Natural Gas Sweetening via Membrane-Assisted Gas Absorption Part 2: A Hollow-Fiber Unit with Dimethyl Diethanolammonium Glycinate-based Absorbent
Corresponding email: kriuchkov.s.s@muctr.ru
Published at : 01 Dec 2025
Volume : IJtech
Vol 16, No 6 (2025)
DOI : https://doi.org/10.14716/ijtech.v16i6.7625
Atlaskina, M., Petukhov, A., Atlaskin, A., Smorodin, K., Zarubin, D., Tsivkovsky, N., Kryuchkov, S., Stepakova, A., Vorotyntsev, A., Kazarina, O., Suvorov, S., Stepanova, E., & Vorotyntsev, I. (2025). Natural gas sweetening via membrane-assisted gas absorption part 2: A hollow-fiber unit with dimethyl diethanolammonium glycinate-based absorbent. International Journal of Technology, 16 (6), 2025–2042.
| Maria E Atlaskina | Mendeleev University of Chemical Technology of Russia, Miusskaya square, 9, Moscow, 125047 Russia |
| Anton N Petukhov | 1. Mendeleev University of Chemical Technology of Russia, Miusskaya square, 9, Moscow, 125047 Russia 2. Lobachevsky State University of Nizhny Novgorod, 23 Gagarin Avenue, Nizhny Novgorod, 603950, Ru |
| Artem A Atlaskin | Mendeleev University of Chemical Technology of Russia, Miusskaya square, 9, Moscow, 125047 Russia |
| Kirill A Smorodin | Mendeleev University of Chemical Technology of Russia, Miusskaya square, 9, Moscow, 125047 Russia |
| Dmitriy M Zarubin | 1. Mendeleev University of Chemical Technology of Russia, Miusskaya square, 9, Moscow, 125047 Russia 2. Lobachevsky State University of Nizhny Novgorod, 23 Gagarin Avenue, Nizhny Novgorod, 603950, Ru |
| Nikita S Tsivkovsky | Mendeleev University of Chemical Technology of Russia, Miusskaya square, 9, Moscow, 125047 Russia |
| Sergey S Kryuchkov | Mendeleev University of Chemical Technology of Russia, Miusskaya square, 9, Moscow, 125047 Russia |
| Anna N Stepakova | Mendeleev University of Chemical Technology of Russia, Miusskaya square, 9, Moscow, 125047 Russia |
| Andrey V Vorotyntsev | Lobachevsky State University of Nizhny Novgorod, 23 Gagarin Avenue, Nizhny Novgorod, 603950, Russia |
| Olga V Kazarina | 1. Mendeleev University of Chemical Technology of Russia, Miusskaya square, 9, Moscow, 125047 Russia 2. Lobachevsky State University of Nizhny Novgorod, 23 Gagarin Avenue, Nizhny Novgorod, 603950, Ru |
| Sergey S Suvorov | Lobachevsky State University of Nizhny Novgorod, 23 Gagarin Avenue, Nizhny Novgorod, 603950, Russia |
| Ekaterina A Stepanova | Lobachevsky State University of Nizhny Novgorod, 23 Gagarin Avenue, Nizhny Novgorod, 603950, Russia |
| Ilya V Vorotyntsev | Mendeleev University of Chemical Technology of Russia, Miusskaya square, 9, Moscow, 125047 Russia |
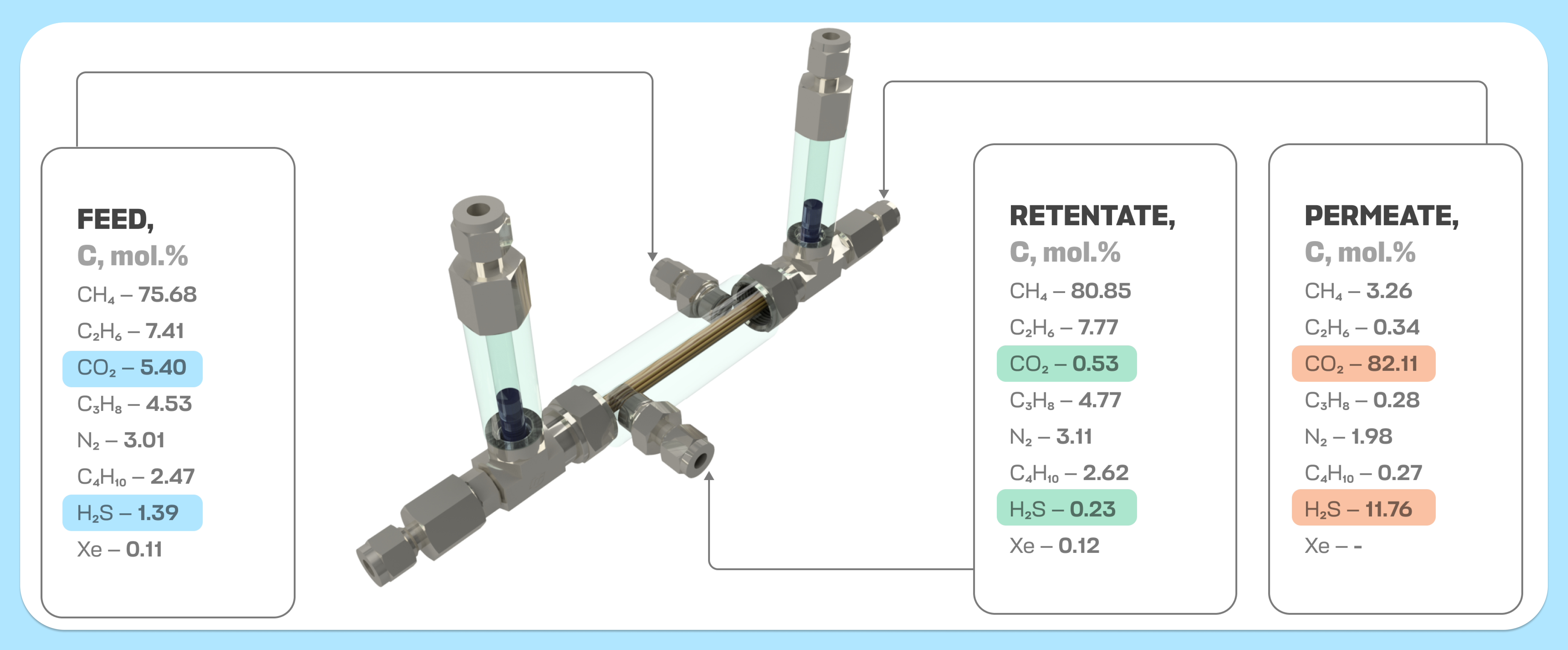
The present study deals with the continuation of the development, enhancement, and optimization of a novel hybrid separation method – membrane assisted gas absorption, which is designed for the removal of acid gases from natural gas processing. The second part focuses on the design of absorbent solutions and their application in the proposed technique to increase the efficiency of acid gas removal and decrease the losses of hydrocarbons. Absorbent systems based on methyldiethanolamine aqueous solutions and containing a novel ionic liquid, dimethyl diethanolammonium glycinate, were proposed and comprehensively studied in terms of the properties that affect the mass transfer rate: sorption capacity, viscosity, and density. As a result of that complex absorbents study, its optimal composition was determined for further separation tests in a membrane-assisted gas absorption unit. On the example of the model ternary gas mixture and quasi-real natural gas separation, the proposed technique provides efficient separation. It not only reduces the concentration of acid gases up to 0.75 mol% but also allows the recovery of 99% of hydrocarbons as a product flow.
Gas separation; Hollow fibers; Ionic liquids; Membrane-assisted gas absorption; Natural gas sweetening
| Filename | Description |
|---|---|
| R2-CE-7625-20250502185339.pdf | --- |
Aghel, B., Janati, S., Wongwises, S., & Shadloo, M.
(2022). Review on CO2 capture by blended amine solutions. International Journal
of Greenhouse Gas Control, 119, 103715. https://doi.org/10.1016/j.ijggc.2022.103715
Ahmady, A., Hashim, M., & Aroua, M. (2010).
Experimental investigation on the solubility and initial rate of absorption of
CO2 in aqueous mixtures of methyldiethanolamine with the ionic liquid
1-butyl-3-methylimidazolium tetrafluoroborate. Journal of Chemical and
Engineering Data, 55 (12), 5733–5738. https://doi.org/10.1021/je1006949
Akhmetshina, A., Gumerova, O., Atlaskin, A., Petukhov, A.,
Sazanova, T., Yanbikov, N., Nyuchev, A., Razov, E., & Vorotyntsev, I.
(2017). Permeability and selectivity of acid gases in supported conventional
and novel imidazolium-based ionic liquid membranes. Separation and Purification
Technology, 176, 92–106. https://doi.org/10.1016/j.seppur.2016.11.074
Akhmetshina, A., Petukhov, A., Gumerova, O., Vorotyntsev,
A., Nyuchev, A., & Vorotyntsev, I. (2019). Solubility of H2S and CO2 in
imidazolium-based ionic liquids with bis(2-ethylhexyl) sulfosuccinate anion.
Journal of Chemical Thermodynamics, 130, 173–182. https://doi.org/10.1016/j.jct.2018.10.013
Akhmetshina, A., Petukhov, A., Vorotyntsev, A., Nyuchev,
A., & Vorotyntsev, I. (2017). Absorption behavior of acid gases in protic
ionic liquid/alkanolamine binary mixtures. ACS Sustainable Chemistry &
Engineering, 5 (4), 3429–3437. https://doi.org/10.1021/acssuschemeng.7b00092
Anggerta, L., Kurniawansyah, F., Tetrisyanda, R., &
Wibawa, G. (2025). Catalytic synthesis of diethyl carbonate from carbon dioxide
using catalyst KI/EtONa with propylene oxide as dehydration agent and process
optimization based on Box–Behnken design. International Journal of Technology,
16 (1), 243–254. https://doi.org/10.14716/ijtech.v16i1.6417
Anselmi, H., Mirgaux, O., Bounaceur, R., & Patisson, F.
(2019). Simulation of post-combustion CO2 capture: A comparison among
absorption, adsorption and membranes. Chemical Engineering & Technology, 42
(4), 797–804. https://doi.org/10.1002/ceat.201800667
Atlaskin, A., Kryuchkov, S., Smorodin, K., Markov, A.,
Kazarina, O., Zarubin, D., Atlaskina, M., Vorotyntsev, A., Nyuchev, A.,
Petukhov, A., & Vorotyntsev, I. (2021). Towards the potential of
trihexyltetradecylphosphonium indazolide with aprotic heterocyclic ionic liquid
as an efficient absorbent for membrane-assisted gas absorption technique for
acid gas removal applications. Separation and Purification Technology, 257,
117835. https://doi.org/10.1016/j.seppur.2020.117835
Atlaskin, A., Kryuchkov, S., Yanbikov, N., Smorodin, K.,
Petukhov, A., Trubyanov, M., Vorotyntsev, V., & Vorotyntsev, I. (2020).
Comprehensive experimental study of acid gases removal process by
membrane-assisted gas absorption using imidazolium ionic liquids solutions
absorbent. Separation and Purification Technology, 239, 116578. https://doi.org/10.1016/j.seppur.2020.116578
Atlaskina, M., Markin, Z., Smorodin, K., Kryuchkov, S.,
Tsivkovsky, N., Petukhov, A., Atlaskin, A., Kazarina, O., Vorotyntsev, A.,
& Vorotyntsev, I. (2025). Optimized co2 cycloaddition to epichlorohydrin
catalyzed by ionic liquid with microwave and ultrasonic irradiation.
International Journal of Technology, 16 (2), 378–394. https://doi.org/10.14716/ijtech.v16i2.7500
Atlaskina, M., Atlaskin, A., Kazarina, O., Petukhov, A.,
Zarubin, D., Nyuchev, A., Vorotyntsev, A., & Vorotyntsev, I. (2021).
Synthesis and comprehensive study of quaternary-ammonium-based sorbents for
natural gas sweetening. Environments, 8 (12), 134. https://doi.org/10.3390/environments8120134
Atlaskina, M., Kazarina, O., Petukhov, A., Atlaskin, A.,
Tsivkovsky, N., Tiuleanu, P., Malysheva, Y., Lin, H., Zhong, G., Lukoyanov, A.,
Vorotyntsev, A., & Vorotyntsev, I. (2023). Amino acid-based ionic liquid as
a promising co2 sorption increasing agent by aqueous mdea solution. Journal of
Molecular Liquids, 395, 123635. https://doi.org/10.1016/j.molliq.2023.123635
Bahadori, A. (2014). Natural gas processing: Technology and
engineering design. Elsevier. https://doi.org/10.1016/C2013-0-13070-X
Baker, R. (2002). Future directions of membrane gas
separation technology. Industrial & Engineering Chemistry Research, 41 (6),
1393–1411. https://doi.org/10.1021/ie0108088
Baker, R., & Lokhandwala, K. (2008). Natural gas
processing with membranes: An overview. Industrial & Engineering Chemistry
Research, 47 (7), 2109–2121. https://doi.org/10.1021/ie071083w
Banat, F., Younas, O., & Didarul, I. (2014). Energy and
exergical dissection of a natural gas sweetening plant using methyldiethanol
amine (mdea) solution. Journal of Natural Gas Science and Engineering, 16, 1–7.
https://doi.org/10.1016/j.jngse.2013.10.005
Belaissaoui, B., Willson, D., & Favre, E. (2012).
Membrane gas separations and post-combustion carbon dioxide capture: Parametric
sensitivity and process integration strategies. Chemical Engineering Journal,
211–212, 122–132. https://doi.org/10.1016/j.cej.2012.09.012
Bernardo, P., Drioli, E., & Golemme, G. (2009).
Membrane gas separation: A review/state of the art. Industrial &
Engineering Chemistry Research, 48 (10), 4638–4663. https://doi.org/10.1021/ie8019032
Cheng, S., Wen, S., Zhao, J., Zhao, C., Li, W., Li, S.,
& Zhang, D. (2017). Mechanism and kinetics study of co2 absorption into
blends of mdea and [c2ohmim][gly] aqueous solution. Energy & Fuels, 31
(11), 12425–12433. https://doi.org/10.1021/acs.energyfuels.7b01942
Donaldson, T., & Nguyen, Y. (1980). Carbon dioxide
reaction kinetics and transport in aqueous amine membranes. Industrial &
Engineering Chemistry Fundamentals, 19 (3), 260–266. https://doi.org/10.1021/i160075a005
Duval, S. (2023). Natural gas sweetening. In Q. Wang (Ed.),
Surface process, transportation, and storage (Vol. 4, pp. 37–78). Gulf
Professional Publishing. https://doi.org/10.1016/B978-0-12-823891-2.00007-7
Feng, Z., Fang, C., Wu, Y., Wang, Y., Li, A., & Zhang,
Z. (2010). Absorption of CO2 in aqueous solutions of functionalized ionic
liquids and MDEA. Chemical Engineering Journal, 160(2), 691–697. https://doi.org/10.1016/j.cej.2010.04.013
Feng, Z., Ma, J., Zhou, Z., Wu, Y., & Zhang, Z. (2012).
Study on the absorption of carbon dioxide in high concentrated MDEA and ILs
solutions. Chemical Engineering Journal, 181–182, 222–228. https://doi.org/10.1016/j.cej.2011.11.066
Flores, R. (2014). Coalification, gasification, and gas
storage. In R. M. Flores (Ed.), Coal and coalbed gas (pp. 167–233). Elsevier. https://doi.org/10.1016/B978-0-12-396972-9.00004-5
Fu, D., Zhang, P., & Wang, L. (2016). Absorption
performance of CO2 in high concentrated [BMIM][Lys]-MDEA aqueous solution.
Energy, 113, 1–8. https://doi.org/10.1016/j.energy.2016.07.049
Hadri, N., Quang, D., & Abu-Zahra, M. (2015). Study of
novel solvent for CO2 post-combustion capture. Energy Procedia, 75, 2268–2286. https://doi.org/10.1016/j.egypro.2015.07.414
Hatchell, D., Namjoshi, O., Fischer, K., & Rochelle, G.
(2014). Thermal degradation of linear amines for CO2 capture. Energy Procedia,
63, 1558–1568. https://doi.org/10.1016/j.egypro.2014.11.165
Ibrahim, M., El-Naas, M., Zhang, Z., & Van Der Bruggen,
B. (2018). CO2 capture using hollow fiber membranes: A review of membrane
wetting. Energy & Fuels, 32(2), 963–978. https://doi.org/10.1021/acs.energyfuels.7b03493
Islam, M., Yusoff, R., Si Ali, B., Islam, M., &
Chakrabarti, B. (2011). Degradation studies of amines and alkanolamines during
sour gas treatment process. International Journal of the Physical Sciences,
6(24), 5877–5890. https://doi.org/10.5897/ijps11.237
Kartohardjono, S., Paramitha, A., Putri, A., &
Andriant, R. (2017). Effects of absorbent flow rate on CO2 absorption through a
super hydrophobic hollow fiber membrane contactor. International Journal of
Technology, 8(8), 1429–1435. https://doi.org/10.14716/ijtech.v8i8.679
Kryuchkov, S., Smorodin, K., Stepakova, A., Atlaskin, A.,
Tsivkovsky, N., Atlaskina, M., Tolmacheva, M., Kazarina, O., Petukhov, A.,
Vorotyntsev, A., & Vorotyntsev, I. (2024). Membrane air separation process
simulation: Insight in modelling approach based on ideal and mixed permeance
values. International Journal of Technology, 15(5), 1218–1236. https://doi.org/10.14716/ijtech.v15i5.6987
Kryuchkov, S., Petukhov, A., & Atlaskin, A. (2021).
Experimental evaluation of the membrane-assisted gas absorption technique
efficiency using an aqueous solution of PEG-400 for the ammonia capture. IOP
Conference Series: Earth and Environmental Science, 666(5), 052071. https://doi.org/10.1088/1755-1315/666/5/052071
Kusrini, E., Sasongko, A., Nasruddin, N., & Usman, A.
(2017). Improvement of carbon dioxide capture using graphite waste/Fe3O4
composites. International Journal of Technology, 8(8), 1436–1444. https://doi.org/10.14716/ijtech.v8i8.697
Li, K., Leigh, W., Feron, P., Yu, H., & Tade, M.
(2016). Systematic study of aqueous monoethanolamine (MEA)-based CO2 capture
process: Techno-economic assessment of the MEA process and its improvements.
Applied Energy, 165, 648–659. https://doi.org/10.1016/j.apenergy.2015.12.109
Li, M., Zhang, P., Chen, G., & Fu, D. (2023). The
performance and mechanism of CO2 desorption and corrosion in
N-methyldiethanolamine aqueous solutions blended with amino acid ionic liquids.
International Journal of Greenhouse Gas Control, 125, 103875. https://doi.org/10.1016/j.ijggc.2023.103875
Mechergui, A., Akhmetshina, A., Kazarina, O., Atlaskina,
M., Petukhov, A., & Vorotyntsev, I. (2020). Acidic gases solubility in
bis(2-ethylhexyl) sulfosuccinate based ionic liquids using the predictive
thermodynamic model. Membranes, 10(12), 429. https://doi.org/10.3390/membranes10120429
Merkel, T., Lin, H., Wei, X., & Baker, R. (2010a).
Power plant post-combustion carbon dioxide capture: An opportunity for
membranes. Journal of Membrane Science, 359(1–2), 126–139. https://doi.org/10.1016/j.memsci.2009.10.041
Merkel, T., Lin, H., Wei, X., & Baker, R. (2010b).
Power plant post-combustion carbon dioxide capture: An opportunity for
membranes. Journal of Membrane Science, 359(1–2), 126–139. https://doi.org/10.1016/j.memsci.2009.10.041
Mokhatab, S., Poe, W., & Mak, J. (2019). Natural gas
fundamentals. In S. Mokhatab, W. A. Poe, & J. Y. Mak (Eds.), Handbook of
natural gas transmission and processing (4th ed., pp. 1–35). Gulf Professional
Publishing. https://doi.org/10.1016/B978-0-12-815817-3.00001-0
Muharam, Y., Kusrini, E., Saubryani, N., & Ulfa, M.
(2018). Simulation of MOF-based adsorbed natural gas storage tank.
International Journal of Technology, 9(2), 412–421. https://doi.org/10.14716/ijtech.v9i2.1100
Nozaeim, A., Tavasoli, A., Mortaheb, H., & Mafi, M.
(2020). CO2 absorption/desorption in DEEA/MDEA and hybrids with sulfolane.
Journal of Natural Gas Science and Engineering, 76, 103219. https://doi.org/10.1016/j.jngse.2020.103219
Petukhov, A., Atlaskin, A., Kryuchkov, S., Smorodin, K.,
Zarubin, D., Petukhova, A., Atlaskina, M., Nyuchev, A., Vorotyntsev, A.,
Trubyanov, M., Vorotyntsev, I., & Vorotynstev, V. (2020). Membrane-assisted
gas absorption for ammonia recovery. Chemical Engineering Journal, 421, 127726.
https://doi.org/10.1016/j.cej.2020.127726
Petukhov, A., Atlaskin, A., Kryuchkov, S., Smorodin, K.,
Zarubin, D., Petukhova, A., Atlaskina, M., Nyuchev, A., Vorotyntsev, A.,
Trubyanov, M., Vorotyntsev, I., & Vorotynstev, V. (2021). Membrane-assisted
gas absorption for ammonia recovery. Chemical Engineering Journal, 421, 127726.
https://doi.org/10.1016/j.cej.2020.127726
Poe, W., & Mokhatab, S. (2017). Introduction to natural
gas processing plants. In Modeling, control, and optimization of natural gas
processing plants (pp. 1–72). Gulf Professional Publishing. https://doi.org/10.1016/B978-0-12-802961-9.00001-2
Sanaeepur, H., Amooghin, A., Bandehali, S., Moghadassi, A.,
Matsuura, T., & Van der Bruggen, B. (2019). Polyimides in membrane gas
separation. Progress in Polymer Science, 91, 80–125. https://doi.org/10.1016/j.progpolymsci.2019.02.001
Shohrat, A., Zhang, M., Hu, H., Yang, X., Liu, L., &
Huang, H. (2022). CO2 capture mechanism of ionic liquids from TFA blended with
MEA and MDEA. International Journal of Greenhouse Gas Control, 119, 103709. https://doi.org/10.1016/j.ijggc.2022.103709
Swatloski, R., Holbrey, J., & Rogers, R. (2003). Ionic
liquids are not always green: Hydrolysis of BMIM-PF6. Green Chemistry, 5(4),
361–363. https://doi.org/10.1039/B304400A
Vorotyntsev, I., Atlaskin, A., Trubyanov, M., Petukhov, A.,
Gumerova, O., Akhmetshina, A., & Vorotyntsev, V. (2017). Absorbing
pervaporation for gas separation using ionic liquids. Desalination and Water
Treatment, 75, 305–313. https://doi.org/10.5004/dwt.2017.20400
Vorotyntsev, I., Drozdov, P., Shablikin, D., &
Gamajunova, T. (2006). Ammonia separation by absorbing pervaporation.
Desalination, 200(1–3), 379–380. https://doi.org/10.1016/j.desal.2006.03.382
Vorotyntsev, V., Drozdov, P., Vorotyntsev, I., & Murav’Ev,
D. (2006). Fine gas purification using membrane module with feed reservoir.
Doklady Chemistry, 411(5), 243–245. https://doi.org/10.1134/S0012500806120068
Zhao, Y., Zhang, X., Zeng, S., Zhou, Q., Dong, H., Tian, X., & Zhang, S. (2010). Density, viscosity, and CO2 capture performance in amine + ionic liquid + water systems. Journal of Chemical & Engineering Data, 55(8), 3513–3519. https://doi.org/10.1021/je100078w
