Influence of Temperature on the Thermal Properties of the Core Material - the Coefficient of Temperature Conductivity, Specific Heat Capacity, Thermal Conductivity
Corresponding email: os_kharitonova@mail.ru
Published at : 04 Apr 2023
Volume : IJtech
Vol 14, No 2 (2023)
DOI : https://doi.org/10.14716/ijtech.v14i2.6009
Burlutsky, E., Balzamov, D., Bronskaya, V., Kharitonova, O., Khairullina, L., Solovyeva, O., 2023. Influence of Temperature on the Thermal Properties of the Core Material - the Coefficient of Temperature Conductivity, Specific Heat Capacity, and Thermal Conductivity. International Journal of Technology. Volume 14(2), pp. 443-454
| Efim Burlutsky | 1. Kazan State Power Engineering University, 51 Krasnoselskaya St., Kazan 420066, Russia, 2. Almetyevsk State Oil Institute, 2 Lenina St., Almetyevsk, 423450, Russia |
| Denis Balzamov | Kazan State Power Engineering University, 51 Krasnoselskaya St., Kazan 420066, Russia |
| Veronika Bronskaya | 1. Kazan National Research Technological University, 68 Karl Marx St., Kazan 420015, Russia, 2. Kazan Federal University, 18 Kremlyovskaya St., Kazan, 420008, Russia |
| Olga Kharitonova | Kazan National Research Technological University, 68 Karl Marx St., Kazan 420015, Russia |
| Liliya Khairullina | Kazan Federal University, 18 Kremlyovskaya St., Kazan, 420008, Russia |
| Olga Solovyeva | Kazan State Power Engineering University, 51 Krasnoselskaya St., Kazan 420066, Russia |
The absorption of heat by rocks is accompanied by an
increase in the kinetic energy of molecules and atoms and is recorded by a
change in rock temperature. The thermal properties of rocks characterize the
ability to transfer and absorb heat and change their size when the temperature
rises. The main thermal properties of rocks are thermal conductivity, heat
capacity, linear thermal expansion, and thermal volumetric expansion. In this
paper, the influence of temperature effects on the thermal properties of reservoirs
– the coefficient of temperature conductivity, specific heat capacity, and
thermal conductivity is studied using the samples of oil-saturated reservoirs
from the good number 19Bp of the Upper Uplift of Sotnikovsky deposit. The
dependence of the thermal properties of the core material on temperature was
revealed using a number of laboratory experiments. The results of these studies
contribute to improving the reliability of data on the relationship of thermal
properties with other physical properties of oil-saturated reservoirs and can
be used to improve the efficiency of the development of fields of super-viscous
oil.
Core material; Core material temperature; Thermal conductivity; Thermal conductivity coefficient; Specific heat capacity
The degree of study of the thermal field of the Earth
and the Earth's crust is still low, and it is incomparable with the modern
results of studies of seismic, magnetic, gravitational and other physical
fields of our planet. This is largely due to the insufficient level of
development of thermal petrophysics (thermal physics of rocks) underlying the
study of natural and artificial thermal fields in the subsurface. To solve such
problems as modeling of sedimentary basins and oil and gas bearing systems,
search and exploration of hydrocarbon deposits, design of thermal methods of
production of high-viscosity oils, interpretation of results of thermometry in
wells, determination of heat flux density from the bowels, etc. requires reliable
database on thermal conductivity, thermal conductivity, the specific heat
capacity of rocks (Abed and Yakhlef,
2020; Adam, 2009).
The situation is complicated by the fact that rocks, as an object of
thermophysical research, have characteristic features, consideration of which
is necessary when creating methods and equipment for determining their thermal
properties. First, this is their essential heterogeneity, multiphase
(saturation of solid rock skeleton by various pore fluids, such as formation
water, gas, oil and their various combinations), dependence on thermodynamic
conditions of occurrence (rock, formation pressure, temperature).
It should be noted that a significant part of the core from shallow
deposits of extra-viscous oils is weakly consolidated and loose samples, which
requires a special research methodology (which, until recently, was practically
absent).
The variety of problems solved in mining thermal physics also determines
the difference in the methods used for experimental determination of the
thermal characteristics of rocks (Alas and
Ali, 2019).
The choice of methods is influenced by many factors, including the purpose
of the study, the range of changes in the thermal properties of rocks,
different degrees of consolidation (from unconsolidated sedimentary reservoirs
of ultra-viscous oils to hard low-porous rocks of the crystalline basement),
the degree of sample saturation, etc. The depth of sampling determines the
values of pressures at which it is necessary to study the rocks. The nature of
the thermal influence determines the temperature range of research (Balzamov et al., 2020b).
The depletion of easily recoverable oil reserves leads to the need to
involve in the development of increasingly complex facilities containing
hard-to-recover oil reserves (HTR reserves). At the moment, the trends are that
due to a decrease in the number of reserves, the lifting of traditional fossil
fuels will be supplemented by the development of unconventional sources of raw
materials (shale oil and gas, high-viscosity oils, bitumen, bituminous sand
oil, coal methane, gas hydrates, etc.).
The world reserves of natural bitumen are estimated at more than 800
billion tons. At the same time, Russia is one of the leaders in reserves, a
third of which is located on the territory of the Republic of Tatarstan.
The extraction of bituminous oil requires an unconventional, unique
approach. There are various ways to develop deposits of heavy oils and natural
bitumen, which differ in technological and economic characteristics.
The research aims to determine the temperature dependence of the specific heat capacity and thermal conductivity of weakly cemented, bituminous-saturated sandstone samples. Data on the thermophysical properties of rocks are a key parameter for numerical models of reservoir systems. In addition, they are needed to determine the rate of advance of the coolant front, assessment of thermal resources of deposits and design development systems.
The efficiency of the
lifting of SVO and natural bitumen using thermal methods largely depends on the
methodological base, based on laboratory studies to determine data on the
thermophysical properties of rocks (Ibragimova et
al., 2017; Ismagilova et al., 2016; Valeeva
et al., 2013). In addition, they are necessary for work
to determine the speed of advance of the heat transfer agent front, the
assessment of thermal resources of deposits and the design of development
systems. All of the above determines the relevance of the topic of this work.
The LFA 467 laser
burst device was used to determine the temperature conductivity. An infrared detector is used to measure
the temperature increase from the back of the sample as a function of time. The
measurement of temperature conductivity, specific heat capacity allows (with a
known or additionally measured volumetric density) to calculate the thermal
conductivity of the sample under study (Balzamov et al., 2020a; Petrov et al., 2019;
Ganeeva et al., 2014).
To determine the specific heat capacity of the
rock, a differential scanning calorimeter DS 204 HP was used in work. The essence of the method consists in measuring the heat of processes
and the specific heat capacity of substances through the heat flux - the
derivative of heat over time (differential). Heat flows are measured
simultaneously by the temperature difference at two points of the measuring
system (Balzamov et al., 2020c; Mirgorodskaya et al., 2018; Gabdrakhmanov et al.,
2015). The determination of
specific heat is carried out by a special program NETZSCH TA4_5.
The
results of this study will optimize the methods of lifting SVO. In this paper,
the influence of temperature on the thermal properties of the core material is
studied – the coefficient of temperature conductivity, specific heat capacity,
and thermal conductivity, the purpose of which is to analyze the influence of
temperature on the thermophysical properties of the core material. The
temperature conductivity is measured on the LFA-467 device, and the specific
heat capacity - on the DSC-204 HP device, the thermal conductivity is
determined by mathematical calculation. The dependence of the thermal
properties of the core material on temperature was revealed using a number of
laboratory experiments. The results of these studies can be used to improve the
efficiency of developing super-viscous oil fields.
The LFA
467 incorporates sophisticated equipment and simple software to provide fast,
accurate and safe measurements. The LFA 467 is based on the laser flash method
according to international standards ASTM E-1461, DIM EN 821 and DIN 30905.
An
infrared detector measures the temperature increase on the sample's backside as
a time function. The measurement of thermal diffusivity and specific heat
capacity allows (with known or additionally measured bulk density) to calculate
the thermal conductivity of the sample under study.
Mathematical
analysis of the measured temperature dependence on time allows us to determine
the thermal diffusivity ?. The analysis is performed by a special program using
a set of differential mathematical models for various applications.
Figure 1 Holder
for thermal conductivity measurements
When the
sample is loaded, the measuring cell LFA 467 is opened, and the samples (round
or square) are placed in the corresponding sample holder. The upper flap of the
furnace is put back in place with the extraction device, and the measuring cell
is closed.
Start the
measurement. Start the measurement by using the NETZSCH software. Determine the
basic settings for the measurement. Set the gas parameters. Set the holder to
be used. Mark the desired sample position in the left window (the sample will
be marked green) and define the parameters of the respective sample:
- Sample
properties
- Stain,
layer properties
-
Template for measurements
-
Thermophysical properties
Temperature
program. Select the start and end temperature of the measurement and determine
the temperature step value (in our work, this corresponds to 50°C).
Set the
required interval for analysis. The calculation interval should be
approximately 10-12 half-periods.
To
ensure correct estimation of the calculation interval, both curves (the
original signal curve and its smoothing) should be combined (Figure 2 see on
supplementary).
Figure 3 Scheme of measurements by DSC 204 HP
method
(F -
furnace (heater), S - sample, R - standard, TF, TmS, TmR - temperatures of
furnace and junction of differential thermocouple of sample and standard, FFS,
FFR - heat fluxes)
The
essence of the method consists in measuring the heat of processes and the
specific heat capacity of substances through the heat flow - the derivative of
heat over time (differential). Heat fluxes are measured by the temperature
difference in two points of the measuring system at one point in time.
Measurements are carried out both in isothermal conditions and in dynamic mode
with a programmable change in the shell's temperature (heater).
Figure 4 Dynamics of changes in the coefficient of specific heat capacity at a depth of 195.9 m
Figure 5 Dynamics of
changes in the coefficient of specific heat capacity at a depth of 196 m
Figure 6 Dynamics of changes in the coefficient of
specific heat capacity at a depth of 196.1 m
Figure 7
Dependence of specific heat capacity on temperature
Where · - 195.9
m; · - 196.0 m; · - 196.1 m
Figure 8 Dynamics of changes in
the temperature conductivity coefficient at a depth of 195.9 m
Figure 9 Dynamics of changes in the
temperature conductivity coefficient at a depth of 196 m
Figure 10 Dynamics of changes in the
temperature conductivity coefficient at a depth of 196.1 m
Figure 11 Results of the temperature conductivity study temperature conductivity mm2/s
Where · - 195.9 m; · - 196.0 m; · - 196.1
m.
The analytical study of thermal
conductivity is reduced to the study of spatio-temporal changes in temperature
and specific heat capacity, i.e., to find equation 2.
When calculating the
thermal conductivity according to the formula using the data of temperature
conductivity and specific heat capacity, the following results were obtained
(Table 1).
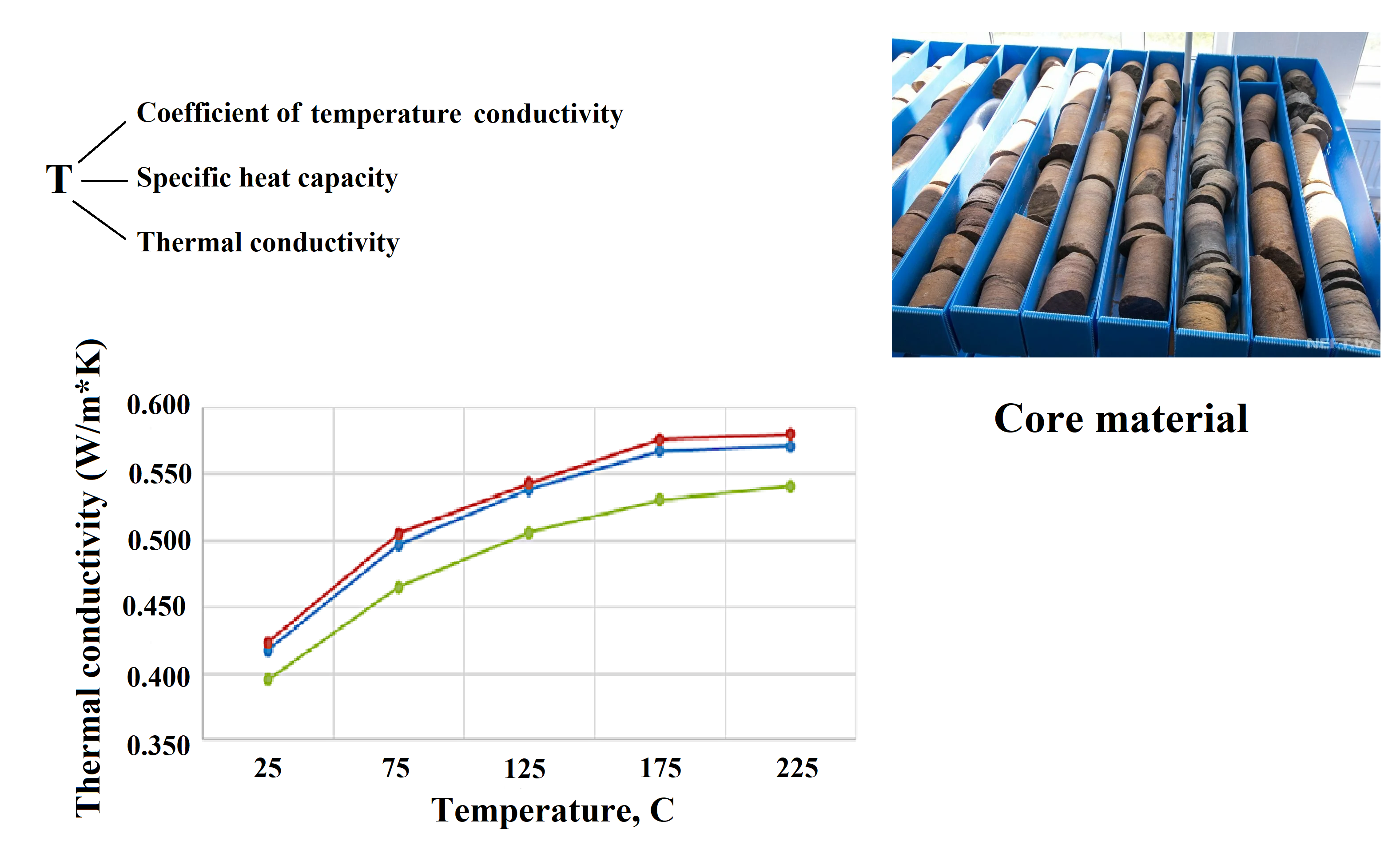
Table 1 Defined
values of thermal conductivity of core material samples temperature
|
Name of
indicators |
T, oC |
Intervals
of sampling of core material | ||
|
195.9 m |
196.0 m |
196.1 m | ||
|
Thermal
conductivity, W/m*K |
25 |
0.417 |
0.423 |
0.395 |
|
75 |
0.497 |
0.505 |
0.465 | |
|
125 |
0.538 |
0.543 |
0.506 | |
|
175 |
0.567 |
0.576 |
0.531 | |
|
225 |
0.571 |
0.580 |
0.541 | |
Figures 6-8 show the direct relationship
between the coefficient of thermal conductivity and
Figure 12 Dynamics of changes in the thermal
conductivity coefficient at a depth of 196.1 m
Figure 13 Dynamics of changes in
the thermal conductivity coefficient at a depth of 196.1 m
Figure 14 Dynamics of changes in the thermal
conductivity coefficient at a depth of 196.1 m
Figure 15
Dependence of thermal conductivity on temperature
Where · - 195.9 m; · - 196.0
m; · - 196.1 m
According to the results of the research, it was found that the
coefficient of thermal conductivity decreases with an increase in the
temperature of the core material, and the coefficient of specific heat capacity
increases with an increase in the temperature of the unconsolidated core. The
highest values of specific heat are observed at a temperature of 225°C. The
coefficient of thermal conductivity also increases with an increase in the
temperature of the core material. The highest thermal conductivity values are
observed at a temperature of 225°C. The increase of the thermal conductivity of
the core material with the temperature change can be explained by the fact that
with increasing temperature, the thermal conductivity of the medium that fills
the gaps between the grains increases (the core material can be attributed to
granular materials), and the heat transfer by radiation inside the granular massif
also increases. The thermophysical properties of the core material obtained
during laboratory studies can be applied in designing and optimizing methods
for lifting viscous oil with thermal effects on the productive reservoir.
The study was carried out
within the framework of a scientific project of the Russian Science Foundation
(RSF) No 21-79-10406 (https://www.rscf.ru/project/21-79-10406/).
| Filename | Description |
|---|---|
| R2-CE-6009-20230330083727.docx | --- |
Abed, A., Yakhlef, M., 2020. Brownfield regeneration
as a strategy for sustainable development: Amman case study. International
Journal of Technology, Volume 11(4), pp. 732–742
Adam, R.B. 2009. Converting oil shale to liquid fuels
with the Alberta Taciuk Processor: energy inputs and Greenhouse Gas Emissions. Energy
& Fuels, Volume 23(12), pp. 6253– 6258
Alas, M., Ali, S.I.A., 2019. Prediction of the high-temperature
performance of a geopolymer modified asphalt binder using artificial neural
networks. International Journal of Technology, Volume 10(2), pp.
417– 427
Balzamov, D., Akhmetova, I., Bronskaya, V., Kharitonova,
O., Balzamova, E., 2020a. optimization of thermal conditions of heat recovery
boilers with regenerative heating in the high-temperature section of Isoamylene
Dehydrogenation. International Journal of Technology, Volume 11(8), pp. 1598–1607
Balzamov, D.S., Balzamova, E.Yu., Bronskaya, V.V., Oykina
G.I., Rybkina E.A., Shaikhetdinova,
R.S., Kharitonova, O.S., 2020b. The beneficial using the heat of the exhaust gases of
the furnaces of the technological unit for the ethylene oxide production. In: IOP Conference Series:
Materials Science and Engineering, Volume 862(6), 062044
Balzamov, D.S., Balzamova, E.Y., Bronskaya, V.V.,
Rybkina, E.A., Kharitonova, O.S., 2020c. Beneficial use of thermal secondary energy resources
in the rectification cycle at ethylene glycol production unit. In: IOP Conference Series:
Materials Science and Engineering, Volume 919(6), 062027
Balzamov, D.S., Balzamova, E. Yu., Bronskaya, V.V.,
Valitov, N.V., 2020d. Possibility of using associated petroleum gas as a
fuel for a production boiler house. In: IOP Conference Series: Materials Science and
Engineering, Volume 791(1), 012006
Chekalyuk, E.B., 1955. The temperature field of the formation when
the coolant is injected into the well. Oil economy, Volume 4, pp. 39–42
Gabdrakhmanov, D.R., Valeeva, F.G., Syakaev, V.V.,
Lukashenko, S.S., Lakharov, S.V., Kuryashov, D.A., Bashkirtseva, N.Y.,
Zakharova, L.Y., Latypov, S.K., Sinyashin, O.G., 2015. Novel supramolecular
system based on a cationic amphiphile bearing glucamine fragment: structural
behavior and hydrophobic probe binding. Mendeleev Communications, Volume
25(3), pp. 174–176
Ganeeva, Y.M., Yusupova, T.N., Romanov, G.V.,
Bashkirtseva, N.Y., 2014. Phase composition of asphaltenes. Journal of
Thermal Analysis and Calorimetry, Volume 115(2), pp. 1593–1600
Ibragimova, D.A., Ivanova, I.A., Sharafieva, Z.F.,
Kharitonova, O.S., 2017. Evaluation of
paraffin and asphaltenes phase equilibrium in petroleum systems. International
Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology
Management, SGEM, Volume 17(15), pp. 147–154
Ismagilov, I.F., Kuryashov, D.A., Idrisov, A.R., Alieva,
M.R, Kashapova, N.E., 2016. Supramolecular system based on cylindrical micelles
of anionic surfactant and silica nanoparticles. Colloids and Surfaces A:
Physicochemical and Engineering Aspects, Volume 507, pp. 255–260
Jamilatun, S., Budhijanto, Rochmadi, Yuliestyan, A.,
Aziz, M., Hayashi, J., Budiman, A., 2020. Catalytic Pyrolysis of Spirulina
platensis Residue (SPR): Thermochemical behavior and kinetics. International Journal of Technology,
Volume 11(3), pp. 522–531
Khisamov R.S., Zakirov I.S., Zakharova E.F.,
Bazarevskaya V.G., Abusalimova R.R., Timirov D.A., 2018. Experience of study
and development of domanik deposits by example of Bavlinskoye field of Republic
of Tatarstan. Petroleum Economy, Volume 11, pp. 78–83
Mirgorodskaya, A.B., Valeeva, F.G., Zakharov, S.V., Kuryashov, D.A., Bashkirtseva, N.Yu., Zakharova, L.Ya., 2018. Aggregation
behavior of morpholinium surfactants in the presence of organic electrolytes. Russian
Chemical Bulletin, Volume 67(2), pp. 291–296
Petrov, S., Nosova, A., Bashkirtseva, N.,
Fakhrutdinov, R., 2019. Features of heavy oil spraying with single evaporation.
In: IOP Conference Series:
Earth and Environmental Science, Volume 282(1), p. 012004
Rubinstein, L.I., 1958. About the temperature field of the
formation when a hot coolant is injected into the formation: (about the
articles by E.B. Chekalyuk). In: Proceedings of the Ufa Petroleum Institute,
Issue 2, pp. 149–173
Sheinman, A.B., Malofeev, G.E., Sergeev, A.I., 1969. The impact of
heat on the reservoir during oil production. M. Nedra
Tugov A.N., Ots A., Siirde A., Sidorkin V.T., Ryabov
G.A. 2016. Development of measures to improve technologies of energy recovery
from gaseous wastes of oil shale processing. Thermal Engineering, Volume
63(6), pp. 430–438
Valeeva, F.G., Kuryashov, D.A., Zakharov, S.V. Vagapova,
G.I., Vasilieva, E.A., Bashkirtseva, N.Yu., Zakharova, L.Ya., Konovalov, A.I., 2013. Supramolecular
system 4-aza-1-hexadecyl-1-azoniabicyclo[2.2.2]octane bromide - Sodium
salicylate. Aggregation and rheological properties. Russian Chemical
Bulletin, Volume 62(4), pp. 989–993
