Tailoring Epoxy Resin Properties Using Glycidyl Methacrylate-Based Reactive Diluents: Viscosity Reduction and Performance Enhancemen
Corresponding email: da_ainakulova@kbtu.kz
Published at : 17 Jul 2025
Volume : IJtech
Vol 16, No 4 (2025)
DOI : https://doi.org/10.14716/ijtech.v16i4.7687
Tulegenkyzy, AD, Megat-Yusoff, PSM, Al Azzam, KM, Kairatovna, BL, Goyal, A, Eshmaiel, G, Negim, E-S, Kusrini, E & Samy, M 2025, ‘Tailoring epoxy resin properties using glycidyl methacrylate-based reactive diluents: viscosity reduction and performance enhancement’, International Journal of Technology, vol. 16, no. 4, pp. 1421-1435
| Ainakulova Dana Tulegenkyzy | School of Materials Science and Green Technologies, Kazakh British Technical University, St. Tole bi 59, 050000, Almaty, Kazakhstan |
| Puteri Sri Melor Megat-Yusoff | Mechanical Engineering Department, Universiti Teknologi PETRONAS, Bandar Seri Iskandar 32610, Perak, Malaysia |
| Khaldun M. Al Azzam | Department of Chemistry, School of Science, University of Jordan, 11942, Amman, Jordan |
| Bekbayeva Lyazzat Kairatovna | National Nanotechnology Open Laboratory, Al-Faraby Kazakh National University, 71, Al-Faraby av., 050040, Almaty, Kazakhstan |
| Arpit Goyal | Civil Engineering Department, Thapar Institute of Engineering and Technology, Patiala, Punjab, India- 147001 |
| Ganjian Eshmaiel | Concrete Corrosion Tech LTD, 12 Humphrey Middlemore Drive, Birmingham, England B17 0JN |
| Elsayed Negim | School of Materials Science and Green Technologies, Kazakh British Technical University, St. Tole bi 59, 050000, Almaty, Kazakhstan |
| Eny Kusrini | 1. Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus Baru UI, Depok 16424, Indonesia 2. Green Product and Fine Chemical Engineering Research Group, Laboratory |
| Moshera Samy | Polymers and Pigments Department, National Research Centre, 33 El Bouhoth St., Dokki, Giza 12622, Egypt |
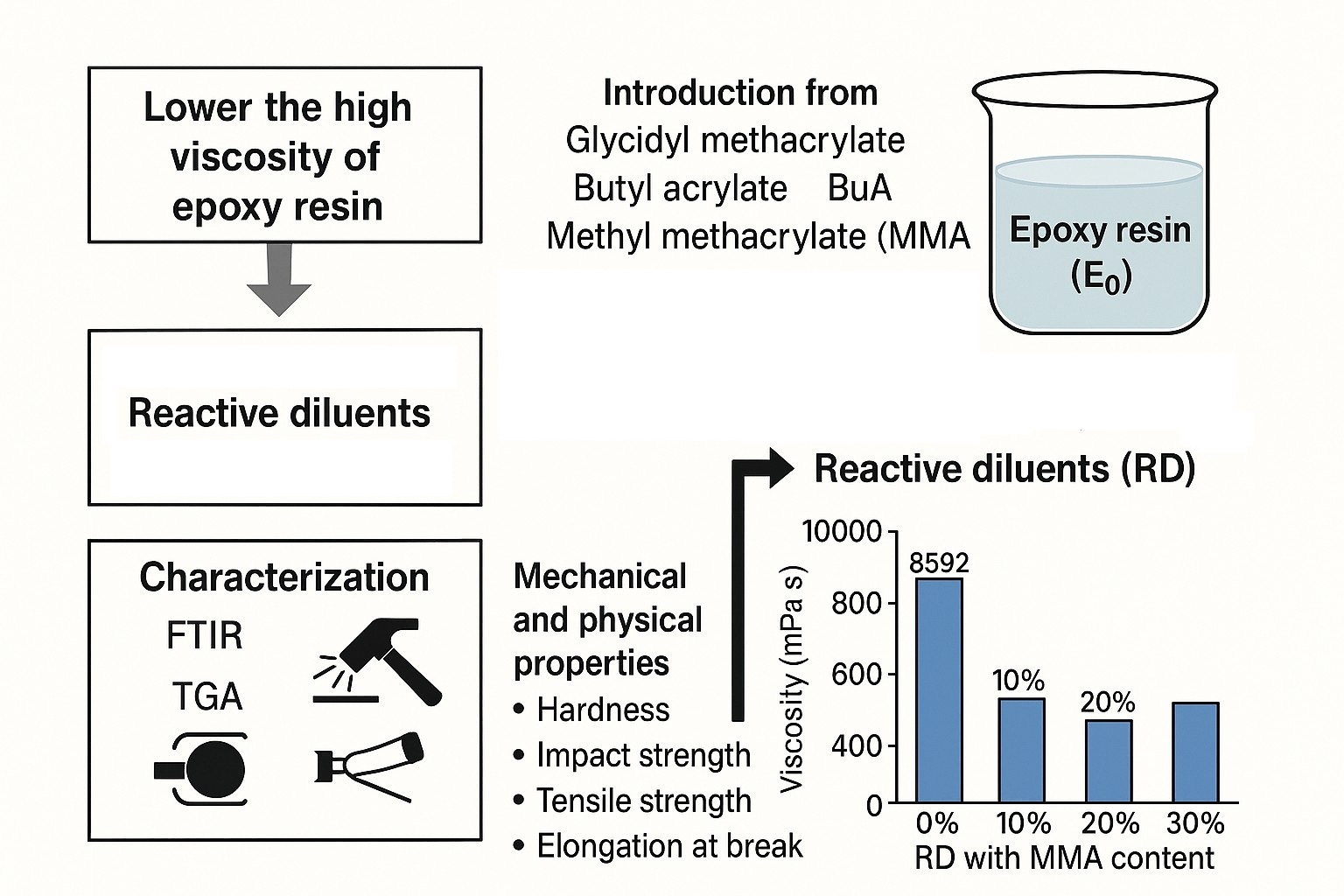
This study aims to lower the high viscosity of epoxy resin for diverse applications and introduces novel reactive diluents synthesized from glycidyl methacrylate (GMA), butyl acrylate (BuA), and methyl methacrylate (MMA) via terpolymerization reaction using different GMA feed ratios with benzoyl peroxide (BPO) acting as a catalyst, BuA, and MMA. Therefore, in the presence of reactive diluents (RD), mechanical and physical tests were performed to determine epoxy resin's (E0) hardness, impact strength, tensile strength, and elongation at break. Fourier Transform Infrared Spectroscopy (FTIR) and thermogravimetric analysis (TGA) were used to characterize the generated reactive diluents, and hardener and epoxy resin were used in the ratio of 1:1, 1:0.5, and 1:0.3 respectively. According to the results, reactive diluents with 20% MMA successfully reduced the viscosity of epoxy resin from 8592 to 1900 mPa·s; thereby, no solvent is needed for coating application. All modified epoxy resin showed excellent adhesion and flexibility on the concrete and metal substrates at a hardener ratio of 1: 0.5. However, the acrylic monomers improved the corrosion and solvent resistance of the metal substrate, and 20% MMA content showed the best corrosion and solvent resistance.
Epoxy; Glycidyl methacrylate; Mechanical properties; Reactive diluents; Terpolymerization
Epoxy resin is widely used in various industrial applications, such as molding compounds, adhesives, electrical laminates, and surface coatings (Roza et al., 2024; Ningrum et al., 2023; Nurhayati et al., 2023; Pramanik et al., 2012; Teh et al., 2007; Wang et al., 2005). This is primarily due to their excellent mechanical qualities and simplicity of processing and curing, even at varying temperatures (Capricho et al., 2019; Pizzi and Mittal, 2017). Moreover, their polar nature enables strong adhesion to a variety of substrates, including most metals, ceramics, concrete, glass, plastics, and wood (Ozgul and Ozkul 2018; Aiello et al., 2002). However, during the hardening process, the resin may undergo limited shrinkage, leading to internal tensions. To address this, the use of the right agents, such as plasticizers or additives, can prevent this difficulty (Izra’ai et al., 2025; Bakar et al., 2010). Once fully cured, epoxy resin exhibits significant mechanical strength as well as strong resistance to weathering and chemical resistance (Rudawska, 2020a; 2020b; Lionetto et al., 2015; Corcione et al., 2014; Frigione and Calò, 2007). In addition, epoxy resin possesses excellent dielectric qualities, high specific resistance, and a low dielectric loss factor are characteristics of hardened epoxy resin (Rudawska and Frigione, 2022). Epoxy resin can be modified with appropriate additives to achieve specific performance characteristics, depending on the intended application (Addina et al., 2022; Rudawska, 2020a; Kusrini et al., 2020; Ikram and Munir, 2012). Therefore, the selection of a suitable curing agent and resin, with proper processing equipment and curing conditions, is important for performance (Arundina et al., 2025; Miturska et al., 2020). According to the results, the commonly used modifiers include diluents, antioxidants, plasticizers, dyes fillers, pigments, and stabilizers (Septriansyah et al., 2025; Asbollah et al., 2021; Khalina et al., 2018; Sinha et al., 2017; Lee et al., 2012; Czub, 2006a; 2006b).
In contrast to conventional solvents or non-reactive diluents, the reactive diluents do not migrate since these diluents are covalently bound into the polymer network and take part in the crosslinking process of epoxy resin. Specifically, the reactive epoxy groups found in the reactive diluents are arranged in glycidyl methacrylate (GMA), benzyl glycidyl ether, 1, 4-butanediol diglycidyl ether, alkylphenols, and aliphatic or aromatic glycidyl ethers of alcohols (Tran et al., 2020). On the other hand, aromatic hydrocarbons such as toluene, xylene, phthalates, styrene, or phenolic chemicals are frequently found in non-reactive diluents. Although non-reactive diluents are often added in relatively high concentrations and are not expected to affect the reactivity of epoxy systems, the addition of toluene has been shown to reduce the mechanical properties of the cured epoxy (Samyn et al., 2023). Furthermore, processing excessively viscous epoxy resin might be challenging, which could be a barrier when transformed into other fiber-containing components. To address this, diluents are introduced to reduce viscosity and improve processability. In this regard, Jagtap and More (2021) highlighted the essential features of viscosity modifiers for epoxy systems. The ability of epoxy resin diluents to improve the cured polymers' strength, elasticity, and deformation in addition to their viscosity, was reported by Lee et al. (2012). Similarly, Kregl et al. (2017) observed that epoxy systems containing diluents had higher tensile strength and strain at break than those modified with toughening agents. However, the final properties depend significantly on both the type and concentration of the diluent used. Epoxy resin (Tzoumani et al., 2022) are polymers containing epoxide groups, including GMA, which have been extensively used in many different applications. Epoxy groups in polymers have a major impact on how well they function in a variety of applications (Maruyama et al., 2001). Therefore, GMA-based copolymers can improve coating adhesion, barrier qualities, corrosion protection, and service life (Riyanto et al., 2023). On the other hand, Asha et al. (2019) reported that free radical solution polymerization provides benefits such as block copolymers. By using free radical polymerization in toluene with BPO as an initiator, Azzahari et al. (2012) produced distinctive copolymers using a range of feed compositions of GMA and tetrahydrofurfuryl acrylate (THFA). According to the results, as the amount of THFA in copolymers grows, their thermal stability also increases. Using the free radical solution polymerization technique. Tulegenkyzy et al. (2024) investigated poly(styrene-co-as glycidyl methacrylate) copolymers as reactive. Epoxy resin's viscosity decreased from 8592 mPa·s to 1656, 680, and 430 mPa·s, showing the outstanding dilution effect of reactive diluents [Poly (St-co-GMA)]. At a hardener ratio of 1:0.3, 70% GMA and 30% St were present in the adhesion, tensile, and hardness properties. This study aims to develop Poly(GMA-co-BuA-co-MMA) copolymers as reactive diluents to improve mechanical and physical qualities, such as adhesion to concrete or metal, and thermal characteristics, while also reducing the viscosity of epoxy resin using custom-synthesized reactive diluents (RD), offering a solvent-free alternative for coating applications. In contrast to conventional diluents, this approach incorporates functionalized copolymers that not only reduce viscosity but also participate in the curing process, improving mechanical performance. Butyl acrylate (BuA) and methyl methacrylate (MMA) were essential to the copolymerization process with GMA because they improved the rate of polymerization and enhanced the mechanical properties of epoxy resin, including their resistance to heat, mechanical stress, and chemicals (Demirors, 2000). Additionally, BuA and MMA are used in copolymerization to modify polymeric properties for various applications. BuA enhances adhesion and flexibility in pressure-sensitive adhesives and flexible coatings (Lee and Lee, 2020), while MMA provides rigidity, transparency, and weather resistance in automotive coatings, optical lenses, and bone types of cement (Deka et al., 2022). The combination creates a balanced material with tunable mechanical properties for optimal industrial and biomedical performance. Poly(GMA-co-BuA-co-MMA) copolymers were characterized by TGA and FTIR.
This study developed and characterized Poly(GMA-co-BuA-co-MMA) terpolymers as reactive diluents for epoxy resin. The objective was to improve mechanical/thermal properties (e.g., adhesion) and reduce viscosity. BuA and MMA were selected for their ability to enhance polymerization and resistance to heat, stress, and chemicals (Demirors, 2000). Copolymers were characterized via TGA and FTIR.
2.1. Methods and materials
Epoxy resin ELM-NG 1000 and hardener ELM-NG 34H were obtained from Elcos Marketing LLP, Kazakhstan. ELM-NG 1000 has an epoxy value of 5.25-5.5 eq/Kg, weight per epoxide of 182 g/eq, viscosity of 8500 mPa·s at 25°C, and density of 1.16 g/cm3. ELM-NG 34H has an amine value of 298 mgKOH/g and a viscosity of 254 mPa·s at 25°C. Glycidyl methacrylate (GMA 97%), butyl acrylate (BuA
99%), methyl methacrylate (MMA
99%), xylene, and benzoyl peroxide (BPO), benzene, xylene, toluene, acetone, NaCl (2%), NaOH (2%) and HCl (2%) were obtained from Sigma-Aldrich. All chemicals were used without further purification. The chemicals and solvents used were of analytical reagent grade.
2.2. Synthesis of the poly (GMA-co-BuA-co-MMA) terpolymer as reactive dilutes
The terpolymerization of GMA, BuA, and MMA with varying feed monomer compositions RD1= 70/10/20 wt.%, RD2 = 70/15/15 wt.% and RD3 = 70/20/10 wt.%, was prepared using free radical polymerization in the presence of xylene as a solvent to reduce the viscosity during the reaction. A 500-mL three-necked flask system, fitted with a stirrer, reflux condenser, and thermometer, was used for the polymerization process. After xylene was introduced into the flask, the catalyst BPO was added while the mixture was mechanically stirred at 500–600 rpm. The flask was maintained at 65°C using an automatically controlled, air-filled water bath. Once the solution reached full solubility, the required amounts of GMA, BuA, and MMA monomers were gradually introduced. The mixture was stirred sequentially at 65°C for one hour, 80°C for two hours, and finally at 90°C for an additional hour.
2.3. Characterization of poly (GMA-co-BuA-co-MMA) terpolymer
The obtained poly (GMA-co-BuA-co-MMA) terpolymers were analyzed using ALPHA FTIR spectroscopy, Bruker, and TGA to determine thermal properties. The dried terpolymers were tested with a Perkin Elmer TGA/SDTA851e from ambient temperature to 900°C at a rate of 10°C per minute in an air atmosphere. A sample weight of 8 mg was taken for all measurements and the weight loss against temperature was recorded.
2.4. Viscosity Tests
The viscosity (mPa·s) of epoxy resin and reactive diluents was tested at room temperature (25oC) with a Brookfield viscometer at 5 and 50 rpm by ISO 12058-1 International Organization for Standardization (ISO, 2018). The titration at room temperature was used to establish epoxy value (eq/Kg) and weight per epoxide, g/eq, of resin by ASTM D1652 (American Society for Testing and Materials (ASTM), (ASTM, 2019a). Tables 1 and 2 show the characteristics of reactive diluents and modified epoxy resin.
2.5. Mixing ratio
To create modified epoxy resin (ERD1, ERD2, ERD3), epoxy resin (E0, 90%) was combined with 10% reactive diluents (RD1, RD2, RD3) for 10 minutes using a stirring stick or spatula. Table 2 shows their properties, and the low-viscosity modified cycloaliphatic ELM-NG 34H hardens epoxy resin in 1.0:1.0, 0.5:1.0, or 0.3:1.0 wt./wt. The components were mixed slowly, scraping the container to ensure complete inclusion. After applying the epoxy mixture to concrete and metal surfaces, it was allowed to cure at room temperature.
Table 2 Characteristics of reactive dilute mixed with epoxy resin
2.6. Preparation for Film
Reactive diluent mixes (ERD) and epoxy resin (E0) were combined in a beaker with hardener ratios of 1:1, 1:0.5, and 1:0.3. After being poured into steel molds measuring 7 mm x 7 mm x 7 mm, the resulting mixes were allowed to cure at room temperature (25°C) with a relative humidity of 59% for 6 days followed by an aerated oven at 60°C for 12 h to entirely remove the solvent.
2.7. Mechanical tests
The tensile properties of the cast films were assessed using an MTS 10/M tensile testing system at a crosshead speed of 50 mm/min and a 1-kN load cell was used. Tensile dumbbell-shaped specimens with a gauge length of 25 mm were punched out from the cast films by using the ASTM Die C. The tests were carried out as per ASTM D412-16 (ASTM, 2021b) method at ambient temperature. The reported values represent the average of at least four measurements.
Mechanical properties, including cracking, impact resistance, and adhesion, were evaluated on steel films with dimensions of 12 cm × 6 cm × 1 mm. A cylindrical Mandrel Tester ASTM D522, (ASTM, 2001b) checked the coating's resistance to cracking or detachment when bent around a mandrel. The tubular impact tester (ASTM D2794) (ASTM, 2019b) assessed film resistance to impact and the crosshatch tester (ASTM D3359) (ASTM, 2001a) evaluated coating adhesion. The adhesion strength of the epoxy and the diluted epoxy mixture was assessed with pull-out tests according to the EN 1542 standard (Krzywi?ski and Sadowski, 2019).
2.8. Corrosion resistance tests
Corrosion resistance tests were carried out on coated panels in solutions of 2% NaCl, 2% NaOH, and 2% HCl; epoxy (Eo) and modified epoxy (ERD) films were tested for corrosion resistance. The test was carried out at a specific time for one week.
2.9. Chemical resistance
Solvent resistance (ASTM D5402-19) (ASTM, 2024) and water resistance (D1647-89) (ASTM, 2021a) tests were also performed. The samples were submerged in each solution for one week. The purpose was to assess corrosion, solvent, and water resistance, and cure times at 25°C were recorded.
3.1. FTIR spectra of terpolymer
Figure 1 shows the FTIR spectra of GMA and poly (GMA-co-BuA-co-MMA). The peak at 940 cm-1 with 60% transmittance corresponds to the epoxy group's stretching vibration in GMA, while in the terpolymer, it appears at 889 cm-1 with 10% transmittance due to the terpolymerization (Abdollahi et al., 2018; Darvishi et al., 2012).
Figure 1 FTIR spectra for poly GMA, and Poly (GMA-co-BuA-co-MMA)
Carbonyl and C–O bonds' stretching vibrations are found at peaks 1711 and 1144 cm-1 in both GMA and terpolymers. Peaks at 3017 and 2868 cm-1 are related to C–H (stretching) in methyl and methylene groups from MMA and BuA. Ester groups appear at 1000–1150 cm-1, C–C (stretching) at 845 cm-1, and C–O bending at 400–600 cm-1. New peaks at 1490 and 1518 cm-1 correspond to CH3 stretching from MMA and BuA. The spectra showing peaks for both monomers and epoxy ring confirm the successful synthesis of poly(GMA-co-BuA-co-MMA). A schematic of terpolymerization is shown in Figure 2.
Figure 2 A terpolymerization reaction schematic to produce Poly (GMA-co-BuA-co-MMA) compound using GMA, BuA and MMA
3.2. TGA analysis
Terpolymer samples (RD) containing different contents of BuA and MMA were tested using a Perkin Elmer TGA/SDTA851e thermogravimeter. Under N2 gas flow, the temperature increased from room temperature to 900°C at a rate of 20°C/min. TGA data shows the material's thermal stability qualitatively. Figure 3(a-c) shows typical TGA measurements for RD, showing two to three degradation stages.
(a)
(b)
(c)
Figure 3 TGA thermogram of poly(GMA-co-BuA-co-MMA) for RD 1 (a), RD2 (b) and RD3 (c)
The degradation process is characterized by two distinct stages for RD1 and RD2 (Figure 3(a) and (b), while RD3 exhibits three degradation stages as the BuA content in the terpolymer increases from 10 to 20% (Figure 3(c)). The initial weight loss starts at a temperature from 29.9oC to 265.3oC for RD1, while the initial weight loss for RD2 starts from 29.8oC to 417.2oC. The weight loss of the first stage was 79% for RD1 and 21% for RD2. As for RD3, the initial weight loss was 75% at temperatures 30.7 – 272.5oC. This is attributed to the evaporated solvent of the samples and the primary decomposition of macromolecular chains. Additionally, RD1 and RD2 exhibit greater initial weight loss before 270°C compared to RD3, attributed to the moisture present in the samples. The second degradation stage for terpolymers varies: RD1 (265.3-790.7°C, 5.1% weight loss), RD2 (417.2-786.8°C, 2.4% weight loss), and RD3 (272.5-479.2°C, 4.6% weight loss). This is due to terpolymer backbone degradation and GMA ester decomposition, yielding CO2, glycidol, dimethyl ketene, and acrolein (Abdollahi et al., 2020). RD2 (15% BuA, 15% MMA) degraded at 417°C with the lowest weight loss (21%), contrasting RD1 and RD3. RD3's third stage degradation was from 479.2 to 792.1°C (1.9% weight loss). Higher MMA content increased thermal decomposition temperature and stability. RD1 (20% MMA) showed higher initial decomposition and PDTmax (460°C) than RD2 (380°C), attributed to monomer type, content, and inter-polymer hydrogen bonds (Miturska et al., 2020; Asha et al., 2019). Table 3 shows decomposition temperatures at various weight loss percentages for all stages.
Table 3 Thermal characteristics of GMA-co-BuA-co-MMA polymers at varying feed ratios
3.3. Viscosity
Epoxy resin viscosity critically impacts coating applications. Low-viscosity epoxy is ideal for solvent-free coatings, improving wetting and adhesion on substrates like metal or concrete. Conversely, high-viscosity epoxy is unsuitable for coatings due to mixing difficulties (Signorini et al., 2020). The effect of terpolymers (RD) on the viscosity of epoxy resin (E0) at speeds 5 and 50 rpm and temperature 25oC is given in Table 4. The viscosity of epoxy resin (E0) was 8592 mPa·s at 5 rpm and 2400 mPa·s at 50 rpm. The viscosity of epoxy resin (E0) mixed with 10% terpolymer decreased to 1900 mPa·s for ERD1, 2250 mPa·s for ERD2, and 2800 mPa·s for ERD3 at 5 rpm. While the viscosity of epoxy resin (E0) decreased to 480 mPa·s for RD1, 610 mPa·s for RD2, and 750 mPa·s for RD3 at 50 rpm. The terpolymer reduces viscosity by affecting the epoxy resin's orientation, entanglement, and bonding. Its unique characteristics also modify the resin's internal structure, disrupting the macromolecular framework and lowering viscosity (Yang et al., 2024). The viscosity of epoxy resin mixed with RD decreased as MMA content in the terpolymer increased at both 5 and 50 rpm. This reduction is attributed to the long-chain rigidity of MMA and the branched structure of RD, which influence the epoxy resin's rheological behavior (Guapacha et al., 2016). The thixotropic index (TI) increased with mixing RD with epoxy resin. ERD1 has the maximum TI followed by ERD3, ERD2, and E0 as shown in Table 4.
Table 4 Viscosity and thixotropic index of epoxy resin mixed with reactive diluents
3.4. Adhesion
Table 5 shows epoxy adhesion results on metal and concrete, testing terpolymers and hardener ratios. Epoxy resin (E0, 90%) and reactive diluent (ERD, 10%) were mixed with 1:1, 1:0.5, and 1:0.3wt./wt. hardener ratios. Strong adhesion is vital for protective, durable epoxy coatings. The E0 resin with a 1:0.3 hardener ratio achieved the best adhesion on metal (2.19 MPa) and concrete (3.33 MPa). The adhesion of modified epoxy with RD mixed with hardener in 1: 05 showed the highest adhesion on metal and concrete substrates. This may be attributed to termoplymers as diluting reactive mixtures with epoxy resin improved the adhesion to the substrates. The highest adhesion values recorded were 6.4 MPa for concrete and 4.7 MPa for metal, showing that ERD1, when mixed with a hardener at a 1:0.5 ratio, exhibited strong adhesion to both substrates. In general, the adhesion of epoxy resin is good or bad depending on many factors, such as hardener types, hardener ratios, viscosity, reactive dilute types, and epoxy resin type (Chen et al., 2013; Bresson et al., 2012). ERD3 mixed with hardener in a ratio of 1: 05 gave the lowest adhesion strength on concrete (4.1 MPa) and metal (2.7 MPa) substrate. Viscosity is the primary factor influencing the adhesion of epoxy resin. Lower viscosity in epoxy resin results in improved bond strength (Xie et al., 2025).
Table 5 The effect of reactive diluent composition on concrete and metal substrate adhesion
Note: A statistically significant difference is given as **** - < 0.0001; *** -
< 0.001; ** -
< 0.01 and no statistically significant difference -
> 0.05
As the MMA content in the terpolymer increases, the viscosity of epoxy resin decreases significantly from 8592 mPa·s (E0) to 1900 mPa·s (ERD1), as shown in Table 4. Additionally, this modification enhances the adhesion of epoxy hybrids, improving their bond strength on concrete from 2.82 MPa (E0) to 6.4 MPa (ERD1) and on metal from 1.51 MPa to 4.7 MPa at a hardener ratio of 1:0.5, as presented in Table 5. According to previously reported results by (Szewczak, 2023), viscosity significantly influences the adhesion of epoxy resin to concrete surfaces. A lower viscosity improves resin’s ability to infiltrate the concrete’s pores and surface irregularities, resulting in enhanced mechanical interlocking and stronger adhesion.
3.5. Elongation at break and Tensile strength
The ratios of reactive diluents and hardeners used in the formulation of epoxy coatings significantly influence the mechanical properties of the coatings on various substrates. The effect of terpolymers and hardener ratios on the tensile and elongation at break is shown in Figure 4. The tensile strength of epoxy films (E0) and modified epoxy films (ERD) increased with increasing hardener ratios from 1: 0.3 to 1.0: 1.0. For E0, when the hardener ratio increases from 0.3 to 1.0, the tensile strength increases from 40.5 MPa to 46.1 MPa. However, the addition of terpolymers as reactive diluents to epoxy resin enhances the tensile strength. For ERD1, the tensile strength increases by approximately 57.5%. In comparison, ERD2 exhibits an increase of about 44.9%, and ERD3 shows an increase of roughly 29.5% compared to E0 at a hardener ratio of 1.0:1.0. The enhancement in tensile strength is attributed to the crosslinking interaction between epoxy, terpolymer, and hardener, resulting in a network surface on both metal and concrete substrates (Ozgul and Ozkul, 2018). The modified epoxy EDR1 with a hardener ratio of 1.0:1.0 exhibits the highest tensile strength (Figure 4). This effect is attributed to the high MMA content (20%) in the terpolymer, which increases film rigidity and consequently enhances tensile strength. In contrast, increasing the BuA content while reducing the hardener ratio led to greater elongation at break in the modified epoxy resin (RD), compared to the unmodified resin (E0), as shown in Figure 5. For instance, ERD3 with 20% BuA has an elongation of 182%, ERD2 with 15% BuA exhibits an elongation at break of 153%, and ERD1 with 10% BuA shows an elongation at break of 114% at a hardener ratio of 1.0:0.3. The flexibility characteristics of BuA are likely responsible for improving the film's elongation at break.
Figure 4 Epoxy resin (E0) and modified epoxy resin (ERD) tensile strengths at various hardener ratios. A statistically significant difference is given as **** - < 0.0001; *** -
< 0.001; ** -
< 0.01, no statistically significant difference -
> 0.05, and ns = not significant
Figure 5 Elongation at break of modified epoxy resin (ERD) and epoxy resin (E0) with varying concentrations of hardeners. A statistically significant difference is given as **** - < 0.0001; *** -
< 0.001
3.6. Hardness
Table 6 shows the hardness values for epoxy resin (E0) and modified epoxy resin (ERD) with varying ratios of hardener. An increase in the hardener ratio enhanced the hardness of resin, attributed to a higher degree of crosslinking between the hardener and epoxy International Organization for Standardization (ISO, 2018). ISO 12058-1; Abdollahi et al., 2018). E0 has the highest hardness (80.9 shore D) at a hardener ratio of 1.0: 1.0, while E0 has the lowest hardness (75 shore D) at a hardener ratio of 1.0: 0.3. However, the reactive dilute has a more significant effect on the hardness of resin films as shown in Table 6.
The MMA content in the reactive dilute shows a more pronounced impact on hardness compared to the ratios of hardeners and the increase in MMA content from 10 to 20%, enhances the hardness from 82.7 to 91.8 shore D. The increase in hardness results from the influence of MMA, which modifies the crosslinking density and molecular structure of epoxy hybrids, leading to a more rigid polymer network (Chaudhary and Dikshit, 2023; Li et al., 2016). While increasing BuA content in the reactive dilute from 10 to 20%, hardness values decreased from 91.8 to 82.7 shore D. The reduction in hardness is attributed to the presence of BuA, which enhances flexibility within the polymer network, thereby decreasing hardness and mechanical properties compared to the more rigid MMA-based systems (Balani et al., 2014). The unmodified epoxy resin (E0) exhibited cracking during the impact test conducted with a 1 kg weight dropped from a height of 1 m at a hardener ratio of 1.0:1.0. In contrast, the modified epoxy resin (ERD) showed impact resistance across all tested hardener ratios and did not fracture under the same testing conditions. According to the results, the terpolymer improved the impact strength of resin at all hardener ratios. Conical mandrel bending tests showed that epoxy resin (E0) failed at hardener ratios of 1.0:0.5 and 1.0:1.0, resulting in cracks. In contrast, all modified epoxy resin (ERD) showed excellent flexibility with no cracks during bending. The hardness of epoxy films is influenced by various factors, such as hardener ratios, diluent type, solvent, and concentrations (Sabergaliyev et al., 2024; Syrmanova et al., 2016).
Table 6 Mechanical characteristics of various reactive diluents mixed with epoxy resin
Keynotes to abbreviations: O = Suitable, = Swelling and blistering, X = Not Suitable, P = Samples passed test, F = Samples Failure the test.
3.7. Chemical and solvent resistance
The resistance of epoxy films (E0) and epoxy resin mixed with relatively dilute (ERD) made from different ratios of GMA, BuA, and MMA to acid, alkali, water, benzene, xylene, toluene, and acetone was studied using an immersion method. The results showed that modified epoxy resin (ERD) had better alkali resistance than non-modified epoxy resin (E0), due to the acrylic monomer's ability to resist alkali and acids as shown in Table 6. Acrylic and vinyl monomers add non-polar characteristics to resin, enhancing their resistance to alkali and water (Gziut et al., 2023; Liu et al., 2013). However, ERD1, which contains 20% MMA, exhibited superior corrosion and solvent resistance compared to ERD3, which contains 10% MMA. This is attributed to the fact that MMA enhances the chemical and solvent resistance of epoxy hybrids by altering their molecular structure and crosslinking density (Chaudhary and Dikshit, 2023; Li et al., 2016). In general, MMA-based adhesives and coatings show strong resistance to various chemicals, including acids and solvents, due to their robust acrylic polymer composition. Furthermore, studies on MMA interactions with organic solvents show that miscibility behavior is a key factor in determining its resistance properties (Vadamalar et. al., 2014). The effect of hardener ratios on the corrosion and solvent resistance is presented in Table 6. The mixing ratio of epoxy resin (E0) and ERD with hardener in ratio 1: 0.5 gave the highest corrosion and solvent resistance. Increased MMA content and cross-linking in ERD resin also boost their alkali and acid resistance. Factors affecting corrosion and solvent resistance include hardener types, epoxy resin, reactive diluents, acrylics, and monomers (Nazari et al., 2022; Kordas, 2022; Jiang et al., 2019).
In conclusion, poly(GMA-co-BuA-co-MMA) terpolymer was synthesized using various monomer ratios (70:10:20, 70:15:15, and 70:20:10wt./wt.) through free radical solution polymerization to enhance epoxy resin properties when combined with an amine-based hardener at ratios of 1:1, 1:0.5, and 1:0.3. The terpolymer significantly influences mechanical and physical characteristics of epoxy resin. The increase in the MMA content in the terpolymer improves adhesion, viscosity, mechanical properties, and corrosion and solvent resistance. In contrast, a higher BuA content leads to reduced mechanical properties, corrosion resistance, and solvent resistance. Higher BuA content lowers crosslinking density, resulting in a softer material with reduced mechanical strength but improved elongation properties. Conversely, increasing MMA content raises crosslinking density, yielding a more rigid material that enhances mechanical properties while decreasing elongation. This is due to MMA’s ability to improve the chemical and solvent resistance of epoxy hybrids by modifying their molecular structure and crosslinking density. The optimal mechanical properties were achieved with a 70:10:20 monomer ratio (ERD1) in epoxy hybrids at a 1:0.5 hardener ratio. ERD1, containing 20% MMA, exhibited the highest corrosion and solvent resistance. These results offer valuable insights for optimizing solvent formulations in the coatings industry, facilitating the development of solvent-free coatings with superior mechanical performance and a lower environmental footprint.
This research is funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. AP 19676595 Development of high electrically conductive paint for corrosion prevention in concrete structures). Authors also thank to Assoc. Prof. Dr. Anwar Usman from Universiti Brunei Darussalam for his contribution to finalize and fine tuning this article.
Abdollahi, H, Najafi, V & Amiri, F 2020, 'Determination of monomer reactivity ratios and thermal properties of poly(GMA-co-MMA) copolymers', Polymer Bulletin, vol. 78, no. 1, pp. 493–511, https://doi.org/10.1007/s00289-020-03123-5
Abdollahi, H, Salimi, A, Barikani, M, Samadi, A, Hosseini Rad, S & Zanjanijam, AR 2018, 'Systematic investigation of mechanical properties and fracture toughness of epoxy networks: Role of the polyetheramine structural parameters', Journal of Applied Polymer Science, vol. 136, no. 9, https://doi.org/10.1002/app.47121
Addina, SN, Alhamid, MI, Usman, A, Prasetyanto, EA, Sufyan M & Kusrini, E 2022, ’Economic analysis of graphene aerogel polyurethane sponge production as adsorbent for oil-water separation’, Materials Today: Proceedings, vol. 51, pp. 1896–1907, https://doi.org/10.1016/j.matpr.2022.01.313
Aiello, MA, Frigione, M & Acierno, D 2002, 'Effects of environmental conditions on performance of polymeric adhesives for restoration of concrete structures', Journal of Materials in Civil Engineering, vol. 14, no. 2, pp. 185-189, https://doi.org/10.1061/(asce)0899-1561(2002)14:2(185)
American Society for Testing and Materials (ASTM) 2024, 'ASTM D5402-19', Standard practice for assessing the solvent resistance of organic coatings using solvent rubs, https://store.astm.org/d5402-19.html
American Society for Testing and Materials (ASTM) 2001a, 'ASTM D3359', Standard test methods for rating adhesion by tap test, https://kta.com/adhesion-astm-d3359/
American Society for Testing and Materials (ASTM) 2001b, 'ASTM D522', Standard test methods for mandrel bend test of attached organic coatings, https://cdn.standards.iteh.ai/samples/111055/1b77d7227f964d4b8825344e762a4dca/ASTM-D522-D522M-17-2021-.pdf
American Society for Testing and Materials (ASTM) 2019a, 'ASTM D1652', Standard test method for epoxy content of epoxy resins, https://store.astm.org/d1652-11r19.html
American Society for Testing and Materials (ASTM) 2019b, 'ASTM D2794', Standard test methods for resistance of organic coatings to the effects of rapid deformation (impact), https://store.astm.org/d2794-93r19.html
American Society for Testing and Materials (ASTM) 2021a, 'ASTM D1647-89', Standard test methods for resistance of dried films of varnishes to water and alkali, https://cdn.standards.iteh.ai/samples/3456/c6816e73acef46578b7b24e492ea8aae/ASTM-D1647-89-1996-e1.pdf
American Society for Testing and Materials (ASTM) 2021b, 'ASTM D412-16', Standard test methods for tensile test method for vulcanized rubber and thermoplastic elastomers, https://storethinghiem.vn/uploads/files/D%20412%20-%2016%20(2021).pdf
Arundina, RY, Marlina, R, Kusrini, E, Usman, A, Subhan, A, Destyorini, F, Prasetyo, AB, Subiyanto, B 2025, ‘Preparation of nitrogen-doped activated carbon from palm oil empty fruit bunches for electrodes in electric double-layer capacitance-type supercapacitors: effect of pyrolysis temperature’, Clean Energy, vol. 9, no. 2, pp. 99–110, https://doi.org/10.1093/ce/zkae100
Asbollah, MA, Mahadi, AH, Kusrini, E, & Usman, A 2021, ‘Synergistic effect in concurrent removal of toxic methylene blue and acid red-1 dyes from aqueous solution by durian rind: kinetics, isotherm, thermodynamics, and mechanism’, International Journal of Phytoremediation, vol. 23 no. 13, pp. 1432–1443, https://doi.org/10.1080/15226514.2021.1901851
Asha, AB, Srinivas, S, Hao, X & Narain, R 2019, 'Enzyme-responsive polymers: Classifications, properties, synthesis strategies, and applications', Smart Polymers and Their Applications, pp. 155-189, https://doi.org/10.1016/b978-0-08-102416-4.00005-3
Azzahari, AD, Yahya, R, Hassan, A & Sheikh, MRK 2012, 'Synthesis and characterization of new copolymers from glycidyl methacrylate and tetrahydrofurfuryl acrylate: Determination of reactivity ratios', Fibers and Polymers, vol. 13, no. 5, pp. 555-563, https://doi.org/10.1007/s12221-012-0555-4
Bakar, M, Szyma?ska, J, Rudecka, J & Fitas, J 2010, 'Effect of reactive diluents and kaolin on the mechanical properties of epoxy resin', Polymers and Polymer Composites, vol. 18, no. 9, pp. 503-510, https://doi.org/10.1177/096739111001800905
Balani, K, Verma, V, Agarwal, A & Narayan, R 2014, 'Physical, thermal, and mechanical properties of polymers', Biosurfaces: A Materials Science and Engineering Perspective (Appendix 1), pp. 329-344, https://doi.org/10.1002/9781118950623.app1
Bresson, G, Jumel, J, Shanahan, MER & Serin, P 2012, 'Strength of adhesively bonded joints under mixed axial and shear loading', International Journal of Adhesion and Adhesives, vol. 35, pp. 27-35, https://doi.org/10.1016/j.ijadhadh.2011.12.006
Capricho, JC, Fox, B & Hameed, N 2019, 'Multifunctionality in epoxy resins', Polymer Reviews, vol. 60, no. 1, pp. 1–41, https://doi.org/10.1080/15583724.2019.1650063
Chaudhary, N & Dikshit, MK 2023, 'Study of the cross-linking density effect on the mechanical properties of h-BNNS reinforced epoxy nanocomposite part-1: A molecular dynamics simulation', Journal of Molecular Modeling, vol. 29, no. 5, p. 146, https://doi.org/10.1007/s00894-023-05552-1
Chen, Q, Zhao, Y, Zhou, Z, Rahman, A, Wu, XF, Wu, W, Xu, T & Fong, H 2013, 'Fabrication and mechanical properties of hybrid multi-scale epoxy composites reinforced with conventional carbon fiber fabrics surface-attached with electrospun carbon nanofiber mats', Composites Part B: Engineering, vol. 44, no. 1, pp. 1-7, https://doi.org/10.1016/j.compositesb.2012.09.005
Corcione, C, Freuli, F & Frigione, M 2014, 'Cold-curing structural epoxy resins: Analysis of the curing reaction as a function of curing time and thickness', Materials, vol. 7, no. 9, pp. 6832-6842, https://doi.org/10.3390/ma7096832
Czub, P 2006a, 'Application of modified natural oils as reactive diluents for epoxy resins', Macromolecular Symposia, vol. 242, no. 1, pp. 60–64, https://doi.org/10.1002/masy.200651010
Czub, P 2006b, 'Characterization of an epoxy resin modified with natural oil?based reactive diluents', Macromolecular Symposia, vol. 245–246, no. 1, pp. 533-538, https://doi.org/10.1002/masy.200651377
Darvishi, A, Zohuriaan Mehr, MJ, Marandi, GB, Kabiri, K, Bouhendi, H & Bakhshi, H 2012, 'Copolymers of glycidyl methacrylate and octadecyl acrylate: Synthesis, characterization, swelling properties, and reactivity ratios', Designed Monomers and Polymers, vol. 16, no. 1, pp. 79-88, https://doi.org/10.1080/15685551.2012.705493
Deka, N, Bera, A, Roy, D & De, P 2022, 'Methyl methacrylate-based copolymers: Recent developments in the areas of transparent and stretchable active matrices', ACS Omega, vol. 7, no. 42, pp. 36929-36944, https://doi.org/10.1021/acsomega.2c04564
Demirors, M 2000, 'Styrene polymers and copolymers', Applied Polymer Science: 21st Century, pp. 93-106, https://doi.org/10.1016/b978-008043417-9/50009-x
Frigione, M & Calò, E 2007, 'Influence of an hyperbranched aliphatic polyester on the cure kinetic of a trifunctional epoxy resin', Journal of Applied Polymer Science, vol. 107, no. 3, pp. 1744-1758, https://doi.org/10.1002/app.27277
Guapacha, J, Vallés, EM, Quinzani, LM & Failla, MD 2016, 'Long-chain branched polypropylene obtained using an epoxy resin as crosslinking agent', Polymer Bulletin, vol. 74, no. 6, pp. 2297-2318, https://doi.org/10.1007/s00289-016-1839-4
Gziut, K, Kowalczyk, A, Schmidt, B, Idzik, TJ & So?nicki, JG 2023, 'Influence of methacrylate and vinyl monomers on radical bulk photopolymerization process and properties of epoxy-acrylate structural adhesives', Polymers, vol. 15, no. 4, article 926, https://doi.org/10.3390/polym15040926
Ikram, S & Munir, A 2012, 'Mechanical and thermal properties of chemically modified epoxy resin', Open Journal of Synthesis Theory and Applications, vol. 1, no. 3, pp. 36-43, https://doi.org/10.4236/ojsta.2012.13007
International Organization for Standardization 2018, 'Plastics — Determination of viscosity using a falling-ball viscometer — Part 1: Inclined-tube method', ISO 12058-1, https://cdn.standards.iteh.ai/samples/74985/32780391e5a545748904264ad7526092/ISO-12058-1-2018.pdf
Izra’ai, SI, Abdullah, AH, Ab Ghani, SM & Ab Ghani, AR 2025, 'The influence of bonding variations on polymerization shrinkage and stress distribution in resin composites: A finite element comparative study', International Journal of Technology, vol. 16, no. 2, pp. 483-495, https://doi.org/10.14716/ijtech.v16i2.7357
Jagtap, AR & More, A 2021, 'Developments in reactive diluents: A review', Polymer Bulletin, vol. 79, no. 8, pp. 5667-5708, https://doi.org/10.1007/s00289-021-03808-5
Jiang, F, Zhao, W, Wu, Y, Dong, J, Zhou, K, Lu, G & Pu, J 2019, 'Anti-corrosion behaviors of epoxy composite coatings enhanced via graphene oxide with different aspect ratios', Progress in Organic Coatings, vol. 127, pp. 70-79, https://doi.org/10.1016/j.porgcoat.2018.11.008
Khalina, M, Beheshty, MH & Salimi, A 2018, 'The effect of reactive diluent on mechanical properties and microstructure of epoxy resins', Polymer Bulletin, vol. 76, no. 8, pp. 3905-3927, https://doi.org/10.1007/s00289-018-2577-6
Kordas, G 2022, 'Corrosion barrier coatings: Progress and perspectives of the chemical route', Corrosion and Materials Degradation, vol. 3, no. 3, pp. 376-413, https://doi.org/10.3390/cmd3030023
Kregl, L, Wallner, GM, Lang, RW & Mayrhofer, G 2017, 'Effect of resin modifiers on the structural properties of epoxy resins', Journal of Applied Polymer Science, vol. 134, no. 44, article 45348, https://doi.org/10.1002/app.45348
Krzywi?ski, K & Sadowski, ? 2019, 'The effect of texturing of the surface of concrete substrate on the pull-off strength of epoxy resin coating', Coatings, vol. 9, no. 2, article 143, https://doi.org/10.3390/coatings9020143
Kusrini, E, Oktavianto, F, Usman, A, Mawarni, DP & Alhamid, MI 2020,’Synthesis, characterization, and performance of graphene oxide and phosphorylated graphene oxide as additive in water-based drilling fluids’, Applied Surface Science, vol. 506, 145005, https://doi.org/10.1016/j.apsusc.2019.145005
Lee, JH & Lee, DW 2020, 'Contact-induced molecular rearrangement of acrylic acid-incorporated pressure sensitive adhesives', Applied Surface Science, vol. 500, article 144246, https://doi.org/10.1016/j.apsusc.2019.144246
Lee, SB, Lee, HJ & Hong, IK 2012, 'Diluent filler particle size effect for thermal stability of epoxy type resin', Journal of Industrial and Engineering Chemistry, vol. 18, no. 2, pp. 635–641, https://doi.org/10.1016/j.jiec.2011.11.030
Li, K, Li, Y, Lian, Q, Cheng, J & Zhang, J 2016, 'Influence of cross-linking density on the structure and properties of the interphase within supported ultrathin epoxy films', Journal of Materials Science, vol. 51, no. 19, pp. 9019-9030, https://doi.org/10.1007/s10853-016-0155-6
Lionetto, F, Timo, A & Frigione, M 2015, 'Curing kinetics of epoxy-deep eutectic solvent mixtures', Thermochimica Acta, vol. 612, pp. 70-78, https://doi.org/10.1016/j.tca.2015.05.004
Liu, M, Mao, X, Zhu, H, Lin, A & Wang, D 2013, 'Water and corrosion resistance of epoxy–acrylic–amine waterborne coatings: Effects of resin molecular weight, polar group and hydrophobic segment', Corrosion Science, vol. 75, pp. 106-113, https://doi.org/10.1016/j.corsci.2013.05.020
Maruyama, T, Katoh, S, Nakajima, M, Nabetani, H, Abbott, TP, Shono, A, & Satoh, K 2001, 'FT-IR analysis of BSA fouled on ultrafiltration and microfiltration membranes', Journal of Membrane Science, vol. 192, no. 1-2, pp. 201-207, https://doi.org/10.1016/s0376-7388(01)00502-6
Miturska, I, Rudawska, A, Müller, M & Valášek, P 2020, 'The influence of modification with natural fillers on the mechanical properties of epoxy adhesive compositions after storage time', Materials, vol. 13, no. 2, article 291, https://doi.org/10.3390/ma13020291
Nazari, MH, Zhang, Y, Mahmoodi, A, Xu, G, Yu, J, Wu, J & Shi, X 2022, 'Nanocomposite organic coatings for corrosion protection of metals: A review of recent advances', Progress in Organic Coatings, vol. 162, article 106573, https://doi.org/10.1016/j.porgcoat.2021.106573
Ningrum, EO, Khoiroh, I, Nastiti, HI, Affan, RA, Karisma, AD, Agustiani, E, Surono A, Suroto, H, Suprapto, S, Taji, LS & Widiyanto, S 2023, 'Surface coating effect on corrosion resistance of titanium alloy bone implants by anodizing method', International Journal of Technology, vol. 14, no. 4, pp. 749-760, https://doi.org/10.14716/ijtech.v14i4.6146
Nurhayati, RW, Laksono, AL, Salwa, A, Pangesty, AI, Whulanza, Y & Mubarok, W 2023, 'The effect of umbilical cord blood serum and platelet-rich plasma coatings on the characteristics of poly(-caprolactone) scaffolds for skin tissue engineering applications', International Journal of Technology, vol. 14, no. 7, pp. 1596-1604, https://doi.org/10.14716/ijtech.v14i7.6709
Ozgul, EO & Ozkul, MH 2018, 'Effects of epoxy, hardener, and diluent types on the hardened state properties of epoxy mortars', Construction and Building Materials, vol. 187, pp. 360–370, https://doi.org/10.1016/j.conbuildmat.2018.07.215
Pizzi, A & Mittal, KL 2017, Handbook of adhesive technology, Third Edition, CRC Press, https://doi.org/10.1201/9781315120942
Pramanik, M, Mendon, SK & Rawlins, JW 2012, 'Determination of epoxy equivalent weight of glycidyl ether based epoxides via near infrared spectroscopy', Polymer Testing, vol. 31, no. 5, pp. 716-721, https://doi.org/10.1016/j.polymertesting.2012.04.004
Riyanto, R, Jazuli, MM, Sahroni, I, Musawwa, MM, Cahyandaru, N & Wahyuni, ET 2023, 'A simple technique for the corrosion inhibition of underwater cannonball from a shipwreck', International Journal of Technology, vol. 14, no. 4, pp. 843-853, https://doi.org/10.14716/ijtech.v14i4.4240
Roza, FN, Herliansyah, MK, Setianto, BY & Githanadi, B 2024, 'Preparation and characterization of curcumin-based coating material on Co-Cr alloy', International Journal of Technology, vol. 15, no. 1, pp. 18-27, https://doi.org/10.14716/ijtech.v15i1.3588
Rudawska, A & Frigione, M 2022, 'Effect of diluents on mechanical characteristics of epoxy compounds', Polymers, vol. 14, no. 11, article 2277, https://doi.org/10.3390/polym14112277
Rudawska, A 2020a, 'Experimental study of mechanical properties of epoxy compounds modified with calcium carbonate and carbon after hygrothermal exposure', Materials, vol. 13, no. 23, article 5439, https://doi.org/10.3390/ma13235439
Rudawska, A 2020b, 'The effect of the salt water aging on the mechanical properties of epoxy adhesives compounds', Polymers, vol. 12, no. 4, article 843, https://doi.org/10.3390/polym12040843
Sabergaliyev, M, Yeligbayeva, G, Khassanov, D, Muradova, S, Orazalin, Zh, Ainakulova, D, Sharipov, R & Negim, E-S 2024, 'Modified bitumen-polymer mastic to protect metal coatings from corrosion', Kompleksnoe Ispol?zovanie Mineral?nogo syr?â/Complex Use of Mineral Resources/Mineraldik Shikisattardy Keshendi Paidalanu, vol. 331, no. 4, pp. 12–20, https://doi.org/10.31643/2024/6445.35
Samyn, P, Bosmans, J & Cosemans, P 2023, 'Role of bio-based and fossil-based reactive diluents in epoxy coatings with amine and phenalkamine crosslinker', Polymers, vol. 15, no. 19, article 3856, https://doi.org/10.3390/polym15193856
Septriansyah, V, Saloma, Nurjannah, SA, Saggaff, A, Usman, AP & Ngian, SP 2025, 'Effect of the nano-silica addition on the mechanical properties of polymer concrete', Science and Technology Indonesia, vol. 10, no. 1, pp. 9-17, https://doi.org/10.26554/sti.2025.10.1.9-17
Signorini, C, Nobili, A, Sola, A & Messori, M 2020, 'Designing epoxy viscosity for optimal mechanical performance of coated glass textile reinforced mortar (GTRM) composites', Construction and Building Materials, vol. 233, article 117325, https://doi.org/10.1016/j.conbuildmat.2019.117325
Sinha, A, Islam Khan, N, Das, S, Zhang, J & Halder, S 2017, 'Effect of reactive and non-reactive diluents on thermal and mechanical properties of epoxy resin', High Performance Polymers, vol. 30, no. 10, pp. 1159-1168, https://doi.org/10.1177/0954008317743307
Syrmanova, K, Negim, E, Kaldybekova, J & Tuleuov, AM 2016, 'Epoxylitane compositions modification with using thermoplastic polyurethane', Oriental Journal of Chemistry, vol. 32, no. 1, pp. 1-7, https://doi.org/10.13005/ojc/320101
Szewczak, A 2023, 'Changes in the rheological and adhesive properties of epoxy resin used in the technology of reinforcement of structural elements with CFRP tapes', Materials, vol. 16, no. 23, article 7408, https://doi.org/10.3390/ma16237408
The, PL, Mariatti, M, Akil, HM, Yeoh, CK, Seetharamu, KN, Wagiman, ANR & Beh, KS 2007, 'The properties of epoxy resin coated silica fillers composites', Materials Letters, vol. 61, no. 11–12, pp. 2156-2158, https://doi.org/10.1016/j.matlet.2006.08.036
Tran, AD, Koch, T, Knaack, P & Liska, R 2020, 'Radical induced cationic frontal polymerization for preparation of epoxy composites', Composites Part A: Applied Science and Manufacturing, vol. 132, p. 105855, https://doi.org/10.1016/j.compositesa.2020.105855
Tulegenkyzy, AD, Megat-Yusoff, PSM, Al Azzam, KM, Kairatovna, BL, Goyal, A, Eshmaiel, G, Negim, E, Samy, M & Ravindran, B 2024, 'Synthesis, characterization, and application of poly(styrene-co-glycidyl methacrylate) as reactive diluents to epoxy resin', International Journal of Technology, vol. 15, no. 4, pp. 903-916, https://doi.org/10.14716/ijtech.v15i4.6920
Tzoumani, I, Soto Beobide, A, Iatridi, Z, Voyiatzis, GA, Bokias, G & Kallitsis, JK 2022, 'Glycidyl methacrylate-based copolymers as healing agents of waterborne polyurethanes', International Journal of Molecular Sciences, vol. 23, no. 15, article 8118, https://doi.org/10.3390/ijms23158118
Vadamalar, R, Mani, P & Balakrishnan, R 2014, 'Molecular mechanics study on the interaction of MMA with higher alcohols and organic solvents', Research Journal of Chemical Sciences, vol. 4, no. 8, pp. 46-53, https://www.isca.me/rjcs/Archives/v4/i8/8.ISCA-RJCS-2014-121.php
Wang, H, Han, W, Tian, H & Wang, Y 2005, 'The preparation and properties of glass powder reinforced epoxy resin', Materials Letters, vol. 59, no. 1, pp. 94-99, https://doi.org/10.1016/j.matlet.2004.09.024
Xie, Z, Tian, Y, Xu, Y, Zhong, F, Li, S, Zhu, X & Yuan, Q 2025, 'Molecular design of epoxy resin and the driving forces in adhesion with cementitious materials', Applied Surface Science, vol. 689, p. 162498, https://doi.org/10.1016/j.apsusc.2025.162498
Yang, K, Zhao, Y & Liu, X 2024, 'Enhanced breakdown strength of epoxy composites by constructing dual-interface charge barriers at the micron filler/epoxy matrix interface', Composites Part B: Engineering, vol. 283, article 111602, https://doi.org/10.1016/j.compositesb.2024.111602
