The Simultaneously Removal of NOx and SO2 Processes through a Polysulfone Hollow Fiber Membrane Module
Corresponding email: sutrasno@che.ui.ac.id
Published at : 09 May 2023
Volume : IJtech
Vol 14, No 3 (2023)
DOI : https://doi.org/10.14716/ijtech.v14i3.5544
Kartohardjono, S., Karamah, E.F., Talenta, G.N., Ghazali, T.A., Lau, W.J., 2023. The Simultaneously Removal of NOx and SO2 Processes through a Polysulfone Hollow Fiber Membrane Module. International Journal of Technology. Volume 14(3), pp. 576-583
| Sutrasno Kartohardjono | Process Intensification Laboratory, Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok 16424, Indonesia |
| Eva Fathul Karamah | Process Intensification Laboratory, Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok 16424, Indonesia |
| Grace Nathalie Talenta | Process Intensification Laboratory, Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok 16424, Indonesia |
| Thariq Ahmad Ghazali | Process Intensification Laboratory, Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok 16424, Indonesia |
| Woei Jye Lau | Advance Membrane Technology Research Center, Universiti Teknologi Malaysia, 81310 Skudai, Johor, Malaysia |
Hazardous pollutants such as NOx (NO and NO2) and SO2
generally come from fossil fuel combustion, harm the human respiratory system,
and damage environmental ecosystems. The conventional technology that has been
used so far consists of two methods: FGD (Flue Gas Desulfurization) and SCR
(Selective Catalytic Reduction) or SNCR (Selective Non-Catalytic Reduction) to
remove SO2 and NOx. The study aims to examine the performance of
polysulfone membranes in removing NOx and SO2 simultaneously using
hydrogen peroxide (H2O2) and sodium hydroxide (NaOH)
solutions as absorbents. The presence of H2O2 and NaOH in
absorbent solutions plays a role in oxidizing NOx into soluble species in water
and in absorbing SO2 gas, respectively. During the experiment, the feed
gas flowed through the lumen fiber and then passed through the fiber to the
shell side of the membrane module, where the reaction happened between NOx and
SO2 and the absorbent. The experimental results showed that the
presence of SO2 affected the NOx reduction efficiency. The NOx and
SO2 removal efficiencies decreased with the feed gas flow. This study's maximum NOx and SO2
reduction efficiencies were 93.9 and 99.8%,
respectively.
Air pollution; H2O2; NOx; Removal efficiency; SO2
Air
pollution in Indonesia increases yearly due to growing public energy
consumption. Hazardous pollutants such as NOx (NO and NO2) and SO2
generally come from fossil combustion, harm the human respiratory system, and
damage environmental ecosystems (Manisalidis et
al., 2020; Wang, Wang, and Shammas, 2020; Sharma et al., 2013). Based
on a study on the emissions prediction from the coal-fired power plants in
Indonesia in 2016-2020, there was an exponential increase of 120.0 and 798.5
ktons of NOx and SO2, respectively, in that period (Sunarno, Purwanto, and Suryono, 2021). The
Indonesian Government's efforts to prevent air pollution nationally set Ambient
Air Quality Standards (BMUA) in Government Regulation No. 41 of 1999 (RI, 1999).
However, the NOx and SO2 emissions produced by the coal-fired power
plants in Indonesia are above the value of the BMUA, so efforts are needed to
reduce emissions in PLTUs made from burning coal. Reducing NOx and SO2
emissions requires two different technologies,
namely SO2
A previous study (Kartohardjono et al.,
2019; Kartohardjono et al., 2017) has shown that the HFMM (hollow
fiber membrane module) can be used as a bubble reactor to remove NOx using H2O2
and HNO3 solutions. The fibers’ role is to distribute the feed gas
into the solutions on the shell side of the HFMM so that reactions happen between
NOx and the absorbent. NO (nitrogen monoxide) in NOx is an insoluble gas in
water, so it needs to be oxidized to increase its solubility. One of the
solutions that can be used to oxidize NO is hydrogen peroxide (H2O2).
The H2O2 is superior as it is very stable under normal
conditions, environmentally friendly, does not leave harmful residues, and the
operating costs are pretty affordable. No conventional technology in the power
generation industry can reduce NOx and SO2 simultaneously (Park et al., 2019; Si et al., 2019).
In order to remove NOx and SO2 simultaneously, an absorbent that can
oxidize NOx into water-soluble species and an alkaline solution that can absorb
SO2 are required. This study utilized the polysulfone hollow fiber
membrane module to remove NOx and SO2 simultaneously using
absorbents consisting of H2O2 as an oxidant and sodium
hydroxide (NaOH) as a base solution. The polysulfone membrane module was chosen
because of its excellent stability over a wide pH range (2-13) and oxidant
resistance (Febriasari et al., 2021; Serbanescu, Voicu, and Thakur, 2021). Therefore, it can
be expected to see the effect of SO2 in the feed gas on NOx removal
compared to NOx removal alone. The NaOH solution absorbs the reaction products
between NOx gas and SO2 and H2O2. Reactions
(1-7) are reactions that may occur in the process of simultaneously removing
NOx and SO2 using a mixture of H2O2 and NaOH
as absorbents: (Kartohardjono et al., 2020;
Sun, Zwoli?ska,
and Chmielewski, 2016):
The hollow fiber membrane module used
contains 50 polysulfone fibers with a diameter of 3 cm and an effective length
of 25 cm, supplied from GDP Filter Bandung, Indonesia. The fibers are 1.8 and 2
mm in the inside and outside diameters, respectively. The feed gas, which
contained 600 ppm of NOx and 500 ppm of SO2 in nitrogen, was supplied from
PT EIN Jakarta, Indonesia. The chemicals used, H2O2 and NaOH, are analytical grades
Merck Indonesia supplies. The feed gas flowed inside the fiber in the membrane
module throughout the experiment. The flow rate was adjusted using a CX Series
mass flow controller. The feed gas diffused across the membrane pores to the
shell side of the HFMM and contacted absorbent solutions so that reactions
occurred between NOx, SO2, H2O2, and NaOH, as shown in Reaction
(1-8). The concentrations of NOx and SO2 gases entering and leaving the
HFMM were recorded by the Gas Analyzer ECOM-D. The schematic of the experiment
is presented in Figure 1.
The amount of absorbed NOx and SO2 gases, GasAbs,
removal efficiency, R, fluxes, J, and NOx and SO2 loading, Gas-loading,
can be calculated by Equations 8-11 (Kartohardjono et
al., 2020; Ding et al., 2014):
Where Xin and Xout, QG,in, T, P, and R are the concentration of gas inlet and outlet of the membrane module, feed gas flow rate, temperature, pressure, and gas constant, respectively.
Figure 1 The experimental diagram schematic
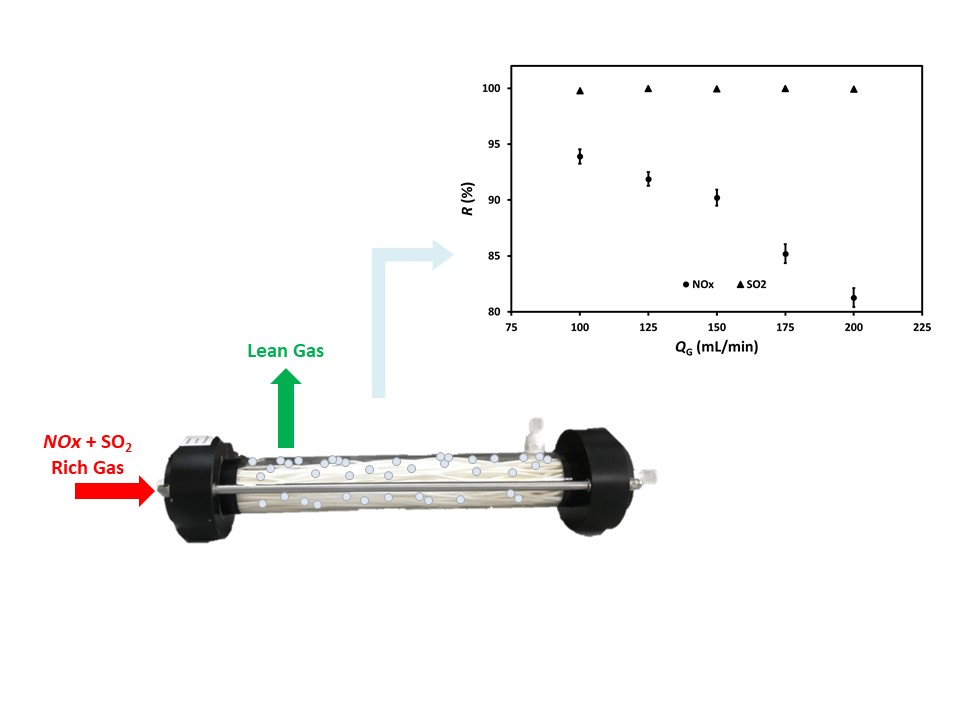
Figure 2 shows the effect of the feed gas flow rate, containing 500 ppm SO2 and 600 ppm NOx, on the simultaneous removal of SO2 and NOx in the HFMM, which contains 0.1M of H2O2 and 0.5 M NaOH each of 200 ml. As demonstrated in Figure 2, the NOx removal efficiency declines with increasing the feed gas flow rate due to the reduced gas residence time in the HFMM (Kartohardjono et al., 2019). Meanwhile, the SO2 removal efficiency is relatively constant to the feed gas flow rate changes because it is already close to 100%. The removal efficiency of SO2 depends not only on the oxidant (H2O2), as expressed in Eq. 6, but also mainly on the alkaline solution present in the adsorbent (NaOH) so that it can be removed entirely (removal efficiency » 100%) (Chen, Chen, and Chiang, 2020; Liu et al., 2019; Huang, Ding, and Zhong, 2015). The NOx absorption efficiency decreases from 93.9 to 81.3% by increasing the feed gas flow from 100 to 200 mL/min. The NOx removal was more complex than the SO2 removal, as the SO2 solubility in water was about 700 times higher than that of NO (Fang et al., 2011). A previous study showed a slight decrease in single NOx removal efficiency from 94.6 to 94.0% by increasing the same feed gas flow rate in a polysulfone HFMM containing 48 fibers using absorbents of H2O2 and HNO3 solutions. It reveals that the presence of SO2 in the feed gas decreases the efficiency of NOx removal due to the competition factor in consuming H2O2 as an oxidation agent, as expressed in Equations 5 and 6 (Chen, Chen, and Chiang, 2020; Kartohardjono et al., 2020).
Figure 2 The dependency of NOx and SO2 reduction
efficiencies, R, on the feed gas
flow, QG
The amount of absorbed NOx and SO2 and mass transfer flux, J, rise with the feed gas flow, as presented in Figure 3. The increase in the feed gas flow increases the number of gas molecules and resulting a higher concentration of the bulk gas. This condition creates a higher concentration driving force, bringing the higher absorbed NOx and SO2 and mass transfer flux (Liu et al., 2019). The absorbed NOx and SO2 rose from 3.8 to 6.6 x 10-5 mmol/s and 4.1 to 8.1 x 10-5 mmol/s, respectively, by increasing the feed gas flow from 100 to 200 mL/min. Meanwhile, the NOx and SO2 flux increased from 4.9 to 8.4 x 10-8 mmol/cm2.s and 5.2 x 10-8 to 1.0 x 10-7 mmol/cm2.s, respectively, when the feed gas flow rate was increased from 100 to 200 mL/min. A previous study exhibited a similar result: single NOx flux increased from 5.6 x 10-8 to 1.1 x 10-7 by doubling the feed gas flow from 100 to 200 mL/min in a polysulfone HFMM with 48 fibers containing absorbents of H2O2 and HNO3 solutions. It is also revealed that the existence of SO2 in the feed gas affects the transfer flux of NOx (Kartohardjono et al., 2019).
Figure 3 The
dependency of absorbed NOx and SO2, and mass transfer fluxes, J, on the feed gas flow, QG
As with flux, NOx and SO2 loading increases with the feed gas flow rate due to the increased amount of absorbed NOx and SO2. Figure 4 shows the dependency of NOx and SO2 loading on the feed gas flow. The NOx and SO2 loading increased from 0.0019 to 0.0033 mmol/mol.s and 0.0020 to 0.0041 mmol/mol.s, respectively, by doubling the feed gas flow from 100 to 200 mL/min. Similar results were also reported that the NOx removal increased from 0.002 to 0.004 mmol/mol.s by doubling the feed gas flow rate, containing NOx 600 ppm, in the PVDF HFMM containing 0.5 wt.% H2O2 and 0.5M HNO3 each of 25 ml (Purnawan et al., 2021).
Figure 4 The
absorbed NOx and SO2 loading dependency on the feed gas flow, QG
Figure 5 shows the absorption efficiency (%R) for NOx and SO2 as a function of H2O2 concentration. The efficiency of NOX removal increases with the increase in H2O2 concentration, while in SO2 gas, the efficiency is relatively constant with the addition of H2O2 concentration. The increase in the concentration of H2O2 causes an increase in the number of moles of O2 produced in the solution to oxidize NOx. The highest absorption efficiency achieved was 97.53% for NOx and 99.79% for SO2 at a 0.1 M H2O2 concentration. This study of simultaneous removal of mixed gases (NOx and SO2) resulted in a lower %R compared to the utilization of hollow fiber membranes on single-gas NOx by H2O2/HNO3 solvents in previous studies (Kartohardjono et al., 2019). The absorption efficiency of NOx gas is about 95% at 0.25% H2O2 by mass. In the same polysulfone membrane module and H2O2 solvent, it is seen that the absorption efficiency decreases between mixed gas (NOx and SO2) and single gas (NOx only) due to SO2 compounds competing with NOx in consuming the absorbent (i.e., H2O2 and NaOH).
Figure 5 The
dependency of NOx and SO2 reduction efficiencies, R, on the H2O2 concentration in the absorbent solutions at the feed gas flow rate of 0.1 L/min
Figure 6 shows the effect of the concentration of H2O2 on the amount of gas absorbed and the mass transfer flux at a feed gas flow rate of 0.1 L/min. The amount of SO2 gas absorbed and the mass transfer flux of SO2 was constant, at about 4.05 x 10-5 mmol/s and 8.98 x 10-8 mmol/cm2.s. Meanwhile, the amount of NOx absorbed and the mass transfer flux of NOx increased with the increase in the concentration of H2O2 in the absorbent solution. The amount of NOx absorbed and the mass transfer flux of NOx increased from 3.72 to 3.96 x 10-5 mmol/s and from 8.24 to 8.77 x 10-8 mmol/cm2.s. The increase in the amount of absorbed gas and flux is relatively small, so it can be categorized as the concentration of H2O2 does not have much effect on the amount of gas absorbed and the flux of NOx and SO2. The increasing concentration of H2O2 only affects the reaction rate between NOx with H2O2. Compared with other studies (Kartohardjono, 2019), the results also show an insignificant mass transfer flux from 1.153 x 10-9 mmol/cm2.s at 0.1% w/w H2O2to 1.486 x 10-9 mmol/cm2.s at 0.4% w/w H2O2.
Figure 6 The
dependency of NOx and SO2 absorbed and flux, J, on the H2O2 concentration in the absorbent solutions at the feed gas flow rate of 0.1
L/min.
NOX and SO2 loading decreased drastically as the feed gas flow rate increased, as shown in Figure 7. The NOx loading at a feed gas flow rate of 100 mL/min was 1.86 x 10-3 mmol NOx per mole H2O2 per second. It decreased drastically to 1.98 x 10-4 mmol NOx per mole H2O2 per second if the concentration of H2O2 in the absorbent solution increased from 0.001 to 0.1 M. Meanwhile, SO2 loading decreased drastically from 2.03 x 10-2 mmol SO2 per mole H2O2 per second to 2.03 x 10-4 mmol SO2 per mole H2O2 per second if the concentration of H2O2 in the absorbent solution increases from 0.001 to 0.1 M. This decrease occurs because the increase in the amount of NOx and SO2 gas absorbed is not proportional to the increase in the concentration of H2O2 in the absorbent. Similar results were also reported: the NOx loading decreased with increasing the concentration of absorbents (Karamah et al., 2021; Purnawan et al., 2021).
Figure 7 The dependency of NOx and SO2loading on
the H2O2 concentration in the absorbent solutions at the
feed gas flow rate of 0.1 L/min
The elimination of NOx and SO2 simultaneously can be
conducted in an HFMM using absorbents such as H2O2 and
NaOH. The presence of SO2 in the feed gas could reduce the removal
efficiency of NOx because of the competition factor in consuming H2O2 in the process. The NOx and SO2 removal efficiencies decrease with
the feed gas flow rate, while the NOx and SO2 absorbed, fluxes, and
loadings increase with the feed gas flow. This study's maximum NOx reduction
efficiency was 93.9%, while SO2 can be almost entirely removed. In
future work, the methods would be applied to remove NOx and SO2 simultaneously from the flue gas resulting from fossil fuel combustion.
The authors wish to
acknowledge the PDUPT Project via the Directorate of Research and Services
Universitas Indonesia through Contract No. NKB-213/UN2.RST/ HKP.05.00/2021.
Brandenberger, S., Kröcher, O., Tissler, A.,
Althoff, R., 2008. The state of the art in selective catalytic reduction
of NOx by ammonia using metal?exchanged zeolite catalysts. Catalysis Reviews, Volume 50(4), pp. 492–531
Chang, M.B., Lee, H.M., Wu, F., Lai, C.R., 2004.
Simultaneous removal of nitrogen oxide/nitrogen dioxide/sulfur dioxide from gas
streams by combined plasma scrubbing technology. Journal of the Air & Waste Management Association, Volume 54(8), pp. 941–949
Chen, T.-L., Chen, Y.-H., Chiang, P.-C., 2020. Enhanced
performance on simultaneous removal of NOx-SO2-CO2 using a high-gravity
rotating packed bed and alkaline wastes towards green process intensification. Chemical Engineering Journal, Volume 393, p. 124678
Cheng, G., Zhang, C., 2018.
Desulfurization and denitrification technologies of coal-fired flue gas. Polish Journal of Environmental Studies, Volume
27(2), pp. 481–489
Ding, J., Zhong, Q., Zhang, S., Song, F., Bu, Y., 2014.
Simultaneous removal of NOx and SO2 from coal-fired flue gas by
catalytic oxidation-removal process with H2O2. Chemical Engineering Journal, Volume 243, pp. 176–182
Fang, P., Cen, C., Tang, Z., Zhong, P., Chen, D.,
Chen, Z., 2011. Simultaneous removal of SO2 and NOx by wet
scrubbing using urea solution. Chemical
Engineering Journal, Volume 168(1), pp. 52–59
Febriasari, A., Huriya, Ananto,
A.H., Suhartini, M., Kartohardjono, S., 2021. Polysulfone-polyvinyl
pyrrolidone blend polymer composite membranes for batik industrial wastewater
treatment. Membranes, Volume 11(1), pp. 1–17
Huang,
X., Ding, J., Zhong, Q., 2015. Catalytic decomposition of H2O2
over Fe-based catalysts for simultaneous removal of NOX and SO2.
Applied Surface Science, Volume 326, pp. 66–72.
Karamah, E.F., Arbi, D.S., Bagas, I.,
Kartohardjono, S., 2021. Hollow fiber membrane modules for NOx removal using a
mixture of NaClO3 and NaOH solutions in the shell side as absorbents.
International Journal of Technology, Volume 12(4), pp. 690–699
Kartohardjono, S., Merry, C., Rizky, MS.,
Pratita, C.C., 2019. Nitrogen oxide reduction through absorbent solutions
containing nitric acid and hydrogen peroxide in hollow fiber membrane modules. Heliyon, Volume
5(12), p. e02987
Kartohardjono, S., Paramitha, A., Putri, AA.,
Andriant, R., 2017. Effects of absorbent flow rate on CO2 absorption
through a super hydrophobic hollow fiber membrane contactor. International Journal of Technology, Volume
8(8), pp. 1429–1435
Kartohardjono, S., Rizky, M.S., Karamah, E.F.,
Lau, W.-J., 2020. The effect of the number of fibers in hollow fiber
membrane modules for NOx absorption. Chemical
Engineering, Volume 11(2), pp. 269–277
Krzyzynska, R. Hutson, N.D., 2012. Effect of
solution pH on SO2, NOx, and Hg removal from simulated coal
combustion flue gas in an oxidant-enhanced wet scrubber. Journal of the Air & Waste Management Association, Volume 62(2), pp. 212–220
Li, Y., Che, D., Zhao, H., Yang, C., Zhao, T.,
Cheng, G., Yao, M., 2020. Tributyl phosphate additive enhancing catalytic
absorption of NO2 for simultaneous removal of SO2/NOx in
wet desulfurization system. Journal of
the Energy Institute, Volume 932, pp. 474–481
Liu, X., Zhu, T., Lv, Q., Li, Y., Che, D., 2019. Simultaneous
removal of NOx and SO2 from coal-fired flue gas based on the
catalytic decomposition of H2O2 over Fe2 MoO43.
Chemical Engineering Journal, Volume 371, pp. 486–499
Manisalidis, I., Stavropoulou, E., Stavropoulos,
A., Bezirtzoglou, E., 2020. Environmental and health impacts of air pollution: a
review. Frontiers in public health, Volume 8, p. 14
Park, J.-H., Ahn, J.-W., Kim, K.-H., Son, Y.-S., 2019. Historic
and futuristic review of electron beam technology for the treatment of SO2
and NOx in flue gas. Chemical Engineering
Journal, Volume 355, pp. 351–366
Purnawan, I., Kartohardjono, S., Wibowo, L.,
Ramadhani, A.F., Lau, W.J., Febriasari, A., 2021. Effect of absorbents on NOx
removal through polyvinylidene fluoride PVDF hollow fiber membrane modules. International Journal of Chemical
Engineering, Volume 2021, p. 8277082
Republic of Indonesia (RI), 1999. Government Regulation No. 41 of 1999
concerning Air Pollution Control. pp. 1–34
Serbanescu, O.S., Voicu, SI., Thakur, V.K., 2020.
Polysulfone functionalized membranes: Properties and challenges. Materials Today Chemistry, Volume 17, p. 100302
Sharma, S.B., Jain, S., Khirwadkar, P., Kulkarni,
S., 2013. The effects of air pollution on the environment and human health. Indian Journal of Research in Pharmacy and
Biotechnology, Volume 13, pp. 391–396
Si, T., Wang, C., Yan, X., Zhang, Y., Ren, Y.,
Hu, J., Anthony, E. J., 2019. Simultaneous removal of SO2 and NOx by a
new combined spray-and-scattered-bubble technology based on preozonation: From
lab scale to pilot scale. Applied Energy, Volume
242, pp. 1528–1538
Sun, Y., Zwoli?ska, E., Chmielewski, A.G., 2016. Abatement
technologies for high concentrations of NOx and SO2 removal from exhaust gases:
A review. Critical Reviews in
Environmental Science and Technology, Volume
46(2), pp. 119–142
Sunarno, S., Purwanto, P., Suryono, S. 2021., Trend analysis of NOX
and SO2 emissions in Indonesia from the period of 1990 -2015 using data
analysis tool. Advances in Science,
Technology and Engineering Systems Journal, Volume 61, pp. 257–263
Wang, M.-H. S., Wang, L.K., Shammas, N.K., 2019. Glossary
of acid rain management and environmental protection. In: Handbook of environment and waste management: Acid rain and greenhouse
gas pollution control, Hung, Y.T., Wang, L.K., Shammas, N.K., (ed.), World
Scientific, Cleveland, USA, pp. 719-749
Wu, Y., Chu, B., Zhang, M., Yi, Y., Dong, L., Fan, M., Jin, G., Zhang, L., Li, B., 2019. Influence of calcination temperature on the catalytic properties of LaCu0.25Co0.75O3 catalysts in NOx reduction. Applied Surface Science, Volume 481, pp. 1277–1286
Zhao, M., Xue, P., Liu, J., Liao, J., Guo, J., 2021. A review of removing SO2 and NOX by wet scrubbing. Sustainable Energy Technologies and Assessments, Volume 47, p. 101451
