significant amount of energy is buildings, where the maximum energy is utilized for the heating and cooling systems (Imessad et al., 2014). The demand for the installation of cooling systems in buildings is growing rapidly in the tropics. This is because the climate zones that receive large amounts of solar radiation have longer sunny days, high humidity, and high temperatures (Al-Obaidi et al., 2014). According to the Regulation of the Ministry of Health of the Republic of Indonesia 2011, it states that the standard temperature range for comfort buildings in Indonesia is 18-30oC. Heat absorption in buildings that are quite high in the tropics causes uncomfortable conditions for humans who are indoors. The uncomfortable thermal conditions can also be caused by the building itself due to the materials used for construction. Currently, many studies have been reported on building materials that can be used in passive methods for achieving energy efficiency and thermal comfort (Latha et al., 2015).
Thermal Energy Storage
(TES) has been widely used to address fluctuations in energy demand and supply
gaps. There are three forms of TES: Latent Heat Thermal Energy Storage (LHTES),
Sensible Heat Thermal Energy Storage (SHTES), and thermochemical storage
systems (Nomura et al., 2015). Phase Change
Materials (PCM) are TES materials currently in great demand by researchers
owing to their large thermal energy storage capacity during the charging and
discharging processes (Jamekhorshid et al., 2014). The correct use of
PCM in the building can minimize peak cooling loads, allow the use of smaller
HVAC technical equipment for cooling, and maintain the indoor temperature
within a comfortable range owing to smaller indoor temperature fluctuations (Souayfane et al., 2016). Many studies have
been related to the application of PCM, especially in buildings. (Zhang et
al., 2017) used PCM
composites as a substitute for sand, (Laaouatni
et al., 2016) used PCM
to build optimal walls made of concrete blocks filled with PCM and ventilated
tubes, and (Saikia et al., 2018) incorporated PCM
into concrete walls to reduce the increase in heat and temperature fluctuations
in the buildings.
PCM are grouped into three
types: organic, inorganic, and eutectic (Kant et al., 2016). Fatty acids as
organic PCM have several advantages. It has a large storage capacity, abundant
in nature, non-toxic, not harmful to health, non-corrosive, and exhibits low
supercooling (Rasta and Suamir, 2018). However, organic
PCM has low conductivity and the possibility of leakage during the phase-change
process (Huang et al., 2017). The stabilized form
of PCM is used to eliminate losses and increase the PCM efficiency in terms of
thermal and physical properties. The
incorporation of nanomaterials into pure PCM can significantly increase their
thermal conductivity and stability (Kalaiselvam
and Parameshwaran, 2014). Many
researchers have carried out mixing PCM with nanomaterials. In a study by (Amin et al., 2017), beeswax PCM was mixed with graphene
nanoparticles; (Meng et
al., 2013)
synthesized a fatty acid/CNT composite, (Wi et al., 2015) studied
shape-stabilized phase change materials using fatty acid esters and exfoliated
graphite nanoplatelets, and (Kim et al., 2016) synthesized
octadecane/expanded graphite composites. Thus, several studies have been
conducted to enhance nano-PCM's material stability and thermal conductivity.
Graphene is the world’s
thinnest material—a single layer of carbon atoms that has excellent electrical
properties graphene can make it play a large role in energy storage, material
composites, sensors, and other fields. The structure of graphene, consisting of
layers, makes graphene highly conductive with carrying mobility of up to
200,000 cm2V-1 s -1 and thermal conductivity
of up to 5,300 Wm-1K -1
(Kusrini et al., 2019). The new
two-dimensional material, MAXene, has hydrophilic properties, excellent
oxidation resistance, high thermal and electrical conductivity, high thermal
stability, and high surface area (Naguib
et al., 2012).
Appropriate shape stabilization methods are required to achieve the desired
modifications in the thermal stability of materials using nanoparticles. The
shape-stabilized PCM (SSPCM) method is divided into three: mixing,
ultra-sonification, and impregnation (Lee et
al., 2018). Mixing is one of the simplest, but there is
no stable connection or bond between the PCM and the supporting material.
Ultra-sonification is a method performed by injecting a PCM into the pores of
the supporting material (Kusrini et al, 2019). Impregnation is a method that removes gas and moisture from pores and
then injects them with the PCM (Putra et al., 2019). The discovery and identification of PCM properties are very important
because PCM has several benefits and applications that can solve energy
problems. In this study, the organic properties of a PCM were modified to
improve its thermal properties. The organic PCM selected in this study was
derived from a group of waxes, namely soy wax, because it has a temperature
range close to room temperature, so it has the potential to be applied on a
wide scale. Based on its application as a heat energy storage material in
buildings subjected to repeated heating and cooling cycles, it is very
important to know the durability of a PCM. In a study conducted by (Trisnadewi et al., 2021), pure soy wax
thermal cycle test results were obtained. Thermal cycling
tests were performed with heating and cooling cycles of 0, 500, 1000, 3000, and
5000. Soy wax experienced an increase in melting and solidification
temperatures with a percentage increase, Tm_soywax 9%, and Ts_soywax
13%, respectively, after testing up to 5000 cycles, which represented the
application of the PCM for approximately 13 years. Soy wax was synthesized with
nanoparticles, such as graphene and MAXene, to increase the effectiveness of
soy wax as a TES material by increasing thermal conductivity.
2.1. Sample Preparation
Soy wax has a melting point of 43.92°C
with a latent heat of 117.59 J/g and a solidification point of 38.49 °C with a
latent heat of 122.18 J/g (Trisnadewi et al., 2021). This
PCM material was synthesized with graphene nanoparticles in the form of a black
powder purchased from XFNANO-China type XFQ021. It had an electrical
conductivity of 800–1100 S/cm, an apparent density of 0.09–0.13 g/cm3,
and a tap density of 0.13–0.16 g/cm3. Another nanoparticle sample,
MAXene Ti3AlC2, was purchased from 2D Semiconductors
(USA). In general, a MAX phase is initially formed by the formula Mn+1AXn,
where n = 1, 2, or 3. M is an early transition metal, A is a group of 13 or 14
elements, and X is either carbon or nitrogen (Barsoum, 2000).
The mixing and
ultra-sonification were used to synthesize soy wax with nanoparticles. The
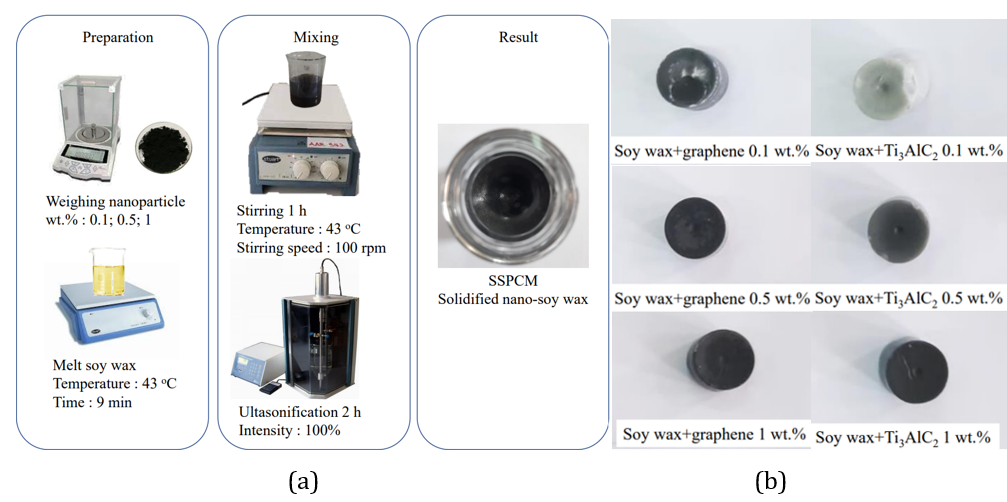
synthesis processes of soy wax+graphene and soy wax+MAXene are shown in Figure
1 (a), and the synthesis result is shown in Figure 1 (b). The first step
involved weighing the mass of nanoparticles using Fujitsu FSR-A320 and soy wax with
three percentage variations, 0.1, 0.5, and 1 wt.%, referring to Equation (1).
Then, soy wax was melted until it was in liquid form using Stuart CB162. In the
third step, the weighed nanoparticles were placed in a beaker, and the soy wax
liquid was poured. The fourth step involved mixing and the synthesis was
carried out using a magnetic stirrer for 1 h with stable heating during a
stirring speed of 100 rpm, followed by ultra-sonification for 2 h using a 20
kHz 950 W ultrasonic processor.
Figure 1 Nano-PCM
synthesis process (a), Synthesis of soy wax+graphene & soy wax+Ti3AlC2 (b)
2.2.
Characterization and property testing method
The characterization of composite
nano-PCMs is important for understanding the impact of nanoparticles. The
nature of the functional groups, their interactions with the molecular
architecture of the support matrix in the composite, and other chemical
properties of the nano-PCM were confirmed using an FT-IR Nicolet™ iS50. The
material's physical properties were characterized using SEM-EDS to determine
the mixed specimens' topography, morphology, composition, crystallography, and
elemental composition. In this test,
SEM-EDS was used with a voltage of 20 kV and a resolution of 512 × 384 pixels. The
instrument used to measure the thermal conductivity of the nano-PCM was a
C-Therm TCI thermal conductivity analyzer with a testing range of 0–100 W/mK.
The modified transient plane source (MTPS)-ASTM D7984 measurement method was
used with a test temperature range of -50–200oC, time testing range
0.8–3 s, precision better than 1%, and accuracy better than 5%. The DSC method
was used to measure the thermal energy storage behavior of the PCM and
composites, including the melting temperature (Tm), solidification
temperature (Tf), latent heat of melting (Hm), and
latent heat of solidification (
Hf). The DSC
(ASTM F 2625-10) measurements were conducted at 5 °C/min heating and
cooling rates and in the temperature ranges of 10–120°C and 120–10°C and were
held for 5 min at 120°C in air.
The chemical structure of PCM composites was analyzed by FTIR spectroscopy. Figure 2 shows the peaks for pure soy wax (a), soy wax adding graphene (b), and Ti3AlC2 (c) with various mass percentages. Pure soy wax has an absorption peak of 2848-2955 cm-1 which indicates the presence of strain vibrations (C-H) of alkanes (Trisnadewi et al., 2021). The absorption peak of soy wax is at 2916 cm-1, which indicates the strong strain frequency of the CH3, CH2, and C-H functional groups (Pethurajan et al., 2018). Soy wax has an absorption peak in 1702–1738 cm-1, which indicates the absorption of the strain vibration of the carboxylate group (C=O). After synthesizing the spectra of the PCM samples, soy wax+graphene (Figure 2(b)) and soy wax+ Ti3AlC2 (Figure 2(c)) show similar peak spectra to those of pure soy wax. The transmittance value decreases with an increase in the weight of the nanoparticles. This decrease indicates a decrease in the percentage of functional groups in soy wax owing to the addition of nanoparticles. This also shows an increase in the presence of nanoparticles in the mixture as the resulting transmittance value decreases. Based on the FTIR results, the addition of graphene and MAXene does not produce any new peaks. These results indicate no chemical interaction between soy wax and graphene or MAXene after the synthesis process.
Figure 2 FTIR spectra of pure
soy wax (a), soy wax + graphene (b), soy wax + Ti3AlC2 (c)
The
morphology of the nano-PCM mixture was analyzed using SEM-EDS. Figure 3 (a)
shows the SEM images of the mixture of soy wax and graphene nanoparticles at a
magnification of 5 µm. The morphology is multilayer or stack-like. There are
lumps, the texture is not very rough, and the corners of the graphene sheet are
not very sharp. Figure 4 (a) shows the results of the SEM test of the mixture
of soy wax and Ti3AlC2 at a magnification of 5 µm. When
compared with graphene, Ti3AlC2 has a wider and more
structured sheet. The texture shown in the Ti3AlC2 SEM
results is almost the same as that of graphene, i.e. sheets with angles that
are not too sharp. The difference is that the particle size of graphene is
smaller than that of Ti3AlC2 . Thus, when viewed from the
perspective of particle size, it can be observed that the thermal conductivity
of the mixture of soy wax and graphene is greater. The multi-layered morphology
of graphene and MAXene allows more nanoparticles to be adsorbed onto the
surface of soy wax. When more nanoparticles are adsorbed, the stability of the
soy-wax material is greater.
Figures 3 (b) and 4 (b) show the results of the EDS mapping test, which aimed to determine the elements that constituted the mixture. Both EDS mapping results show that these two mixtures have very high C (carbon) content. In addition to similarities in element C, these two materials contain O. The EDS results of these two samples follow the results of the FTIR test, which shows the presence of hydrocarbon bonds. The difference between these two samples is the Al content of Ti3AlC2 . Figure 4 (b) shows aluminum scattered along the carbon sheet. The presence of carbon and aluminum in PCM soy wax can increase the thermal conductivity of the material, increasing the ability of PCM soy wax to conduct heat. Based on the EDS test, the carbon value of MAXene can approach the carbon content in graphene, namely 82.2 wt.% in Ti3AlC2 and 84.49 wt.% in graphene. These results indicate that MAXene Ti3AlC2 is feasible and has excellent potential for use as a supporting material with characteristics like those of graphene.
Figure 3 (a). Morphology of a mixture of soy wax and graphene nanoparticles; (b) mapping distribution elements of graphene
Figure 4 (a). Morphology of a mixture of soy wax and Ti3AlC2 nanoparticles; (b) mapping distribution elements of Ti3AlC2
Thermal
conductivity testing was carried out at a temperature of approximately 20oC,
and data collection was repeated 10 times to obtain accurate results. The
average thermal conductivity test results are shown in Table 1. Figure 5 (a)
shows the ratio of the increase in the average thermal conductivity of soy wax
after synthesis with graphene and Ti3AlC2. Soy
wax+graphene results in an increase of 5% at a mass percentage of 0.5 wt.% with
a ratio of 6.01 with pure soy wax. Soy wax+ Ti3AlC2 results in an increase of 4.7% at a mass percentage of 1 wt.% with a ratio of
5.71 with pure soy wax. The results show an increase in thermal conductivity
with an increase in the weight of the nanoparticles (Putra et al., 2016).
However, a mixture must have an optimal point such that in the event of
exceeding the optimum limit, there is no change or deterioration in properties.
This condition occurs in the soy wax and graphene mixture, with a decrease of
0.01 W/mK at 1 wt.%.
The addition of a particular mass
fraction of nanoparticles to the PCM results in a decrease in the melting
point, solidification point, and latent heat of the PCM (Huang et al., 2017). The graph for the
soy wax and graphene mixture shows the same trend for the three mass
variations, namely, heat flow degradation and changes in melting point and
solidification point, and the same trends are observed for soy wax + Ti3AlC2.
Figure 5(b) shows the effect of the nanoparticles on the melting and
solidification rates. This demonstrates that the melting temperature of the nano-PCM
slowly decreases, but the solidification rate does not change significantly
with the addition of nanoparticles. The mixture of soy wax + graphene
experiences an increase in melting point concerning that of pure soy wax by 6.4oC
and by 5.5oC for the solidification point, with a constant value for
each increase in the percentage of graphene. For soy wax + Ti3AlC2,
the melting point is increased by 7.4oC, but the solidifying point
reduces by 5.3oC from that of pure soy wax. Based on the results of
these thermal properties, it is evident that the addition of nanoparticles
enhances thermal conductivity but reduces its melting point. The thermal
properties and enthalpy of the PCM were investigated using a DSC test. The test
results are shown in Figure 6, in which the graphs are generated using dynamic
mode (Barreneche et al., 2013). The enthalpy value
based on the DSC result is representative of the peak area, but this result
shows the data with
In
contrast to the solidification peak area, the soy wax + graphene mixture
decreased after the wt% of 0.5 wt% by 560.1 µVs/mg but increased after 1 wt% to
585.9, but in the soy wax + Ti3AlC2 mixture, there was a
linear decrease. From the values of thermal conductivity, melting, and
solidification temperatures, the melting and solidification peak areas show the
same pattern where at 0.5 wt% there is a decrease and a non-linear increase
with an increase in the proportion of the amount. Thus, it can be solved that
the concentration of 0.5 wt% graphene+soy wax mixture is the maximum/best
mixture to increase the thermal properties of soy wax.
Figure 5 (a) The enhancement percentage of thermal conductivity of soy wax + graphene and soy wax + Ti3AlC2, (b) Effect of nanoparticles on melting and solidification temperatures of nano-PCMs
Figure 6 DSC curves of (a)
pure soy wax, (b) soy wax + graphene, (c) soy wax +
Table 1 Thermal
properties of soy wax + graphene and soy wax +
Synthesis of soy wax with nanoparticles
using the stirring and ultra-sonification methods does not change the chemical
structure of soy wax, which is composed of fatty acids. The morphology of
graphene and MAXene Ti3AlC2 is a layered sheet, which
allows the soy wax to bind and trap more nanoparticles. The highest thermal
conductivity value of 0.89 W/mK was obtained for soy wax-graphene of 0.5 wt.%
and 0.85 W/mK for soy wax-Ti3AlC2 of 1 wt.%. The soy wax
and graphene mixture showed the same tendency as the soy wax + Ti3AlC2 synthesis in heat flow degradation and changes in melting and freezing points.
The soy wax + graphene mixture increases the melting point of pure soy wax by
6.4oC and the solidification point increases by 5.5oC for
each increase in the percentage of graphene. The melting point for soy wax + Ti3AlC2 increases by 7.4oC, but the solidification point decreases by 5.3oC
from pure soy wax. This study concludes that soy wax modified with graphene and
MAXene can improve the properties and thermal conductivity of the material and
increase the stability of the soy wax material when it undergoes a phase change
process. The best percentage of graphene+soy wax is 0.5 wt%, with the highest
thermal conductivity with stable thermal properties.
Al-Obaidi, K.M., Ismail, M., Rahman, A.M.A. 2014. Passive Cooling Techniques Through Reflective And Radiative Roofs In Tropical Houses In Southeast Asia: A Literature Review. Frontiers of Architectural Research, Volume 3(3), pp. 283–297
Amin, M., Putra, N., Kosasih, E.A., Prawiro, E., Luanto, R.A., Mahlia, T.M.I., 2017. Thermal Properties Of Beeswax/Graphene Phase Change Material As Energy Storage For Building Applications. Applied Thermal Engineering, Volume 112, pp. 273–280
Barreneche, C., Solé, A., Miró, L., Martorell, I., Fernández, A.I., Cabeza, L.F. 2013. Study On Differential Scanning Calorimetry Analysis With Two Operation Modes And Organic And Inorganic Phase Change Material (PCM). Thermochimica Acta, Volume 553, pp. 23–26
Barsoum, M.W. 2000. The MN+1AXN Phases: A New Class Of Solids: Thermodynamically Stable Nanolaminates. Progress in Solid State Chemistry, Volume 28, pp. 201–281
Huang, X., Alva, G., Liu, L., Fang, G., 2017. Preparation, Characterization And Thermal Properties Of Fatty Acid Eutectics/Bentonite/Expanded Graphite Composites As Novel Form–Stable Thermal Energy Storage Materials. Solar Energy Materials and Solar Cells, Volume 166, pp. 157–166
Imessad, K., Derradji, L., Messaoudene, N.A., Mokhtari, F., Chenak, A., Kharchi, R., 2014. Impact Of Passive Cooling Techniques on Energy Demand For Residential Buildings In A Mediterranean Climate. Renewable Energy, Volume 71, pp. 589–597
Jamekhorshid, A., Sadrameli, S.M., Farid, M., 2014. A Review Of Microencapsulation Methods Of Phase Change Materials (PCMs) as a Thermal Energy Storage (TES) Medium. Renewable and Sustainable Energy Reviews, Volume 31, pp. 531–542
Kalaiselvam, S., Parameshwaran, R., 2014. Thermal Energy Storage Technologies For Sustainability: Systems Design, Assessment and Applications. Boston: Academic Press.
Kant, K., Shukla, A., Sharma, A., Kumar, A., Jain, A., 2016. Thermal Energy Storage Based Solar Drying Systems: A Review. Innovative Food Science & Emerging Technologies, Volume 34, pp. 86–99
Kim, D., Jung, J., Kim, Y., Lee, M., Seo, J., Khan, S.B., 2016. Structure And Thermal Properties Of Octadecane/Expanded Graphite Composites as Shape-Stabilized Phase Change Materials. International Journal of Heat and Mass Transfer, Volume 95, pp. 735–741
Kusrini, E., Putra, N., Siswahyu, A., Tristatini, D., Prihandini, W.W., Alhamid, M.I., Yulizar, Y., Usman, A., 2019. Effects of Sequence Preparation of Titanium Dioxide–Water Nanofluid Using Cetyltrimethylammonium Bromide Surfactant and TiO2 Nanoparticles for Enhancement of Thermal Conductivity. International Journal of Technology, Volume 10(7), pp. 1453-1464
Laaouatni, A., Martaj, N., Bennacer, R., Elomari, M., El Ganaoui, M., 2016. Study of Improving The Thermal Response of a Construction Material Containing a Phase Change Material. Journal of Physics: Conference Series, Volume 745, p. 032131
Latha, P.K., Darshana, Y., Venugopal, V., 2015. Role of Building Material In Thermal Comfort In Tropical Climates – A Review. Journal of Building Engineering, Volume 3, pp. 104-113
Lee, J., Wi, S., Yun, B.Y., Chang, S.J., Kim, S., 2019. Thermal and Characteristic Analysis Of Shape-Stabilization Phase Change Materials By Advanced Vacuum Impregnation Method Using Carbon-Based Materials. Journal of Industrial and Engineering Chemistry, Volume 70, pp. 281–289
Meng, X., Zhang, H., Sun, L., Xu, F., Jiao, Q., Zhao, Z., Zhang, J., Zhou, H., Yutaka, S., Liu, Y., 2013. Preparation and Thermal Properties of Fatty Acids/CNTs Composite As Shape-Stabilized Phase Change Materials. Journal of thermal analysis and calorimetry, Volume 111, pp. 377–384
Naguib, M., Come, J., Dyatkin, B., Presser, V., Taberna, P.L., Simon, P., Barsoum, M.W., Gogotsi, Y., 2012. MXene: a Promising Transition Metal Carbide Anode For Lithium-Ion Batteries. Electrochemistry Communications, Volume 16, pp. 61–64
Nomura, T., Zhu, C., Sheng, N., Tabuchi, K., Sagara, A., Akiyama, T., 2015. Shape-Stabilized Phase Change Composite By Impregnation Of Octadecane Into Mesoporous Sio2. Solar Energy Materials and Solar Cells, Volume 143, pp. 424–429
Pethurajan, V., Sivan, S., Konatt, A.J., 2018. Facile Approach To Improve Solar Thermal Energy Storage Efficiency Using Encapsulated Sugar Alcohol Based Phase Change Material. Solar Energy Materials and Solar Cells, Volume 185, pp.524–535
Putra, N., Prawiro, E., Amin, M., 2016. Thermal Properties of Beeswax/CuO Nano Phase-change Material Used for Thermal Energy Storage. International Journal of Technology, Volume 7(2), p. 244–253
Putra, N., Rawi, S., Amin, M., Kusrini, E., Kosasih, E.A., Mahlia, T.M.I., 2019. Preparation Of Beeswax/Multi-Walled Carbon Nanotubes As Novel Shape-Stable Nanocomposite Phase-Change Material For Thermal Energy Storage. Journal of Energy Storage, Volume 21, pp. 32–39
Rasta, I.M., Suamir, I.N., 2018. The Role of Vegetable Oil In Water Based Phase Change Materials for Medium Temperature Refrigeration. Journal of Energy Storage, Volume 15, pp. 368–378
Saikia, P., Azad, A., Rakshit, D., 2018. Thermodynamic Analysis Of Directionally Influenced Phase Change Material Embedded Building Walls. International Journal of Thermal Sciences, Volume 126, pp. 105–117
Souayfane, F., Fardoun, F., Biwole, P.H., 2016. Phase Change Materials (PCM) for Cooling Applications in Buildings: A Review. Energy and Buildings, Volume 129, pp. 396–431
Trisnadewi, T., Kusrini, E., Nurjaya, D.M., Putra, N., Mahlia, T.M.I., 2021. Experimental Analysis Of Natural Wax As Phase Change Material By Thermal Cycling Test Using Thermoelectric System. Journal of Energy Storage, Volume 40, p. 102703
Vennapusa, J.R., Konala, A., Dixit, P., Chattopadhyay, S., 2020. Caprylic Acid Based PCM Composite With Potential for Thermal Buffering and Packaging Applications. Materials Chemistry and Physics, Volume 253, p. 123453
Wi, S., Seo, J., Jeong, S.G., Chang, S.J., Kang, Y., Kim, S., 2015. Thermal Properties of Shape-Stabilized Phase Change Materials Using Fatty Acid Ester and Exfoliated Graphite Nanoplatelets For Saving Energy in Buildings. Solar Energy Materials and Solar Cells, Volume 143, pp. 168–173
Zhang, L., Yang, W., Jiang, Z., He, F., Zhang, K., Fan, J., Wu, J., 2017. Graphene Oxide-Modified Microencapsulated Phase Change Materials with High Encapsulation Capacity and Enhanced Leakage-Prevention Performance. Applied Energy, Volume 197, pp. 354–363
