Adhesives Type on the Burning Rate and Emission Properties of Honeycomb Sandwich Composite
Corresponding email: arsubmt@unud.ac.id
Published at : 10 Jul 2024
Volume : IJtech
Vol 15, No 4 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i4.5728
Adhi, I.G.A.K.C., Atmika, K.A., Dwidiani, N.M., Subagia, I.D.G.A., 2024. Adhesives Type on the Burning Rate and Emission Properties of Honeycomb Sandwich Composite. International Journal of Technology. Volume 15(4), pp. 999-1011
| I Gusti Agung Ketut Catur Adhi | Department of Mechanical Engineering, Mataram University, Nusa Tenggara Barat, 83121, Indonesia |
| Ketut Adi Atmika | 1. Mechanical Engineering Study Program, Udayana University, Bukit Jimbaran 80361, Badung-Bali, Indonesia 2. Technology and Material Research Centre, the Institution of Research and Community Servic |
| Ni Made Dwidiani | Mechanical Engineering Study Program, Udayana University, Bukit Jimbaran 80361, Badung-Bali, |
| I Dewa Gede Ary Subagia | 1. Mechanical Engineering Study Program, Udayana University, Bukit Jimbaran 80361, Badung-Bali, Indonesia 2. Materials Engineering Laboratory, Engineering Faculty, Udayana University, Bukit Jimbaran |
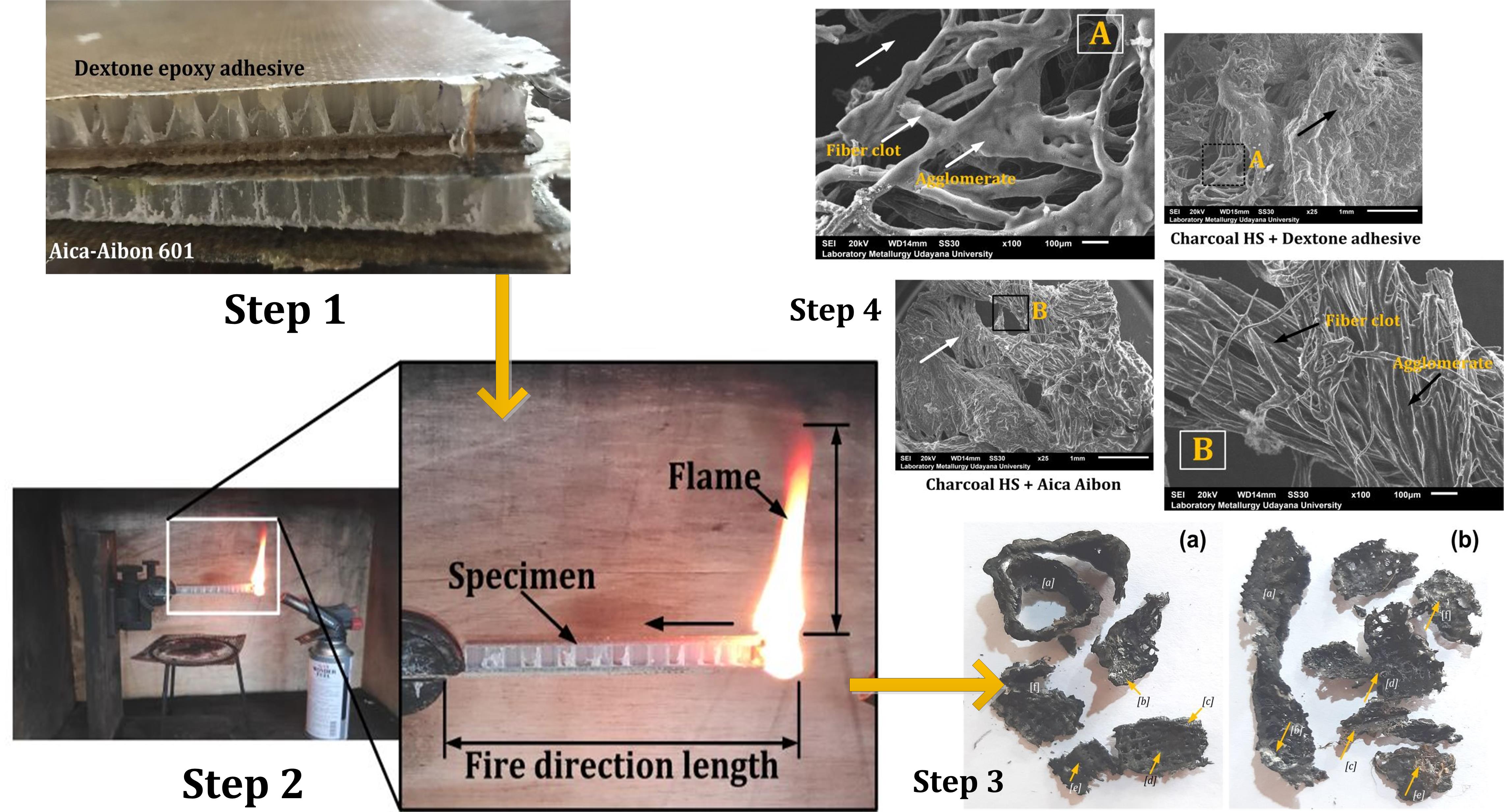
Sandwich composite is an important part of engineering products capable
of replacing metallic composite. It consists of two types of material, namely
polypropylene honeycomb core and skin made from jute fiber-reinforced epoxy
composite (JFRP), which are joined with glue. This study presented a unique
discussion about adhesives that focused on the burning rate and emission
performance of SA-A and SD-E. The burning rate performance was
assessed with the UL-94 HB test in accordance with the ASTM D 635 standard.
Emission value of both adhesives was also examined in line with the ASTM D 2863
standard using a Gasboard-3100P Syngas analyzer. In addition, FTIR and SEM
analyses were used to determine the characteristics of the SA-A and
SD-E adhesives. The results showed a significant difference in
adhesives rates, with SA-A burning 0.5% faster than SD-E
in addition to a 0.58% reduced weight loss. Emission test confirmed that both
adhesives have similar LOI values of 22.6% and 22.8%, respectively. SA-A
adhesives contained LSD, which is dangerous to human health. In conclusion, SD-E
adhesives should be used on sandwich composite due to its epoxy-based potential
as a flame retardant because SA-A adhesives has more potential to
trigger firing due to the fuel content.
Adhesives; Burning rate; Emission; Fire; Sandwich composite
Sandwich composite structures are widely used in manufacturing
engineering products, and it consist of three components, namely skin, core and
adhesives (Jeevi, Nayak, and Abdul-Kader, 2019;
Novotný, Doubrava, and Ružicka, 2017). The thick
core is covered by a pair of thin skins (Wei et al., 2020). Sandwich
composite structures are characterized by the lightweight nature, production
ease, strong mechanical properties and are highly used in manufacturing
airplanes, automobiles, ships, and packaging, including electrical insulators,
energy absorption, and other industrial purposes (Rupani, Acharya, and Jani,
2017).
Failure caused by fire has become increasingly relevant in recent years (Suwondo et al., 2021), despite the numerous benefits of composite materials, one significant disadvantage is the highly inflammable nature (Zhu et al., 2020). Fire performance was determined based on factors such as ignition, self-extinguishing ability, flame spread, burn through, heat release, smoke obscuration, toxicity, and related scenarios. Therefore, the process of assessing the resistance of composite materials is needed to examine the ability to perform the intended load-bearing functions when exposed to fire. Several studies have extensively examined fire performance of composite materials (Ortega et al., 2020; Hörold et al., 2017; Suoware, Ezema, and Edelugo, 2017; Salmeia et al. 2016; Bar, Alagirusamy, and Das, 2015; Szolnoki et al., 2015). However, the most widely accepted study focused on an experiment conducted by incorporating fire retardant into the polymer matrix. The aim was to suppress heat release, increased temperature, and gas emission, perceived as toxic sources, through the solid and gaseous phase mechanism (Ogabi et al., 2021; Kim, Dutta, and Bhattacharyya, 2018).
These properties need to be considered
when studying the combustibility of honeycomb-based composite materials. According
to Kim, Dutta, and Bhattacharyya (2018), several
factors such as the chemical composition, physical features, load-bearing
capacity of adhesives, surface condition of the joints, and the use of
materials that do not adhere to health standards significantly affect the
toxicity of emitted smoke (Jeevi, Nayak, and Abdul-Kader, 2019; Ledesma et al., 2018). Preliminary
studies stated that synthetic adhesives, such as structural silicone, or
stiffer substances namely acrylic or epoxy are commonly used for assembling
composite materials (Shang et
al., 2020; Valente et al., 2019; Ledesma et al., 2018;
Machalická and Eliášová, 2017). However,
thermosetting types such as phenol-formaldehyde (PF), urea-formaldehyde (UF),
and polyurethane (PU) are currently been used due to the water-resistant
properties (Chanda, Kim, and Bhattacharyya, 2022; Shavandi and Ali, 2018). The
application of adhesives in non-metallic structural materials has numerous
benefits, including uniform stress distribution, eliminating the need for
drilling, and enabling the bonding of substances with varying mechanical and
thermal properties. Sugiman and Sulardjaka (2016), stated that
adhesives play a critical role in the bonding of materials. Arenas, Narbón, and Alía (2010), stated that joint strength
is inversely proportional to the thickness of adhesives, meaning the shear
strength increases as the thickness of adhesives decreases. Davies et al. (2009), examined the physical,
chemical, and mechanical properties of Aluminium substrates bonded with epoxy
adhesives of varying thicknesses ranging from 0.2 to 1.3 mm. However, there is
no proof that varying thickness can weaken composite materials (Kostin, Nasonov, and Zinin, 2021;
Momber, Fröck, and Marquardt, 2021; Shang et al. 2020; Jeevi, Nayak, and
Abdul-Kader, 2019; Shavandi and Ali, 2018).
Adhesives are an essential aspect of
sandwich composite structures that bond the core and skin. Generally, the
liquid types such as SA-A and SD-E are commonly used due
to ease of application. Aica-Aibon (SA-A), a kind of poly-chloroprene-based
glue with toluene characteristics (C7H8) (Tualeka et al., 2019; Djurendic-Brenesel, Stojiljkovic,
and Pilija, 2016), contains lysergic acid
diethylamide (LSD) with the chemical formula C20H25N3O.
LSD is a synthetic narcotic drug that causes mental disorders when consumed
excessively. Liao et al. (2020) stated that when SA-A
is burned, it tends to have minimal impact on the surrounding environment.
Meanwhile, Dextone-Epoxy (SD-E) is an
epoxy-based adhesive dependent on epoxy resin and one of the most essential
polymer classes due to the multiple binding capacities provided by the oxirane
ring. Due to the significant differences in the source materials, studying the
flame and emission of these adhesives becomes interesting. This study mainly focused on examining the impact of flammability and emission on
adhesives used in sandwich composite. Furthermore, both adhesives were tested
concerning the application process in honeycomb composite to evaluate burning
rate and emission. The aim is to investigate the potential roles as triggers
for fuel fire and the resulting emission. The tests were carried out based on
ASTM D 635 and 2863 standards to determine both adhesives burning rate and
emission.
2.1.
Materials
Sandwich composite panel comprised three
main parts, namely skins, adhesives, and honeycomb core. A typical example is
the natural jute fiber, produced by Casthanal Textile CIA in Brazil, shown in
Figure 1 (Gupta, Srivastava, and Bisaria, 2015).
The fiber has relatively low conductivity, ranging from 0.29 to 0.32/mK, as
well as composite matrix materials, namely Bakelite Korea epoxy resin
Bakelite-EPR-174 and Justus Kimia-Raya cycloaliphatic amine curing agent
Bakelite-EPH-555, shown in Tables 1 and 2.
Polypropylene honeycomb (PPH),
particularly a Nomex 8 mm mesh, was used as the core of composite sandwich.
This material has a low density and good performance function as a shear
load-carrying core in sandwich composite construction. PPH is a hexagonal
structure with equal sides and six interior angles of the same dimension
measuring 1200, as shown in Figure 2.
Figure 2 Geometry and
cross-section of honeycomb
After
SD-E is an epoxy-based adhesive containing epichlorohydrin
bisphenol-A (DGEBA) in the formulation. Epoxy resin is characterized by
low-molecular-weight comprised of oxirane or epoxide rings as functional
groups, imparting thermosetting properties. This makes epoxy resin a commonly and widely used material in various applications,
including adhesives, coatings, semiconductor packaging, and composite matrices.
The specific SD-E adhesive characteristics
are shown in both Tables 2 and 3.
2.2.
Sandwich composite structures
Sandwich composite panel used in the experiment was 350 mm x 350 mm in
size. A panel with three layers of 2 mm thick jute fiber reinforcement was used
as the skin of composite. In addition, the panel was manufactured using the
vacuum injection process (VaRTM), with the core bonded to both skins using two
different types of synthetic adhesives. Figure 3 and Table 4 show sandwich
composite and specimen structures using both SA-A and SD-E
adhesives, respectively.
Figure
4 shows the geometry of the sample and burning test schema following the ASTM D
635 standards. The specimen used was composed of three segments measuring 125
mm in length. The first segment, positioned 25 mm from the free end, was the
initial burning area. The subsequent and final ones, which served as burning
test and clamping areas, were 75 mm and 25 mm long, respectively.
2.3. Analytical
Method
where LOI is the limiting oxygen index (%) (Parmar et al. 2014), O2 and N2 are
denoted as oxygen and nitrogen. L is the distance between two marking lines (75 mm), t is fire
spreading time (minutes), FP is the flame propagation (mm/second), Dp
is the propagation distance (mm), Pt is fire propagation time
(second), and Lt is burning time (second).
where W is the total weight loss after burning (gram/sec), w
is the weight loss of the specimen during the burning process, w0
is the initial weight (gram), w1 is the final weight (gram),
and t is the burning time (second).
2.4. FT-IR spectroscopic analysis
The characteristics of adhesives in
sandwich composite were measured using Fourier transform infrared spectroscopy
(FTIR) spectra obtained with a Shimadzu spectrometer. The signal resolution of
the FTIR was 1 cm-1, and a minimum of 16 scans were obtained
and averaged within the range of 500 to 4000 cm-1.
2.5. Scanning
electron microscopy (SEM)
The residue
from the horizontal burning test on sandwich composite was subjected to SEM
analysis using a JSM-6510 from Japan. In addition, the residue surface was
coated with gold before being tested.
3.1. FTIR analysis
Figure 5 FT-IR spectra pattern adhesives of SA-A
and SD-E
Analysis of the
FTIR spectra comparing the SA-A (red line) and SD-E adhesives
(blue line) shows significant differences. The O-H stretching region in the
absorbance range of 2500 cm-1 to 3000 cm-1 showed there
was a peak shift of 2936 cm-1 and 2924 cm-1 for SD
–E and SA-A. However, within an absorbance range of 2000 cm-1
to 2500 cm-1, SD-E had increased energy compared to SA-A,
depicting a greater tendency for bond breaking, thereby contributing to
enhanced stability and reduced flammability. The characteristic peaks for C-O-C
ether and aromatic C-C stretching were observed at 1036 cm-1 and
1509 cm-1, respectively. The stretching C=C in the aromatic ring was
observed at 1608 cm-1, while C-H bending bands occurred within the
absorbance range of 500 cm-1 to 1000 cm-1.
3.1. Burning
Rate
The
recent focus on building fire has proven the importance of understanding the
material types used (Suwondo et
al., 2021). Building materials and equipment types have
been identified as the main factors contributing to the frequency of these fire
(Nugroho, Latief, and Wibowo, 2022). The
combustibility of a material depends on various factors related to the
constituents, which impact characteristics such as heat release, flame spread,
and ignitability (Pausas, Keeley, and Schwilk, 2017). In addition, the presence of adhesives used to bond the skin and core
of sandwich composite was considered fire reaction properties, as shown in
Table 5.
Figure 6 Comparison of adhesives
on burn test and weight loss of honeycomb composite
Figure 6 shows a bar graph of burning
rate and weight-loss combination of sandwich composite using SA-A
and SD-E adhesives. The use of dark colour shows there is a
significant difference in burning rate between sandwich composite using SA-A
and SD-E. This was caused by the different chemical compound
properties of both adhesives, as shown in Figure 5. SA-A is an
elastomer-based adhesive that can function as thermoplastic and thermosetting
types, depending on the required cross-linked structure. It is also
characterized by rapid
adhesion and belongs to a group of elastomers with rubber-based adhesives,
including butyl, butadiene, styrene-butadiene, and nitrile rubber, as well as
silicone, and neoprene (Wang et al., 2022). However, adhesiveness and cohesion levels are
limited at temperatures greater than 70°C, and a stabilizer is needed to
withstand environmental effects such as UV and ozone. Adhesives can be dried at
normal temperatures and has good heat, water, and chemical resistance,
depending on the contents of the hardening compounds. SA-A adhesives
characterized based on FTIR analysis, showed features of a rubber-based
composition,
for example, C=C aromatic
bonds observed at a wavenumber of 1656.92 cm-1. Meanwhile, the
aliphatic C-H or C-C hydrocarbon groups that trigger the flame were observed in
the wavenumber range of 2000 cm-1 to 2500 cm-1 with a
prominent peak at 2358.08 cm-1 and an intensity of 79.64 mm. SD-E adhesives are epoxy-based, mainly
synthesized from active hydrogen reactions in phenols, alcohols, amines, and
acids with epichlorohydrin under well-controlled conditions. Generally, SD-E
adhesives were prepared by packing epoxy and curing agent composition
separately before use, with curing occurring briefly after mixing in accordance
with the mixing ratio. Epoxy adhesives such as SD-E often have a higher
glass transition temperature, making it suitable for applications requiring
high-temperature resistance. In FTIR analysis, the epoxy tends to react with amines and amides
(NH) at frequencies ranging from 3140 to 3320 cm-1. The deformation
band of C-O occurred at 828.46 cm-1 within the wavenumber range of 750 cm-1
to 1000 cm-1. In the spectrum range, characteristics of the aromatic
ring were observed, double chain, benzene and C-C aromatics, C-O-C chain from
the ether, C-O chain of oxirane group, and CH2 chain were formed at
wavenumbers of 1606 cm-1, 1506 cm-1, 1031 cm-1,
913 cm-1, 862 cm-1 and 769 cm,-1 respectively.
The stretching of hydroxyl groups of O-H was depicted by broadband at 3500 cm-1,
suggesting the presence of species and dimers of high molecular weight. In
addition, a band corresponding to the ether linkage was observed within the
1000 to 1100 cm-1 spectrum range.
The average combustion
length of the SA-A and SD-E adhesives was 75 mm and 72
mm, respectively. The results showed the carbon composition of the SA-A adhesives
possessed higher fuel propagation capacity compared to the SD-E,
which had greater fire resistance. This result is in line with the study conducted by Chanda, Kim, and
Bhattacharyya (2022), focusing on the significant influence of adhesive
formulation materials on fuel value. The burning rate of each sandwich
composite sample using the SA-A adhesives was 0.5% faster than the SD-E.
The difference was caused by certain thermos-physical properties, such as the
reaction rate depending on the temperature threshold or higher amount of oxygen
(O2) during the combustion process, leading to increased heat energy
(Ogabi et al., 2021). Table 5 shows the burning time of the two
adhesives used.
In Figure 6, the result of
the weight loss measures in the bar graph is shown in grey. The result is
similar to the burning rate, and the weight loss of SA-A adhesive
samples has a higher value than SD-E, with a significant difference
of 0.58%. This difference directly correlates with the degradation of the
samples caused by increased heat and smoke production. The higher weight loss
and low percentages of mass residues observed in the SA-A samples
were attributed to the pyrolysis and burning phases, depicting a prolonged
exposure to heat flux. These results are in line with previous studies (Vinod et al., 2022).
3.2. Burning Emission
Table 6 shows emission from burning
sandwich composite, which were tested to determine the environmental effects of
burning adhesives. When burned, adhesives in composite emits carbon dioxide (CO2)
and carbon monoxide (CO) as combustion products, which is proven by the results
of the FTIR test shown in Figure 5. However, both SA-A and SD-E
adhesives generated the same quantity of oxygen, measured at 20.9 mg/m3.
The NO2 produced by the SA-A adhesives is slightly
greater than SD-E by 0.01%, the two materials have similar LOI
values of approximately 22.6% and 22.8%, respectively. The difference in
burning behavior was caused by the higher carbon content in SD-E,
which formed a denser carbon layer, preventing heat transfer during the burning
process. There is a definite relationship between burning rate and oxygen
content, with a threshold of 21%. Materials with an LOI less than this
threshold are typically regarded as flammable or combustible and would burn
readily in an open-air setting, while a higher index showed lower flammability (Raajeshkrishna and Chandramohan, 2020). When the oxygen content drops below the critical LOI values, the
material ceases to burn because the available oxygen is insufficient for
combustion. However, a high LOI value, minimizes burning potential and oxygen
concentration, in order to sustain combustion (Misnon et al., 2018). LOI is a distinguishing characteristic of materials, often used to rank
the relative flammability of polymer composite materials (Raajeshkrishna and Chandramohan, 2020;
Chukwunwike et al., 2019; Bhattacharyya and Kim, 2017).
3.3.
Burning residue
Figures 7a and b show the charcoal from sandwich composite with SA-A
and SD-E adhesives, respectively. The characteristics of the
charcoals depend on the quantity of lignin and cellulose content in the jute
fiber and properties of the polypropylene in the core. Furthermore, Charcoal
produced from poly-propylene is more reflective and contains the following
compounds CO, CO2, HC2, NO2, and CxHx.
The elements produced from combustion are also described in the spectral range
of the FTIR test for each adhesive applied in the bonding of sandwich
composite. This implied that the chemical breakdown of each adhesive sample
contributed to the formation of oxides and other degradation products, forming
the crosslinking network that produced the coal. Meanwhile, the LSD element
contained in the SA-A adhesives was in the form of CxHx,
which is harmful when inhaled.
Figure 8 Photography SEM adhesive residue after the burning process. a) SEM SD-E
adhesives, b) SEM SA-A adhesives
In
conclusion, adhesives were critical in sandwich composite structures, directly
impacting the burning rate. To address the flammability of composite, it was
necessary to incorporate materials or substances capable of inhibiting the
burning rate. Synthetic adhesives such as SA-A and SD-E were
used to bond the skin to the core of sandwich composite. The results showed
that sandwich composite's burning rate using SA-A adhesives was 0.5%
faster, with a weight loss of 0.58% greater than SD-E. Emission test
results for both samples had a comparatively similar LOI value of 22.6% and
22.8%, respectively. Therefore, adhesives significantly impacted burning rate
and composite emission, depending on the presence of flammable substances
contained in the constituents. The
SA-A adhesives posed a significant risk when burned due to its
aromatic nature and the presence of LSD elements, which could be harmful when
inhaled. However, SD-E adhesives only showed the aromatic properties
when burned. The use of SA-A adhesives on sandwich composite
materials posed a greater risk compared to SD-E. This was due to the
potential of SA-A adhesives to ignite more intense fire attributed
to the contained benzene. So, it was suggested that using SA-A
adhesives in materials subjected to high temperatures and susceptible to
flammability be avoided.
The authors are grateful to the Institution of Research and
Community Service for their financial support. The authors are also grateful to
the Udayana University and the Indonesian Ministry of Education, Culture, and
Research for the grant under contract number B/136-8/UN14.4.A/PT.01.05/2021.
| Filename | Description |
|---|---|
| R3-MME-5728-20230817232633.jpg | Replace Figure 5 |
Arenas, J.M., Narbón, J.J., Alía, C., 2010.
Optimum Adhesive Thickness in Structural Adhesive Joints Using Statistical
Techniques Based on Weibull Distribution. International Journal of Adhesion
and Adhesives, Volume 30(3), pp. 160–165.
Bar, M., Alagirusamy, R., Das, A., 2015.
Flame Retardant Polymer Composites. Fibers and Polymers, Volume 16(4),
pp. 705–717
Bhattacharyya, D., Kim, N.K., 2017.
Flammability of Natural Fiber-Reinforced Polymeric Composites. In: ICCM
International Conferences on Composite Materials, Volume 2017, pp. 1–9
Chanda, A., Kim, N.K., Bhattacharyya, D.,
2022. Effects of Adhesive Systems On The Mechanical and Fire-Reaction
Properties of Wood Veneer Laminates. Composites Science and Technology,
Volume 230, p. 109331
Chukwunwike, S.A., Ike-Eze, I.E., Aigbodion,
V.S., Okafor, K.J., 2019. Flammability
Properties of Flame Retarded Natural Fiber Reinforced Polymer Composites: An
Overview. Journal of Materials and Environmental Sciences, Volume 10(7),
pp. 647–656
Davies, P., Sohier, L., Cognard, J.Y.,
Bourmaud, A., Choqueuse, D., Rinnert, E., Créac’hcadec, R., 2009. Influence of
Adhesive Bond Line Thickness on Joint Strength. International Journal of
Adhesion and Adhesives, Volume 29(7), pp. 724–736
Djurendic-Brenesel, M., Stojiljkovic, G.,
Pilija, V., 2016. Fatal Intoxication with Toluene Due to Inhalation of Glue. Journal
of Forensic Sciences, Volume 61, pp. 875–878
Gupta, M.K., Srivastava,
R.K., Bisaria, H., 2015. Potential of Jute Fibre
Reinforced Polymer Composites?: A Review. International Journal of Fiber and
Textile and Research, Volume 5(3), pp. 30–38
Hörold, A., Schartel, B., Trappe, V., Korzen,
M., Bünker, J., 2017. Fire Stability Of Glass-Fibre Sandwich Panels: The
Influence of Core Materials And Flame Retardants. Composite Structures,
Volume 160, pp. 1310–1318
Jeevi, G., Nayak, S.K., Abdul-Kader, M.,
2019. Review on Adhesive Joints and Their Application In Hybrid Composite
Structures. Journal of Adhesion Science and Technology, Volume 33(14),
pp. 1497–1520
Kim, N.K., Dutta, S., Bhattacharyya, D.,
2018. A review of Flammability of Natural Fibre Reinforced Polymeric
Composites. Composites Science and Technology, Volume 162, pp. 64–78
Kostin, V., Nasonov, F., Zinin, A., 2021.
Influence of Adhesive Bond Line Thickness on Joint Strength of Composite
Aircraft Structures. Journal of Physics: Conference Series, Volume
1925(1), pp. 724–736.
Ledesma, R., Yost, W., Palmieri, F., Cataldo,
D., Connell, J., 2018. Correlation of Optically Stimulated Electron Emission
with Failure Mode of Adhesively Bonded Epoxy Composites. International
Journal of Adhesion and Adhesives, Volume 84, pp. 257–264
Lee, C. H., Salit, M.S., Hassan, M.R., 2014.
A Review of The Flammability Factors of Kenaf and Allied Fibre Reinforced
Polymer Composites. Advances in Materials Science and Engineering,
Volume 2014, p. 514036
Liao, B., Yang, L., Ju, X., Peng, Y., Gao,
Y., 2020. Experimental Study on Burning and Toxicity Hazards of a PET Laminated
Photovoltaic Panel. Solar Energy Materials and Solar Cells, Volume 206,
p. 110295
Machalická, K., Eliášová, M., 2017. Adhesive
Joints in Glass Structures: Effects of Various Materials in The Connection,
Thickness of The Adhesive Layer, and Aging. International Journal of
Adhesion and Adhesives, Volume 72, pp. 10–22
Misnon, M.I., Islam, M.M., Epaarachchi, J.A.,
Chen, H., Goda, K., Khan, M.T.I., 2018. Flammability Characteristics of
Chemical Treated Woven Hemp Fabric Reinforced Vinyl Ester Composites. Science
and Technology of Materials, Volume 30(3), pp. 174–188
Momber, A.W., Fröck, L., Marquardt, T., 2021.
Effects of Adhesive Type on The Mechanical Properties of Adhesive Joints
Between Polyurethane Top Coats and Polyurethane-Based Adhesives After
Accelerated Atmospheric Aging. Marine Structures, Volume 79, p. 103022
Novotný, C., Doubrava, K., Ružicka, M., 2017.
Testing Of Adhesive Joints In Sandwich Structures. Materials Today:
Proceedings, Volume 4(5), pp. 5898–5903
Nugroho, P.S., Latief, Y., Wibowo, W., 2022.
Structural Equation Modelling For Improving Fire Safety Reliability through
Enhancing Fire Safety Management on High-Rise Building. International
Journal of Technology, Volume 13(4), pp. 740–750
Ogabi, R., Manescau, B., Chetehouna, K.,
Gascoin, N., 2021. Composites and Their Gaseous Emission Assessment. Energy,
Volume 14, pp. 1–32
Ortega, R., Monzón, M.D., Ortega, Z.C.,
Cunningham, E., 2020. Study and Fire Test of Banana Fibre Reinforced Composites
with Flame Retardance Properties. Open Chemistry, Volume 18(1), pp.
275–286
Parmar, M.S., Singh, M., Tiwari,
R.K., Saran, S., 2014. Study on Flame Retardant Properties Of
Poly(Lactic Acid) Fiber Fabrics. Indian Journal of Fibre and Textile
Research, Volume 39(3), pp. 268–273
Pausas, J.G., Keeley, J.E., Schwilk, D.W.,
2017. Flammability as an Ecological and Evolutionary Driver. Journal of
Ecology, Volume 105(2), pp. 289–297
Raajeshkrishna, C.R., Chandramohan, P., 2020.
Effect of Reinforcements and Processing Method on Mechanical Properties of
Glass and Basalt Epoxy Composites. SN Applied Sciences, Volume 2 (5), p.
959
Rupani, S.V., Acharya, G., Jani,
S.S., 2017. Design, Modelling and Manufacturing aspects of Honeycomb
Sandwich Structures: A Review. International Journal of Scientific
Development and Research, Volume 2(4), pp. 526–532
Salmeia, K.A., Jovic, M., Ragaisiene,
A., Rukuiziene, Z., Milasius, R., Mikucioniene, D., Gaan, S., 2016. Flammability of Cellulose-Based Fibers and The Effect of Structure of
Phosphorus Compounds On Their Flame Retardancy. Polymers, Volume 8 (8),
p. 293
Shang, X., Marques, E.A.S., Carbas, R.J.C.,
Barbosa, A.Q., Jiang, D., da Silva, L.F.M., Chen, D., Ju, S., 2020. Fracture
Mechanism of Adhesive Single-Lap Joints with Composite Adherends Under
Quasi-Static Tension. Composite Structures, Volume 251, p. 112639
Shavandi, A., Ali, A., 2018. A New Adhesive
From Waste Wool Protein Hydrolysate. Journal of Environmental Chemical
Engineering, Volume 6(5), pp. 6700–6706
Sugiman, Sulardjaka, 2016. Water Absorption
and Desorption Behavior and Their Effect on The Tensile Properties of FM 73M
Adhesive Film. International Journal of Technology, Volume 7(3), pp.
438–446
Suoware, T.O., Ezema, I.C., Edelugo, S.O.,
2017. Flammability of Flame Retarded Natural Fibre Composites and Application
in Automobile Interior: A Review. Imperial Journal of Interdisciplinary
Research, Volume 3(8), pp. 2454–1362
Suwondo, R., Cunningham, L., Gillie, M.,
Suangga, M., Hidayat, I., 2021. Model Parameter Sensitivity for Structural
Analysis of Composite Slab Structures in Fire. International Journal of
Technology, Volume 12(2), pp. 339–348
Szolnoki, B., Bocz, K., Soti, P.L., Bodzay,
B., Zimonyi, E., Toldy, A., Morlin, B., Bujnowicz, K., Marosi, G., 2015.
Development of Natural Fibre Reinforced Flame Retarded Epoxy Resin Composites. Polymer
Degradation and Stability, Volume 119, pp. 68–76
Tualeka, A.R., Wibrata, D.A., Ilmi,
B., Ahsan, A., Rahmawati, P., 2019. Association Between
Toluene Inhalation Exposure and Demography Towards Risk of Neurotoxic: A
Cross-Sectional Study at Plastic Sack Industry Workers in Indonesia. Global
Journal of Health Science, Volume 11(2), p. 20
Valente, J.P.A., Campilho, R.D.S.G., Marques,
E.A.S., Machado, J.J.M., Da Silva, L.F.M., 2019. Adhesive Joint Analysis Under
Tensile Impact Loads By Cohesive Zone Modelling. Composite Structures,
Volume 222, p. 110894
Vinod, A., Tengsuthiwat, J., Gowda, Y.,
Vijay, R., Sanjay, M.R., Siengchin, S., Dhakal, H.N., 2022. Jute/Hemp Bio-Epoxy
Hybrid Bio-Composites: Influence of Stacking Sequence on Adhesion of
Fiber-Matrix. International Journal of Adhesion and Adhesives, Volume
113, p. 103050
Wang, W., Wang, D., Xia, B., Li, T., Wang,
Y., Zhang, X., Bai, H.,Chen, M., Dong, W., 2022. Rigid Polyurethane Foams Based
on Dextrin and Glycerol. Industrial Crops and Products, Volume 177, p.
114479
Wei, X., Xiong, J., Wang, J., Xu, W., 2020.
New Advances in Fiber-Reinforced Composite Honeycomb Materials. Science
China Technological Sciences, Volume 63(8), pp. 1348–1370
Zhu, C., Li, S., Li, J., Clement, M., Rudd,
C., Yi, X., Liu, X., 2020. Fie Performance of Sandwich Composites with
Intumescent Mat Protection: Evolving Thermal Insulation, Post-Fire Performance
and Rail Industry Testing. Fire Safety Journal, Volume 116, p. 103205
