Effect of Reducing Agents on The Performance of AgNPs and PANI Flexible Conductive Fabrics
Published at : 03 Nov 2022
Volume : IJtech
Vol 13, No 6 (2022)
DOI : https://doi.org/10.14716/ijtech.v13i6.5853
Nurlis, A.G., Adhika, D.R., Suprijanto, 2022. Effect of Reducing Agents on The Performance of AgNPs and PANI Flexible Conductive Fabrics. International Journal of Technology. Volume 13(6), pp. 1163-1172
| Aulia Ghifari Nurlis | 1. Engineering Physics, Faculty of Industrial Technology, Institut Teknologi Bandung, Jl. Ganesa 10, Bandung 40132, Indonesia 2. National Research Center for Nanotechnology, Institut Teknologi Bandu |
| Damar Rastri Adhika | 1. Engineering Physics, Faculty of Industrial Technology, Institut Teknologi Bandung, Jl. Ganesa 10, Bandung 40132, Indonesia 2. National Research Center for Nanotechnology, Institut Teknologi Bandu |
| Suprijanto | Engineering Physics, Faculty of Industrial Technology, Institut Teknologi Bandung, Jl. Ganesa 10, Bandung 40132, Indonesia |
Cotton
fabric with the addition of silver nanoparticles (AgNPs) and polyaniline (PANI)
was developed as a flexible electrode to measure muscle biopotential signals
using Electromyography (EMG) in
this study. AgNPs are synthesized using two reducing agents, Gum Acacia and
Hydrazine Hydrate. The effect of the two different reducing agents will be
studied by measuring the electrical conductivity of each sample and also by
comparing flexible electrode performance during EMG measurements. The results
obtained from this study are that the AgNP electrodes synthesized using the Gum
Acacia have very high SNR and conductivity. The absence of an electrolyte gel
during EMG measurement using the developed flexible electrode can provide
comfort to the user without any risk of skin irritation due to electrolyte gel
when the measurement process is carried out for a long duration. The AgNP
fabric electrode with Gum Acacia as the reducing agent is expected to be used
to replace commercial electrodes in the
future.
Conductivity; Flexible electrodes; Gum acacia; Hydrazine hydrate; Reducing agents
The flexible
electrode has a good conductivity value and high flexibility. The problem that
is taken as the basis for developing this flexible electrode is that the
electrodes commonly used to carry out biopotential measurements have their
respective drawbacks. The dry electrode is fairly rigid and does not require an
electrolyte gel during the measurement process, but the measures will produce a
high noise signal
Functionalization of fabrics with the
addition of conductive materials could be performed to achieve a fabric-based
dry flexible electrode. Conductive materials added to the fabrics should have
good conductivity and maintain the fabrics' flexibility. The choice of
conductive polymer material is because most polymers already have a high degree
of flexibility and are elastic
AgNP with high purity and small size is
necessary for medical applications (Khumaeni et al., 2019). The
addition of AgNP to the fabric electrode increases the electrode conductivity.
The small diameter size AgNP can get into crevices and stick to the surface of
the fabric substrate. If the measurement conductivity increases, the noise
reduction in the EMG will increase. Adding AgNP and polyaniline to cotton
fabrics will produce a flexible electrode with good flexibility and
conductivity that can be used for biopotential signal measurements such as Electroencephalography
(EEG), Electrocardiography (ECG), and Electromyography (EMG) signals. The main
advantage of this fabric-based flexible electrode is that the fabric electrode
can follow the shape of the user's body curve when measuring the signals. Therefore,
the skin-to-electrode contact could be improved, and the measurement results
can be more accurate. In addition, the absence of an electrolyte gel can
provide comfort to the user without giving symptoms of skin irritation when the
measurement process is carried out for a long duration. Comfort in measurement
is also very important because the biopotential signal can possibly change due
to fatigue (Puspasari et al., 2017).
Plain white cotton fabric and aqua-dm were
obtained from a local market in Indonesia. Aniline, Ammonium Persulfate (APS),
NaOH, and HCl were purchased from Merck, Germany. Gum Acacia and Hydrazine
Hydrate were obtained from a local chemical store in Indonesia. Silver nitrate
(AgNO3) was purchased from PT. Antam Indonesia.
2.1. Preparation of Fabric-Based Flexible Electrode with Addition of
AgNP with Different Reducing Agents and PANI
At this stage, AgNP will be synthesized
using two different reducing agents to compare the performance of the
electrodes with each other. In this research, the dimensions of the white
cotton fabric used are 4 cm x 2 cm. After the cotton fabric was prepared, the
AgNP solution was designed using different reducing agents. AgNP reducing
agents used were gum acacia and hydrazine hydrate. To increase the
conductivity, the electrodes coated with
AgNP are varied with an additional layer, namely PANI. Electrode performance
will be seen based on the results of the SEM EDS characterization,
conductivity, and EMG signal of each sample. Based on the characterization,
variations of reducing agents that produce better performance will be selected
for the next optimization stage. The procedure for preparing AgNP and PANI
solutions is described in the following subsections.
2.1.1. Green
synthesis of AgNPs with Gum Acacia
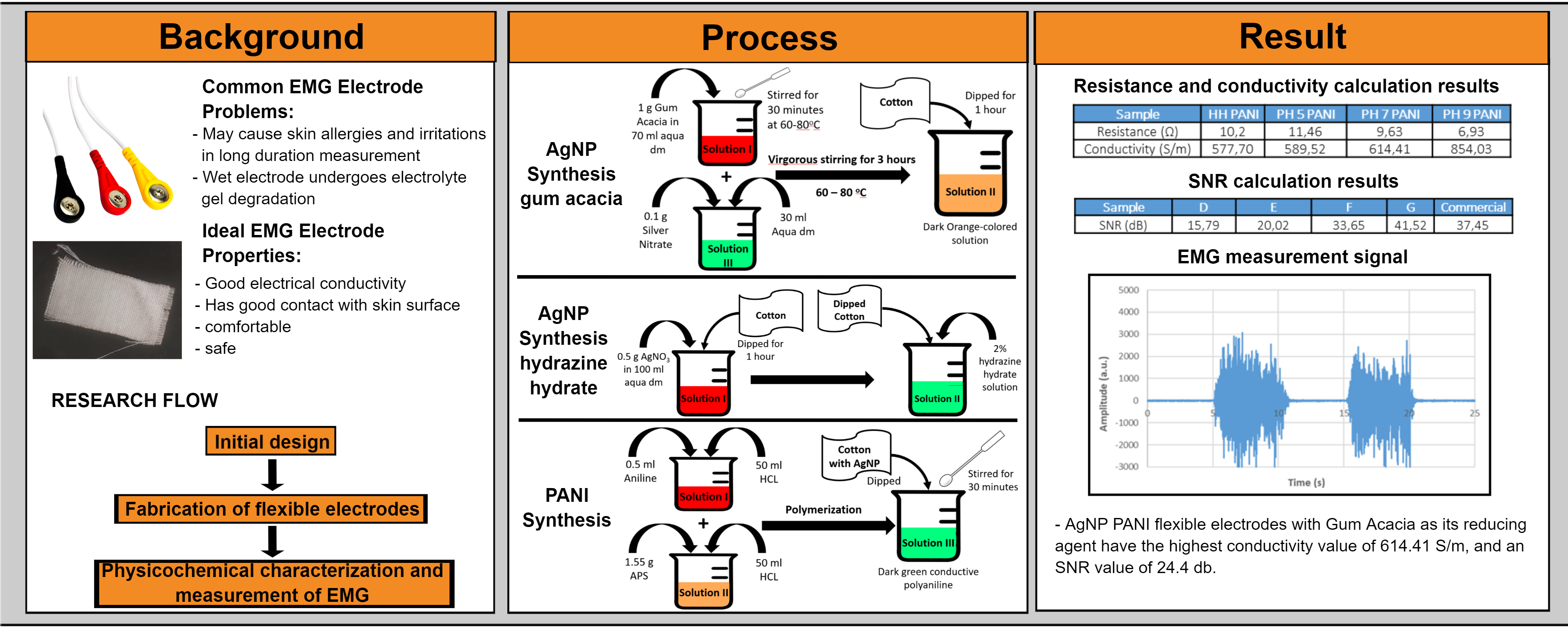
Gum Acacia is commonly used as a green synthesis-reducing agent to produce AgNP. Besides gum acacia, Rosman et al. (2021) has synthesized AgNP with another green synthesis-reducing agent, namely polychaete (Marphysa moribidii) (Rosman et al., 2021). However, gum Acacia has a low, reducing property; therefore, the AgNP synthesis process with Gum Acacia also requires a longer time. A total of 1%-wt (1 g) of Gum Acacia was added to 70 mL of distilled water in a flask. The solution is then stirred with a magnetic stirrer at the desired temperature (60-80oC) for about half an hour to obtain a homogeneous solution. At the same time, 0.1% by weight (0.1 g) of silver nitrate was dissolved in 30 mL of distilled water at room temperature and then rapidly injected into the above Gum Acacia solution with vigorous stirring (Dong et al., 2014). The solution was maintained at the desired temperature and allowed to react for 3 hours until a dark-colored solution was obtained. Then, the cotton fabric was dipped in the AgNP solution for 1 hour so that the solution was well absorbed into the cotton fabric. After soaking in AgNP solution, the sample will be dried in an oven at 60oC for 2 hours.
Figure 1 AgNP coating procedure on fabric
with gum acacia (Dong et al., 2014)
2.1.2. Green synthesis of AgNPs with Hydrazine Hydrate
Figure 2 AgNP
coating procedure on fabric with hydrazine hydrate
Hydrazine
hydrate is one of the reducing agents which is quite strong in reducing Ag
solution. However, hydrazine hydrate solution is a very toxic solution and can
trigger cancer cells when in contact with body surfaces. The AgNP fabrication
process using a reducing agent is carried out in a fumehood so that the gas
from hydrazine hydrate is not inhaled. The fabrication begins with dissolving
silver nitrate powder into aqua dm to produce a solution of AgNO3
with a solubility of 5 g/l for 1 hour. Then, the cotton fabric is rapidly
dipped in the 2% hydrazine hydrate solution. The sample was soaked until there
were no bubbles resulting from reducing AgNO3 to Ag on the fabric.
After the sample is saturated, the sample will be dried in an oven at a
temperature of 60oC for 2 hours. Fabric samples coated with AgNP
with hydrazine hydrate will have a gray cotton fabric; the gray color indicates
AgNP has adhered to the cotton fabric.
2.1.3. Synthesis of PANI
The synthesis procedure was started by
preparing cotton fabric as a substrate, 0.5 mL of aniline monomer in 50 mL 1 M
HCl (solution I), and 1.55 grams of APS in 50 mL HCl 1 M (solution II) (Abu-Thabit, 2016). The solutions I and II were
mixed while stirring using a magnetic stirrer to keep the stirring rate constant
so that the polymerization that occurred was evenly distributed. Next, the
AgNP-treated fabric substrate was immersed in the solution during the
polymerization process for approximately 30 minutes to obtain an average
polymerization conversion of 90%. The success of the polymerization is
indicated by a change in color to dark green, like the color of emerald salt.
After the cotton fabric substrate is dark green, the cotton fabric is dried in an oven at 60oC to dry.
Figure 3 PANI coating procedure on fabric
Based on the synthesis procedure
described previously, the electrode samples will be varied into four models the
nomenclature of the samples obtained from the synthesis process can be seen in Table
1.
Table 1 Nomenclature of the
sample
|
Sample |
Composition |
||
|
A |
Cotton |
AgNP Gum Acacia |
|
|
B |
Cotton |
AgNP Hydrazine Hydrate |
|
|
C |
Cotton |
AgNP Gum Acacia |
PANI |
|
D |
Cotton |
AgNP Hydrazine Hydrate |
PANI |
The fabric-based flexible electrodes
from each sample have different colors due to other treatments. The color
change of white cotton fabric after being coated by AgNP solutions from other
reducing agents and PANI can be seen in Figure 1.
|
|
Figure 4 Fabric substrate in (a) after being coated with AgNP acacia gum (b) after being coated with AgNP hydrazine hydrate (c) after PANI coating
2.2. Characterization of Fabric-Based Flexible Electrode
SEM (Hitachi SU3500) was used to analyze PANI-coated fabrics' morphology and composition. In addition, electrical conductivity characterization was carried out using a digital multimeter (Keithley DMM7510) and a DC source generator using the four-point probe (FPP) method (Figure 5). FPP is suitable for measuring the resistance of thin films or substrates in ohms per square by forcing a current through the two outer probes and reading the voltage across the two inner probes (Chlaihawi, et al., 2018). Using this FPP configuration can avoid errors in measurement due to contact resistance between the probe and sample. In this method, the value of the voltage generated by the electrode is measured, which is given an electric current with a constant value
Figure 5 (a) Keithley DMM7510 digital multimeter and (b) four-point probe
After the tool has been prepared, the DC source generator is turned on.
The value of the input current source used is 0.5 mA, which is estimated
because the source meter used has a maximum value limit of the measured
voltage, which is 22 Volts. Measurements were repeated until 100 data were
obtained for each electrode. The data obtained are then tabulated into a table
in the form of the average voltage value, standard deviation, maximum voltage
value, minimum voltage value, and peak-to-peak value of all electrode samples.
2.3. Biopotential EMG Measurement
Testing of the electrodes that have been
generated in the EMG signal measurement instrument was carried out using the
FlexComp Infiniti – 10 Channel System device, which was produced and displayed
on the Infiniti BioGraph software.
Figure 6 (a) Set-up of EMG signal measurement with FlexComp Infiniti – 10
Channel System device (b) the placement of the EMG measurement probe for the
detection electrodes is placed on A and B while the reference electrode is
placed on C
The
placement of the EMG measurement probe can be observed in Figure 3 where two
electrodes were attached to the biceps muscle, and one electrode was attached
to the elbow, which served as a reference electrode. The three electrodes
installed on the arm are then connected to the EMG test equipment, and the
obtained measurement signals are displayed on a computer. The measurement
signals were received by clenching the hands to get muscle signals when
contracting and releasing fists to get muscle signals when relaxing for 5 seconds
each. This process was repeated twice so that the total measurement was carried
out for about 20 seconds. When the hand is relaxed, the biopotential signal
shows a value close to zero, but when the hand contracts, the signal moves to a
peak.
3.1. SEM EDS Observation
SEM-EDS is a tool for
characterizing the morphology of the sample surface and calculating the
composition of the material making up the sample. The design of each sample (A,
B, C, and D) follows the composition in Table 1. Samples A and C were coated
with silver nanoparticles synthesized using acacia gum, while samples B and D
were synthesized with hydrazine hydrate. In addition, samples C and D were
given an additional PANI layer. From the obtained SEM images in Figure 7, it
can be seen that the variation of the electrode without the addition of PANI
has flashes due to the charging effect. The charging product seen in the SEM
image is due to the low conductivity of the observed sample. Qualitatively, the
results of SEM A did not show a charging effect as large as the SEM results of
sample B, so it can be concluded that the conductivity in sample A is higher
than B. In addition, SEM samples C and D have better SEM results because no
charging effect occurred. Absence of the charging effect indicates a higher
conductivity on samples C and D than that of A and B due to the addition of
PANI. Silver particles could be identified as small particles on the surface of
the fabric on the SEM image.
Figure 7 SEM characterizations of
(a) sample A (b) sample B (c) sample C (d) sample D
The
SEM results that have been obtained are then analyzed using EDS. Based on the
results of the EDS analysis in Table 2, it was found that there was a decrease
in silver content during the PANI coating process on AgNP electrodes. Samples A
and C, which use gum acacia as the reducing agents, decreased about 0.8% silver
particles during the PANI coating process. Otherwise, samples B and D decreased
silver content by 0.7%. The silver content decreased due to the addition of C,
O, N, S, and other PANI atoms and the possibility of silver particles being
released during the PANI coating process. Samples A and B do not have N and S
atomic compositions because samples A and B are only composed of AgNP and
cotton fabric without PANI addition. According to Monier's research, cotton is
only composed of C, H, and O atoms (Monier
et al., 2014).
Table 2 EDS characterization
of AgNP electrode with a variation of reducing agents and PANI addition
|
Sample |
Atomic Composition (%) |
||||
|
C |
N |
O |
S |
Ag |
|
|
A |
47.2 |
- |
51.7 |
- |
1.1 |
|
B |
46.9 |
- |
52.1 |
- |
1 |
|
C |
46 |
3.9 |
42.7 |
7.1 |
0.3 |
|
D |
41.5 |
8.5 |
39.2 |
10.5 |
0.3 |
3.2. Electrical Conductivity
Measurements
The measured voltage data
for the four samples amounted to 100, then averaged. The following is the
result of the voltage data obtained from the measurement results, as listed in Table
3.
Table 3 Voltage measurement of flexible electrode sample
(units in Volts)
|
Sample |
A |
B |
C |
D |
|
n Data |
100 |
100 |
100 |
100 |
|
Average |
0.01036 |
0.00723 |
0.00143 |
0.00153 |
|
Standard
Deviation |
0.00147 |
0.00324 |
0.00007 |
0.00026 |
The dimensions of each
tested sample were measured using a ruler to measure length and width and using
the CHY-CA thickness measurement instrument to measure the thickness of the
sample. The dimensions of the electrode samples can be seen in Table 4.
Table 4 Fabric electrode
sample size dimension (units in meters)
|
Sample |
A |
B |
C |
D |
|
Thickness |
0.00018 |
0.00017 |
0.00017 |
0.00017 |
|
Length |
0.04 |
0.04 |
0.04 |
0.041 |
|
Width |
0.02 |
0.02 |
0.02 |
0.02 |
The electrode resistance could be
calculated using the formula 1 below:
(1)
R is the sample resistance (?), V is the
read voltage (Volt), and I is the electric current (0.5 mA). CF1, CF2, and CF3
were the correction factor values due to the influence of sample size, sample
thickness, and temperature, respectively, when FPP measurement was performed.
So that each has a value of CF1 = 3.2246; CF2 = 1,000; and CF3 = 1.0410. The
sample's electrical conductivity value is obtained using equation 2 below.
(2)
Where ? is the electrical
conductivity of the electrode, p is the resistivity of the electrode, Rs is the
resistance of the electrode, and t is the thickness of the electrode. Each
sample was tested for conductivity. The result is tabulated in Table 5.
Table 5 The flexible
electrode's electricalconductivity (?) and resistivity (?) table
|
Sample |
A |
B |
C |
D |
|
Resistance
(?) |
48.5 |
69.6 |
9.6 |
10.2 |
|
Conductivity
(S/m) |
119.2 |
113.2 |
614.4 |
577.7 |
Based on the test results,
sample C showed the highest conductivity value compared to other variations,
which was 614.41 S/m. The number and
uniform distribution of conductive molecules across the electrodes, such as
polyaniline and silver nanoparticles, increased.
3.3. EMG Measurement of
Flexible Electrode
Signal-to-Noise Ratio
(SNR) can be calculated using formula 3:
(3)
Where SNR is the signal-to-ratio, S is the RMS voltage of the measured
EMG signal, and N is the RMS voltage of the measured noise signal. EMG
measurements were carried out only on the electrode samples with the best
conductivity. In this case, the electrodes added with PANI, samples C and D,
gave the highest conductivity values. The EMG measurement of samples C and D
are tabulated in Table 6.
Table 6 EMG measurement
table of AgNP PANI electrode
|
Sample |
C |
D |
|
SNR (dB) |
24.4 |
15.8 |
The results of the EMG measurements show a correlation between the conductivity values of the electrodes and the obtained SNR values. The SNR value indicates that the noise signal does not interfere with the EMG measurement process. The higher the SNR value, the smaller the effect of the noise signal. Based on the results that have been processed, sample C shows the highest SNR value compared to the other sample. The SNR value obtained from sample C is 24.4 dB, while sample D shows 15.8 dB. The biopotential signal is shown in Figure 8.
Figure 8 EMG
measurement signal of (a) sample C [AgNP Gum Acacia + PANI] (b) sample D [AgNP
Hydrazine Hydrate + PANI]
After getting the SNR results from fabric-based flexible electrode
samples C and D, a comparative study of electrode performance was carried out
to compare the performance of the electrodes produced by this research with the
results of other researchers. The results of the comparative study can be seen
in Table 7 below.
Based on the comparative study above, fabric-based flexible electrodes made of AgNP PANI with acacia gum as a reducing agent have a higher SNR value than commercial electrodes, which is 24.4 dB. While commercial electrodes have an SNR value of 21.2 dB Therefore, the performance of AgNP PANI is better than the common electrodes.
Table 7 Comparative electrode performance study for body biopotential signals
measurement
|
No |
Material |
Substrate |
Type |
SNR (dB) |
References |
|
1 |
MWCNTs/PDMS composites on Ag |
TPU on fabric |
Dry |
23.1 |
|
|
2 |
Laser Induced Graphene |
PDMS |
Dry |
32 |
|
|
3 |
Ag/AgCl |
Parylene C |
Dry |
23.8 |
|
|
4 |
Ag/AgCl |
Commercial |
Wet |
21.2 |
(Masihi, et al.,
2021) |
|
5 |
AgNP PANI with Gum Acacia |
Cotton fabric |
Dry |
24.4 |
This Study |
|
6 |
AgNP PANI with Hydrazine
Hydrate |
Cotton fabric |
Dry |
15.8 |
This Study |
The
conclusion that can be drawn from this research is that the combination of AgNP
and PANI in cotton flexible electrodes can perform quite well for measuring
biopotential muscle signals. The flexible electrode has the advantage of good
electrode flexibility and conductivity; therefore, it does not need to use an
electrolyte gel to provide good skin-to-electrode contact. These advantages can
solve problems that often occur when using common Ag/AgCl commercial
electrodes, such, as high noise signals, uncomfortable usage, and skin
allergies. AgNP PANI flexible electrode with Gum Acacia as a reducing agent has
the highest conductivity and SNR value among other variations, which is 614.41
S/m, and an SNR value of 24.4 dB. Future work is focused on improving the
attachment of AgNP and PANI to the cotton fabric and the sustainability of
flexible electrodes.
Authors
express their gratitude to PPMI Engineering Physics ITB 2020 Project for
financial support.
Abu-Thabit, N.Y., 2016. Chemical Oxidative Polymerization of
Polyaniline: A Practical Approach for Preparation of Smart Conductive Textiles.
Journal of Chemical Education, Volume 93(9), pp. 1606–1611
Ahn, S., Han, T-H., Maleski, K., Song, J., Kim, Y-H., Park, M-H., Zhou, H., Yoo, S., Gogotsi, Y., Lee, T-W., 2020. A 2D Titanium Carbide MXene Flexible
Electrode for High?Efficiency Light-Emitting Diodes. Advanced Materials, Volume 32(23), p. 2000919
Chlaihawi, A. A. et al., 2018. Development of Printed and Flexible Dry ECG
Electrodes. Sensing and Bio-Sensing Research, Volume 20, pp. 9–15
Dong, C., Zhang, X., Cai, H., Cao, C., 2014. Facile and One-Step Synthesis of Monodisperse Silver Nanoparticles Using Gum Acacia in Aqueous Solution. Journal of
Molecular Liquids, Volume 196, pp. 135–141
Fu, X., Li, T., Qi, F., Zhang, S.,
Wen, J., Shu, W., Luo, P., Zhang, R., Hu, S., Liu Q., 2020. Designing High Electrochemical Surface
Area Between Polyaniline and Hydrogel Polymer Electrolyte for Flexible
Supercapacitors. Applied Surface Science, Volume 507, p. 145135
Gicevicius, M., Celiesiute, R., Kucinski, J., Ramanaviciene, A., Bagdziunas, G., Ramanavicius, A., 2018. Analytical Evaluation of
Optical Ph-Sensitivity of Polyaniline Layer Electrochemically Deposited on ITO
Electrode. Journal of The Electrochemical Society, Volume 165(14), pp. H903–H907
Khumaeni, A., Sutanto, H., Budi, W.S., 2019. The
Role of Laser Irradiance, Pulse Repetition Rate, And Liquid Media in The
Synthesis of Gold Nanoparticles by The Laser Ablation Method Using an Nd: YAG
Laser 1064 Nm at Low Energy. International Journal of Technology, Volume 10(5), pp. 961–969
Masihi, S., Panahi, M., Maddipatla, D., Hanson, A.J., Fenech, S., Bonek, L., Sapoznik, N., 2021. Development of a Flexible Wireless ECG
Monitoring Device with Dry Fabric Electrodes for Wearable Applications. IEEE
Sensors Journal, Volume 22(12), pp. 11223–11232
Monier, M., Akl, M. A., Ali, W.M., 2014. Modification and Characterization of Cellulose Cotton Fibers for Fast Extraction
of Some Precious Metal Ions. International Journal of Biological
Macromolecules, Volume 66, pp. 125–134
Peng, H.L., Liu, J.Q., Dong, Y.Z., Yang, B., Chen, X., Yang, C.S.,
2016. Parylene-Based Flexible Dry Electrode for Bioptential Recording. Sensors
and Actuators B: Chemical, Volume 231, pp. 1–11
Penhaker, M., Polomik, J., Kubicek, J. Kasik, V., 2017.
Biopotential Conducting Polymer Electrodes Design and Realization for ECG Measurement. In: 10th International
Conference on Biomedical Electronics and Devices
Puspasari, M.A., Iridiastadi, H., Sutalaksana, I.Z., Sjafruddin,
A., 2017. Effect of Driving
Duration on EEG Fluctuations. International Journal of Technology, Volume 8(6), pp. 1089–1096
Rosman, N.S.R., Masimen, M.A.A., Harun, N.A., Idris, I., Ismail,
W.I.W., 2021. Biogenic Silver Nanoparticles (AgNPs) from Marphysa Moribidii
Extract: Optimization of Synthesis Parameters. International Journal of
Technology, Volume 12(3), pp. 635–648
Saude, M.A. Morshed, B.I., 2016. Polypyrrole (PPy) Conductive
Polymer Coating of Dry Patterned Vertical CNT (pvCNT) Electrode to Improve
Mechanical Stability. In: 2016 IEEE Topical Conference on Biomedical
Wireless Technologies, Networks, and Sensing Systems (BioWireleSS)
Tseghai, G.B., Melengier, B., Fante, K.A. Langenhove, L.V., 2020.
The Status of Textile-Based Dry EEG Electrodes. AUTEX Research Journal, Volume 21(1), pp. 63–70
Wang, C., Yang, Y., Li, R., Wu, D., Qin, Y., Kong, Y., 2020. Polyaniline Functionalized Reduced
Graphene Oxide/Carbon Nanotube Ternary Nanocomposite as a Supercapacitor Electrode. Chemical
Communications, Volume 56(28), pp. 4003–4006
Yang, J., Zhang, K., Yu, J., Zhang, S., He, L., Wu, S., Deng, Y.,
2021. Facile Fabrication of Robust and Reusable Pdms Supported Graphene Dry
Electrodes for Wearable Electrocardiogram Monitoring. Advanced Materials
Technologies, Volume 6(9), p. 2100262
Yokus, M.A. Jur, J.S., 2016. Fabric-Based Wearable Dry Electrodes
for Body Surface Biopotential Recording. IEEE Transactions on Biomedical
Engineering, pp. Volume 63(2), pp. 423–430
