Optimized CO2 Cycloaddition to Epichlorohydrin Catalyzed by Ionic Liquid with Microwave and Ultrasonic Irradiation
Corresponding email: atlaskina.m.e@gmail.com
Published at : 25 Mar 2025
Volume : IJtech
Vol 16, No 2 (2025)
DOI : https://doi.org/10.14716/ijtech.v16i2.7500
Atlaskina, M, Markin, Z, Smorodin, K, Kryuchkov, S, Tsivkovsky, N, Petukhov, A, Atlaskin, A, Kazarina, O, Vorotyntsev, A & Vorotyntsev, I 2025, ‘Optimized CO2 cycloaddition to epichlorohydrin catalyzed by ionic liquid with microwave and ultrasonic irradiation’, International Journal of Technology, vol. 16, no. 2, pp. 378-394
| Maria Atlaskina | Laboratory of SMART Polymeric Materials and Technologies, Mendeleev University of Chemical Technology of Russia, Miusskaya square, 9, Moscow, 125047 Russia |
| Zakhar Markin | Chemical Engineering Laboratory, Lobachevsky State University of Nizhny Novgorod, 23 Gagarin Avenue, Nizhny Novgorod, 603022, Russia |
| Kirill Smorodin | Laboratory of SMART Polymeric Materials and Technologies, Mendeleev University of Chemical Technology of Russia, Miusskaya square, 9, Moscow, 125047 Russia |
| Sergey Kryuchkov | Laboratory of SMART Polymeric Materials and Technologies, Mendeleev University of Chemical Technology of Russia, Miusskaya square, 9, Moscow, 125047 Russia |
| Nikita Tsivkovsky | Laboratory of SMART Polymeric Materials and Technologies, Mendeleev University of Chemical Technology of Russia, Miusskaya square, 9, Moscow, 125047 Russia |
| Anton Petukhov | Chemical Engineering Laboratory, Lobachevsky State University of Nizhny Novgorod, 23 Gagarin Avenue, Nizhny Novgorod, 603022, Russia |
| Artem Atlaskin | Laboratory of SMART Polymeric Materials and Technologies, Mendeleev University of Chemical Technology of Russia, Miusskaya square, 9, Moscow, 125047 Russia |
| Olga Kazarina | Laboratory of SMART Polymeric Materials and Technologies, Mendeleev University of Chemical Technology of Russia, Miusskaya square, 9, Moscow, 125047 Russia |
| Andrey Vorotyntsev | Chemical Engineering Laboratory, Lobachevsky State University of Nizhny Novgorod, 23 Gagarin Avenue, Nizhny Novgorod, 603022, Russia |
| Ilya Vorotyntsev | Laboratory of SMART Polymeric Materials and Technologies, Mendeleev University of Chemical Technology of Russia, Miusskaya square, 9, Moscow, 125047 Russia |
The capture, storage, and conversion of anthropogenic carbon dioxide (CO2) is one of the urgent environmental challenges. Among the various methods available, direct conversion is the most preferable. An example is the production of cyclic carbonates which are important products in the chemical industry. Considering that this method relies on catalysis, different catalysts can be used. Ionic liquid has been proposed as a promising candidate but the application in the process remains underexplored. Therefore, this study aimed to synthesize and comprehensively characterize ionic liquid 1-(2-hydroxyethyl)-3-methylimidazolium bromide using spectral techniques, including 1H and 13C NMR, Fourier transform infrared spectroscopy (FTIR), and matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF). This compound was used as a catalyst in the cycloaddition reaction of CO2 with epichlorohydrin to produce 1-chloromethylpropylenecarbonate, comprising an important and in-demand class of organics namely cyclocarbonates. Key reaction parameters including temperature, pressure, and catalyst loading were systematically evaluated for the effects on epichlorohydrin conversion, product yield, and selectivity. The results showed that optimal conditions were found at 90 °C and 650 kPa, with a catalyst loading of 2 mol%. The main product was obtained at a yield of 94% with a selectivity of 97% within 2 hours. Additionally, methods to intensify the catalytic reaction were explored through the application of ultrasonic and microwave irradiation. Both forms of irradiation significantly accelerated the conversion of epichlorohydrin, increasing the catalyst turnover frequency (TOF) from 3.85 h-1 in the blank experiment to 4.50 h-1 and 8.38 h-1 with microwave and ultrasonic irradiation, respectively. The practical ease of integrating these approaches into industrial setups suggests promising potential for technological application. Finally, the feasibility of catalyst recycling was investigated. The 1-(2-hydroxyethyl)-3-methylimidazolium catalyst had stable catalytic activity, with only minimal activity loss observed over five consecutive cycles.
Carbon dioxide utilization; Cyclic carbonates; Cycloaddition; Ionic liquid
Carbon dioxide (CO2) is a greenhouse gas and the increasing atmospheric concentration disrupts the planet thermoregulation, making it the primary cause of global climate change (Nunes, 2023). The search and development of technologies for CO2 utilization is a major task of science and technology (Fatah et al., 2020; Guo et al., 2020; Vega et al., 2020). In recent years, there have been quite a few methods of CO2 extraction from emission sources. The traditional method for the removal of CO2 from gas mixtures is chemical absorption by alkanolamine solutions. However, new energy-efficient approaches for example, membrane gas separation (Kryuchkov et al., 2024; Petukhov et al., 2021; Dmitrenko et al., 2020; Trubyanov et al., 2019; Penkova et al., 2018; Dmitrenko et al., 2017; Kartohardjono et al., 2017; Vorotyntsev et al., 2006) are attracting the attention of different studies and the industry.
More unconventional methods are also being developed, such as CO2 absorption using ionic liquids (Atlaskina et al., 2024; Vorotyntsev et al., 2017). Although these methods provide ways to effectively remove CO2 from the source, direct recycling into any useful products does not occur. This is because the conversion is a substantial challenge.
Due to the high thermodynamic and kinetic stability of CO2, the utilization is a very non-trivial task (Valluri et al., 2022; Lu et al., 2022; Mustafa et al., 2020; Anwar et al., 2020). In this regard, catalytic processes play a special role. For example, the conversion of CO2 occurs into carbonates by the interaction between epoxides and CO2 (Anggerta et al., 2025; Lopes et al., 2020; Luo et al., 2020; Pal et al., 2020; Zhang et al., 2020).
Lewis acids can catalyze these transformations quite efficiently. However, common Lewis acids, such as aluminum chloride, are often toxic and highly corrosive, complicating technological processes. The presence of chlorine ions in the reaction medium can also lead to the formation of phosgene. Therefore, the development of catalysts devoid of the above-mentioned disadvantages is of particular interest. Numerous transition metal-based catalysts have been developed to produce cyclic carbonates by coupling CO2 with epoxides, metal-organic frameworks (MOFs) (Zhang and Xu, 2024; Shah et al., 2022; Maina et al., 2017), transition metal complexes (Kinzel et al., 2021) and metal oxides. However, due to the toxicity of some transition metals, leaching leads to serious environmental pollution. Various non-metallic catalysts such as functionalized SiO2 (Ye et al., 2023), covalent organic frameworks (COFs) (He et al., 2020), and modified carbon materials have been developed to make the catalysis reaction more environmentally friendly. The disadvantages of these catalysts are related to the complicated and overly expensive preparation, as well as the need to use a special solvent during the catalytic reaction.
Another possible option is catalysis by ionic liquids (Lei et al., 2022; Mujmule and Kim, 2022; Qu et al., 2022; Li et al., 2021; Vorotyntsev et al., 2019). Metal-free ionic liquids have catalytic activity due to the presence of ions. For example, halogen anions act as nucleophiles in the reaction between CO2 and oxiranes (Ghorai et al., 2024). An additional advantage of using ionic liquid as catalyst is the ability to act not only as a catalyst but also a reaction medium and a sorbent for CO2 capture and retention (Akhmetshina et al., 2019). One of the most popular homogeneous salt-based catalysts for the synthesis of cyclic carbonates is imidazolium salts (Guo et al., 2021).
For CO2 conversion processes, the use of intensifying external forcing is typical (Islam et al., 2021). In the context of catalytic reactions, intensification of the reaction to produce cyclic carbonates is also possible by microwave or ultrasonic irradiation. Due to the polar and ionic nature, ionic liquids absorb microwave radiation efficiently (Orrling, 2009). A previous study (de la Hoz et al., 2005) reported that one of the effects of microwave radiation is the acceleration of reactions, provided polar substances or transition complexes participate in the process. In addition, microwave radiation is considered an environmentally friendly method that accelerates the time of various reactions (Aviantara et al., 2024; Sediawan et al., 2023). The effect of microwave irradiation power and time on the reaction parameters of cycloaddition of phenyl glycidyl ether (PGE) and CO2 using silica-supported ionic liquid was studied in (Dharman et al., 2011). With increasing microwave radiation power, the conversion and reaction yield decreased, but the selectivity remained constant. This is probably because the solubility of CO2 decreased with increasing microwave irradiation power. Substrate conversion increased with increasing irradiation time, reaching a maximum at 20 minutes, after which it did not significantly improve.
The use of ultrasound induces a physicochemical phenomenon, known as cavitation, in which cavities in liquid medium are sequentially formed, developed, and collapsed. When cavities collapse, energy is released. Due to the effect of cavitation arising from the use of ultrasonic ultrasound, there is an intensification of physical and chemical processes (Nisya et al., 2024; Ibrahim et al., 2020). In general, cavitation increases the reaction rate and product yield (Holkar et al., 2019).
Based on the description above, this study was conducted to investigate the possibility of intensifying the catalytic conversion of CO2 into cyclic carbonates using epichlorohydrin and ionic liquid as substrate and catalyst respectively. For this purpose, ionic liquid 1-(2-hydroxyethyl)-3-methylimidazolium bromide was synthesized and comprehensively characterized using spectral techniques, including 1H and 13C NMR, FTIR, and MALDI-TOF spectroscopy.
The effect of intensifying irradiation (ultrasonic and microwave) on the reaction of obtaining cyclic carbonates using this ionic liquid as a catalyst was studied for the first time. A UWave-2000 reactor was used and the process was carried out under the conditions of a reference experiment at 90 °C, constant CO2 pressure of 110 kPa, and catalyst concentration of 2 mol.%. The results showed that the use of these methods allows the reaction to be accelerated up to 2.5 times without significant losses in selectivity or product yield.
2.1. Chemicals and Materials
1-Methylimidazole (99 wt %, Acros Organics (Belgium)), 2-bromoethanol (99 wt %, Sigma-Aldrich (Germany)), ethyl acetate (< 99 wt %, Aldosa, Russia), phosphoric anhydride (98 wt%, CJSC Chimreaktiv (Russia)) and nitrogen ( 99.999 vol.%, and Monitoring LLC (Russia)) were used to synthesize ionic liquid 1-(2-hydroxyethyl)-3-methylimidazolium bromide. Epichlorohydrin (> 99 wt %, Chemical Line, Russia), CO2 (
99.99 vol.%, Monitoring LLC (Russia)) and the synthesized in the present work ionic liquid 1-(2-hydroxyethyl)-3-methylimidazolium bromide, were used to carry out the catalytic reaction for the production of cyclic carbonates. The reagents were used without further purification.
2.2. Catalysts Preparation and Characterization
NMR Spectroscopy. 1H NMR and 13C NMR spectra were recorded on an Agilent DD2 400 MHz spectrometer. Chemical shifts () are given in parts per million (ppm) for a solution of the compounds in DMSO-d6, with the residual solvent peak as an internal standard.
IR spectroscopy. IR spectra of the samples were recorded using an IRTracer-100 FTIR spectrophotometer (Shimadzu, Kyoto, Japan) equipped with a modified FTIR Attenuated Total Reflectance (ATR) attachment and a ZnSe crystal plate (PIKE Miracle, North Carolina, USA) in transmission mode at ambient temperature. A minimum of 30 scans were averaged with a resolution of 4 cm-1 in the range of 4000-600 cm-1.
MALDI. MALDI mass spectra were recorded on Bruker Microflex LT mass spectrometer in linear mode using trans-2-[3-(4-tert-Butylphenyl)-2-methyl-2-propenylidene]malononitrile (DCTB) as a matrix on a ground steel target plate.
Elemental analysis. The elemental composition of ionic liquid was determined using the Elemental Vario EL cube (Elementar Analysensysteme GmbH, Germany), which allows for the simultaneous analysis of hydrogen, carbon, nitrogen, bromine, and oxygen.
Procedure of ionic compound synthesis. Ionic liquid was obtained by the Menshutkin reaction (Figures S1 (Supplementary Information)). A recent study by (Koutsoukos et al., 2022) described an alternative synthesis method that entails the use of a solvent (ethyl acetate). However, further details on the synthesis route and process conditions were not prescribed. The reported product yield was 79%. In this study, the modified synthesis method was used by adding 2-bromoethanol into 1-methylimidazole in a molar ratio of 1:1 in an inert medium (nitrogen atmosphere). The reaction was carried out in a heat-resistant vial with a lid and Teflon septum, also under a nitrogen atmosphere. The completeness of the reaction was monitored using a Fourier Transform Infrared Spectrophotometer (Shimadzu IT-Racer-100) by the decrease in the intensity of the C-Br band (656 cm-1), showing the breaking of the C-Br bond in the initial halogen alcohol and the formation of imidazolium compound. After completion of the reaction (after 72 hours), the resulting ionic liquid was washed 3 times with ethyl acetate from the residues of unreacted initial compounds and dried over phosphoric anhydride in the desiccator. The isolated 1-(2-hydroxyethyl)-3-methylimidazolium bromide is white crystals with a melting point of 65-70 °C, and a yield of 94 %.
[C2OHmim][Br]: 1H NMR (400 MHz, DMSO-d6) 9.18 (d, J = 1.6 Hz, 1H), 7.75 (dt, J = 12.7, 1.8 Hz, 2H), 5.16 (s, 1H), 4.23 (dd, J = 5.7, 4.5 Hz, 2H), 3.87 (s, 3H), 3.74 - 3.67 (m, 2H). 13C NMR (101 MHz, DMSO-d6)
136.79, 123.32, 122.65, 59.30, 51.57, 35.72. Elemental Analysis C6H11BrN2O found (%): C, 34.78; H, 5.34; Br, 38.61; N, 13.57; O, 7.73; calculated (%): C, 34.80; H, 5.35; Br, 38.59; N, 13.53; O, 7.73; MALDI-TOF: m/z 127.2 (cation peak).
2.3. Catalytic tests
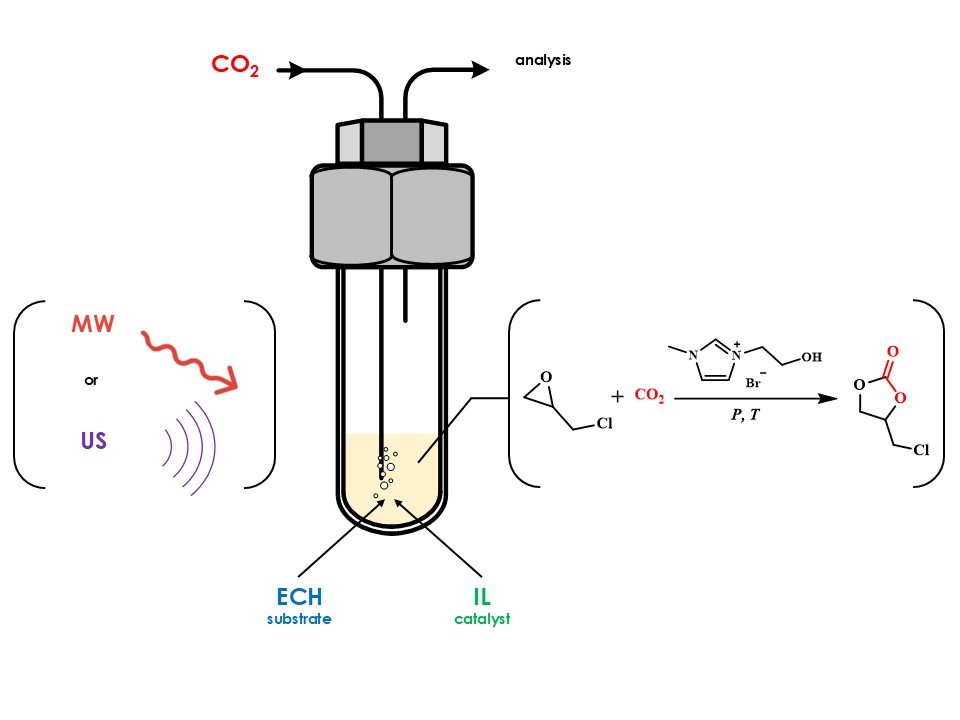
To study the effect of ultrasonic and microwave irradiation on the process of epichlorohydrin carboxylation, a model reaction was first carried out under set model conditions of temperature 90 °C, constant CO2 pressure 110 kPa, catalyst concentration 2 mol.% (control experiment). This catalyst was designed for the reaction of CO2 conversion to cyclic carbonates as shown in Figures S2 (Supplementary Information). The conversion of epichlorohydrin to products was carried out to approximately 80 %. The main product yield and selectivity were determined using GC method. Sampling was performed every 20 min for the first 2 hours, then every 30-60 min. The experimental setup scheme is given in Figure 1.
Figure 1 Schematic diagram of the unit for catalytic tests at constant CO2 pressure 1 - gas cylinder, 2 - mass flow controller, 3 - reactor, 4 - magnetic stirrer, 5 - back pressure regulator, 6 - gas chromatograph, 7 – computer
The unit shown in Figure 3 was designed for the carboxylation of unsaturated compound oxides at atmospheric pressure and continuous gas flow. The model setup consists of a thermostatically controlled reactor containing a catalyst, a starting substance (epichlorohydrin), as well as the necessary control and measuring equipment. In this system, CO2 gas is supplied from a cylinder to the reactor through the El-Flow Prestige FG-201CV gas flow regulator (Bronkhorst, the Netherlands). At the reactor outlet, there is a gas back pressure regulator El-Press P-702CM (Bronkhorst, the Netherlands). The outlet from the reactor is connected to the mass spectrometric complex consisting of diaphragm and turbomolecular vacuum pumps (Pfeiffer Hi-Cube ECO 300), which provide the primary discharge in the zone of sample input. The pressure in this cavity is determined using a pressure transmitter (Pfeiffer MPT200). The vacuum post is further connected to the mass spectrometer chamber (Pfeiffer PrismaPro QMG 250 M2), where the vacuum is provided by a second vacuum post (Pfeiffer Hi-Cube 80 Eco) and the level is determined by a second pressure transmitter of the same model. The gas stream leaving the reactor is sent for analysis to a mass spectrometric complex to determine the composition. The reactor is located on a C-Mag HS 7 control magnetic stirrer (IKA-Werke, Germany) for constant stirring of the reaction mixture.
To evaluate the influence of pressure and temperature on the yield of reaction products, the reaction was carried out on a setup similar to the one shown in Figure 1. The setup differs only in the fact that the reactor, made of stainless steel 08X16H11M3, has one additional port for gas inlet and outlet. A three-port, two-position SS-83XTS4 valve (Swagelok, USA) is used to switch between the reactor operation modes (gas inlet, gas analysis). At the start of the experiment, CO2 was introduced into the internal volume of the reactor, and then, after some time, the three-port valve was switched to the position connecting the reactor to the mass spectrometric complex for analyzing the gas composition of the medium. After the reaction, the mixture was cooled to room temperature, and excess CO2 was removed.
2.4. Effect of irradiation
The UWave-2000 reactor (Sineo, China) was used to study the effect of ultrasonic and microwave irradiation on the process of carboxylation of chloropropylene oxide. A schematic diagram of the reactor is shown in Figure 2. The reaction was carried out under constant stirring in a quartz flask, in which a sonicator was submerged (in reactions where the effect of ultrasound was studied). The temperature was controlled by a thermocouple (when the set temperature was exceeded, cooling by air blowing was switched on). For visual online control of the reaction, a built-in camera with a display was used. Samples were taken with the same regularity as in the control experiment.
Figure 2 Schematic diagram of the reactor for studying the influence of intensifying radiation 1 - gas cylinder, 2 - pressure controller, 3 - mass flow controller , 4 - irradiation generator, 5 - temperature sensor, 6 - quartz flask, 7 - valve, 8 - gas chromatograph, 9 - IR spectrometer
3.1. Catalyst synthesis and characterization
The 1H,13C NMR spectra are shown in Figures S3 and S4 (Supplementary Information). The mass spectrum was recorded on a Trace DSQII instrument with MALDI-TOF ionization and is presented in Figure S5 (Supplementary Information). The spectra contain the C2OHmim+ cation signal, observed at 127.2 m/z. In the IR spectrum (Figure S6), the characteristic vibrations are visible including a high-intensity band at 1778 cm-1 corresponding to the valence asymmetric vibrations of the C=O bond in the carbonate moiety and bands associated with the C-O-C ester bonds at 1158, 1062 and 1037 cm-1. Additionally, the C-Cl bond appeared at 763 cm-1, the valence vibration bands of the C-H bonds were found at 2920-3020 cm-1 and the C-H strain bonds were detected at 1500-1250 cm-1.
3.2. Effect of temperature, pressure and catalyst loading
To optimize the technological parameters of the process, several screenings were conducted in which certain parameters were varied, while the others were kept constant. The varied parameters include:
- reaction temperature: varied between 50 and 90 °C;
- reaction time: varied from 1 to 4 hours;
- pressure: varied from 110 to 1300 kPa.
3.2.1. Investigation of the influence of temperature
The effect of temperature was evaluated under the following conditions namely pressure 650 kPa, catalyst content 2 mol.%, reaction time of 3 hours, and the temperature of 50 to 110 °C in steps of 20 °C. For each experiment, the amount of catalyst was screened with loading variations of 0.5 to 5 mol.% (relative to epichlorohydrin). At 50 °C and interaction time of 4 h, a maximum conversion of about 60% was achieved, with an average main product selectivity of 96% and independent of catalyst loading (Figure 3a). At a temperature of 70 °C and reaction time 3 of hours, the conversion of epichlorohydrin predictably increased. Increasing the catalyst concentration from 3 to 5 mol% did not raise the conversion proportionally, but at the same time, the selectivity for the main product decreased to 89 % (Figure 3b). Further increase of temperature up to 90 °C (reaction time 2 hours) increased the conversion of epichlorohydrin, but increasing the concentration of catalyst above 2 mol.% slightly decreased the conversion and the selectivity for the main product up to 93 % (Figure 3c). The graphs with multiple "y" axes should be read as follows epichlorohydrin conversion - green circles and green scale bar, product yield - red squares and red scale bar, and selectivity - blue triangles and blue scale bar.
a) b)
Figure 3 Dependence of selectivity, epichlorohydrin conversion, and product yield on reaction temperature and catalyst concentration: a - 50°C, 4h; b - 70°C, 3h; c - 90°C, 2h
c)
Figure 3 Dependence of selectivity, epichlorohydrin conversion, and product yield on reaction temperature and catalyst concentration: a - 50°C, 4h; b - 70°C, 3h; c - 90°C, 2h (cont.)
Since increasing the catalyst concentration above 2 mol% did not yield a positive effect, and the reaction time of up to 4 hours led to a rise in product yield and conversion of epichlorohydrin, the effect of temperature at these parameters was evaluated (pressure was constant 650 kPa) (figure 4).
Figure 4 Dependence of selectivity, epichlorohydrin conversion and product yield on temperature (650 kPa, C cat = 2 mol.%, 4h)
The results showed that increasing the temperature to 90 0C under the conditions had a positive effect. The values of conversion and product yield increased to 85%. Further increase in temperature enhanced the conversion of epichlorohydrin but reduced the yield of the product. The selectivity decreased to 86%, showing the inexpediency of further increase in temperature.
In general, temperature increase had a positive effect on the conversion of epichlorohydrin. An increase from 50 to 110 °C led to elevated conversion from 28 to 99 %. The most significant increase in conversion was observed in the transition from 50 to 70 °C, reaching 28 to 83 %. A further increase from 70 to 90 °C had a negligible effect on conversion but nearly completed the reaction, achieving a 97% conversion of the initial epichlorohydrin. Selectivity for the main product remained high up to 90 °C, reaching 97%. Conducting the reaction at 110 °C resulted in a 99% conversion of the initial compound into products, but the selectivity dropped to 90 % and the yield of the main product, chloropropylene carbonate was lower than at 90 °C (89 % compared to 94 %).
3.2.2. Investigation of the influence of pressure and catalyst concentration
Comparison of the catalyst concentration effect was carried out at the same temperature of 90°C, reaction time of 1 hour, and different pressures (Figure S7 (Supplementary Information)). At 650 kPa, the conversion of epichlorohydrin and selectivity for the main product decreased with increasing catalyst concentration above 2 mol% (Figure S7 a). At this catalyst concentration, a conversion of 85% with a selectivity of 98% was achieved in 1 hour, while in 2 hours under the same conditions, the conversion was 90% with a selectivity of 97%. This implied that a 2-fold increase in reaction time produced a less significant increase in conversion.
The reaction was carried out at a pressure of 1300 kPa and the results are shown in Figure S7 b. The conversion did not significantly change at pressures 650 and 1300 kPa, reaching 85%, while selectivity decreased across all catalyst concentrations, dropping to 94% or lower. The increase in pressure did not cause a significant rise in the reaction rate but reduced the selectivity for the main product. This suggests that the rate-determining step in the carboxylation of oxides for unsaturated compounds is the opening of the epoxide cycle.
The effect of pressure was evaluated under the conditions of temperature 70 °C, catalyst content 2 mol.%, reaction time 4 hours, pressure variation from 110 to 1300 kPa (figure 5). In all cases except 110 kPa, the pressure was set at the start of the reaction and the system was closed throughout the reaction time. In the case of 110 kPa, the pressure was maintained at this value throughout the reaction.
Under the same conditions, increasing pressure resulted in a higher conversion of the main product, while the selectivity remained practically unchanged and averaged 90 %. Doubling the pressure from 650 to 1300 kPa led to an 11% increase in the yield of the main product from 71 to 82 %.
Figure 5 Effects of pressure on epichlorohydrin conversion, product yield and selectivity (Ccat = 2 mol.%, T = 70°C)
The effect of temperature and pressure on the conversion of epoxide in reaction with CO2 in the presence of ionic liquid 1-Butyl-3-methylimidazolium tetrafluoroborate [Bmim][BF4] was studied in (Peng and Deng, 2001). Increasing temperature from 65 to 110 °C (P CO2 = 2500 kPa) led to a rise in the conversion, reaching a maximum of 100% at 110 °C (TON = 40.0). At the same temperature with increasing pressure from 1500 to 2000 kPa, the conversion increased to 90.1% (TON = 60.1) and decreased to 69.4% (TON = 46.3) with a further increase to 2500 kPa.
This character of reaction efficiency dependence on temperature and pressure was also confirmed in (Wang et al., 2013), where the temperature dependence of product yield in the reaction between CO2, epichlorohydrin, and aniline in the presence of 1-Butyl-3-methylimidazolium chloride [Bmim][Cl] was studied. Initially, increasing temperature led to elevated reaction yield by up to 60% in the range of 40-60 °C, but further increase did not affect the yield of cyclic carbonate. Increasing the pressure up to 1500 kPa resulted in an elevated yield of 55-60%, but a further increase up to 2500 kPa led to a 45% decrease.
Based on the comparison of the results obtained with literature data, materials including ionic liquids, polymers, MOFs and zeolite imidazolate frameworks (ZIF) can be used as catalysts in reactions of CO2 conversion into cyclic carbonates with epichlorohydrin substrate. A study by (Ziaee et al., 2019) investigated the catalytic application of UIIP, ionic polymer based on amine-containing imidazolium salt (TAIB) functionalized with urea in the cycloaddition reaction of CO2 and epichorohydrin. The reaction was carried out in a pre-dried autoclave reactor then the mixture of epoxide (38.4 mmol) and UIIP (0.15 mmol) was flushed using CO2 several times to remove air. After heating the reactor to the desired temperature with continuous stirring, the reactor was placed in cold water and slowly depressurized. The highest conversion values (99%) were achieved when the reaction was carried out for 1 hour at a temperature of 110ºC and a pressure of 1000 kPa.
Another study (Sun et al., 2008) compared the catalytic activity of different ionic liquids, as well as various solvents and metal-containing catalysts for the reaction under investigation. The reaction was carried out under conditions of 0.2 mol epoxide and 3.2 mol HEMIMB was introduced into the reactor. CO2 was supplied at a pressure of 2000 kPa, and the temperature of the reaction system was 125 °C. Under these conditions, the yield of the reaction between CO2 and epichlorohydrin was 92 %. The highest yield was achieved in the conversion of cyclohexene oxide - 99 %.
In (Xiao et al., 2014), reactions between epoxides and CO2 in the presence of various ionic liquids were also studied. The catalytic activity of HMimBr was investigated in reactions with various epoxides. For example, in the reaction of CO2 with epichlorohydrin, the reaction yield was 93.5 %. Similarly, Wu et al. (2010) used ionic liquid based on 1-butyl-3-methylimidazolium cation alongside amino acid anions as catalysts for the reaction with propylene oxide. Ionic liquid [bmim][Lys] had the highest activity probably due to the additional amino group in the side chain structure, while [bmim][Ser] with an additional hydroxyl group had poor results. On the other hand, [bmim][Glu] with a carboxylic acid functional group of the side chain and [bmim][Ala] with a methyl group, showed moderate activity. The use of [bmim][Ala] as a catalyst at a reaction time of 18 hours achieved a yield of 96% and a selectivity of 97% for both the reaction with propylene oxide and epichlorohydrin.
In another study (Olaniyan and Saha, 2020), the cycloaddition reaction of CO2 and epichlorohydrin was also studied using zeolite imidazolate frameworks (ZIF) as catalysts including ZIF-8 based on zinc nitrate hexahydrate and Zirconium-doped ZIF-8 (Zr/ZIF-8). In a typical cycloaddition reaction, a certain amount of catalyst and limiting reagent, epichlorohydrin, were loaded into a high-pressure reactor. The reactor was sealed and stirred continuously at a known stirring rate and temperature then a certain amount of liquid CO2 was loaded. After completion of the reaction, the reactor was cooled to room temperature, and the mixture was collected and filtered. The catalyst was separated, washed with acetone, and dried in a vacuum oven. A known amount of methanol used as an internal standard, was added to the product and analyzed through gas chromatograph.
In (Hu et al., 2015), the reaction of cyclic carbonate formation using propylene oxide as a substrate was studied. The cycloaddition was carried out in the presence of ionic liquids as catalysts based on 1-[2-(2-hydroxyethoxy)ethyl]-3-methylimidazolium (Heemim), 1-hexyl-3-methyl-imidazolium (Hmim), 1-butyl-3-methylimidazolium (Bmim) and 1-butyl-3-methypyridinuim (BMPy). CuCl2, ZnCl3, ZrCl5, CoCl3, TiCl5, AlCl4, Cl, Br, BF4 or PF6 were used as anions. The results showed that the types of cations or anions of the ILs had a great influence on the cycloaddition. IL [Heemim][ZrCl5] was identified as the most efficient ionic liquid catalyst among those investigated. The selected catalyst was evaluated for the synthesis of cyclic carbonates from various epoxides such as oxirane, 2-propyloxirane, 2-(methoxymethyl)oxirane, 2-(chloromethyl)oxirane, 2-(phenoxymethyl)oxirane, 2-phenyloxirane and cyclohexene oxide. The selectivities for all reactions over cyclic carbonates using [Heemim][ZrCl5] as catalysts were more than 98%. The data on the results of the aforementioned studies are summarized in Table 1.
UIIP appears to be the most promising catalyst for the carboxylation reaction of epichlorohydrin among those considered, achieving a maximum conversion and selectivity values of 99% (Table 1). These values were achieved at a high pressure of 1000 kPa. Performing the reaction with the [C2OHmim][Br] catalyst allowed the same conversion value (99%) to be achieved at lower pressure (650 kPa). Although the reaction time with UIIP is shorter than [C2OHmim][Br], the reaction requires a pressure higher than that used in this present work. The synthesis of [C2OHmim][Br] is one step, against the multistep and complex synthesis of UIIP, using expensive precursors, which also shows the economic viability of the catalyst.
The simplicity of synthesis, low required pressure, and high conversion value (99%) suggest the possibility and feasibility of using [C2OHmim][Br] as a catalyst for the carboxylation reaction of epichlorohydrin.
Table 1 Catalytic activity of various catalysts.
3.2. Effect of irradiation
To study the intensification capabilities for catalytic conversion of CO2 into cyclic carbonates, a model reaction was carried out under selected reference conditions of temperature 90 °C, constant CO2 pressure 110 kPa, and catalyst concentration 2 mol.% (control experiment). The reaction was carried out until the conversion of epichlorohydrin reached approximately 80 %. The dependence of conversion, yield of the main product, and selectivity of the reaction are presented in Figure S8 (Supplementary Information). When the reaction was carried out under the described model conditions, the conversion of epichlorohydrin into the products was estimated at 84% in 10 hours. The selectivity for the main product namely chloropropylene carbonate was stable and quite high reaching 90% on average, except for at the beginning of the reaction, where it was lower and amounted to 80%.
The reaction order for epichlorohydrin and the constant were determined. The CO2 concentration was constant and pressure was maintained at 110 kPa. For this purpose, the dependences of epichlorohydrin concentration on time were plotted in linear and logarithmic coordinates as presented in Figures S9 a and b, respectively.
Considering the dependence in the coordinates lnCECH = f(t) is a straight line, the reaction order for epichlorohydrin is 1. The equations describe the relationships quite accurately (R2 > 0.99), and the value of the reaction constant found from the logarithmic from linear relationships coincide and are 2.9 -10-3 mol-1.
Ultrasound and microwave radiation were applied as reaction intensifiers. At first, the reaction mixture was exposed to ultrasonic irradiation to intensify the carboxylation of epichlorohydrin. Irradiation was carried out with 60% power ultrasound (input power 54 W) with frequency 27.76 kHz in the mode 2 min/10 min. The effect of irradiation was evaluated under the same conditions as the control experiment including a temperature of 90 °C, constant CO2 pressure of 110 kPa, and catalyst concentration of 2 mol.%. The dependences of epichlorohydrin conversion, yield of the main product (chloropropylene carbonate), and selectivity for the main product are presented in Figure 6.
Figure 6 Dependence of conversion, main product yield and selectivity for chloropropylene carbonate on the reaction time of carboxylation of epichlorohydrin under ultrasonic radiation
As shown in Figure 6, ultrasound tended to accelerate the reaction. Compared to the control experiment without intensifying radiation, the reaction proceeded more rapidly achieving epichlorohydrin conversion of 84 % within 240 minutes (4 hours). However, the selectivity for chloropropylene carbonate decreased significantly and averaged 80 %, while the yield was 67 % in the same 4 hours. The selectivity for the main product remained almost constant throughout the reaction.
To further investigate the effects of intensified irradiation, the reaction mixture was exposed to microwave radiation. The power was set to 300 W, heating time to 90 °C - 3 min, and irradiation mode was set to pulsed. Dependence of epichlorohydrin conversion, yield of the main product (chloropropylene carbonate), and selectivity for the main product are presented in Figure 7.
Figure 7 Dependence of conversion, main product yield and selectivity for chloropropylene carbonate of epichlorohydrin carboxylation reaction under microwave radiation
As shown in Figure 12, exposure to microwave radiation accelerated the carboxylation reaction of epichlorohydrin. Compared to the control experiment without intensifying radiation, the reaction proceeded more rapidly achieving epichlorohydrin conversion of 84 % within 360 minutes (6 hours). However, the selectivity for chloropropylene carbonate decreased significantly compared to the control and the experiment with ultrasonic irradiation, averaging 65%, with a yield of 72% in 6 hours. Table 2 and Figure 8 provide the values of selectivity, epichlorohydrin conversion, and chloropropylene carbonate yield for a comparative assessment of different types of radiation.
Table 2 Effect of irradiation on the carboxylation reaction of ECH under different experimental conditions with the catalyst- [C2OHmim][Br].
Figure 8 Studies on the possibility of reuse of the [C2OHmim][Br] catalyst
As shown in Figure 7, ultrasound exposure proved to be the most effective method. Although the method resulted in reduced selectivity, the carbonate yield was the highest in a shorter time. Meanwhile, microwave irradiation slightly changed the conversion trend. Up to 180 min, the conversion rate was slightly lower than without exposure, and afterward, it facilitated a faster conversion of epichlorohydrin into products. By 360 minutes, the yield of the main product was comparable to that of the reaction without radiation exposure, as microwave radiation resulted in the lowest selectively among all the methods considered. Similar conversion rates were achieved at different times, while the selectivity varied significantly.
Important catalyst characteristics such as turn-over number (TON) and turn-over frequency (TOF) were also calculated. TON characterizes the ability of the catalyst to turn over the substrate. The higher the TON, the more the catalyst can process the substrate and subsequently consume or lose catalytic activity. Meanwhile, TOF is a measure of the catalyst activity, determining how fast it works and turnovers. Since the catalyst loading was the same in all cases, the TON values for close reaction yields were also close, but the TOFs varied according to the patterns discussed. The highest value of TOF (8.38) was achieved for the reaction intensified by ultrasound, which is twice as high compared to the model reaction without irradiation. These results show the benefits of using ultrasonic irradiation in epichlorohydrin conversion reactions. The implementation in the plant processes is not energy-consuming. Ultrasonic irradiation allows the achievement of higher epichlorohydrin conversion and selectivity while significantly reducing the reaction time where necessary.
3.4. Reusability of catalyst
Evaluation of catalyst reusability is an essential characteristic to understand feasibility in industrial applications. The reusability of ionic liquid in cycloaddition reaction was investigated. The experiments were carried out under optimal reaction conditions, including 90°C, 650 kPa with fresh 2% (w/w) catalyst for 2 hours. Following the initial reaction cycle, the catalysts were washed, centrifuged, and oven-dried at 60°C for 14 h before reuse. The recovered catalysts were reused in five subsequent experiments following the same experimental procedure (Figure S10). [C2OHmim][Br] showed no loss of activity, indicating the stability of the catalyst for epichlorohydrin CO2 cycloaddition reaction. There was no significant change in ECH conversion, selectivity, and yield with [C2OHmim][Br]. After five uses of ionic liquid, the yield decreased by only 13% from 77% (fresh) to 67% (recycled). Similarly, the selectivity did not reduce significantly from 91.6% (fresh) to 88.2% (recycled). These results show the potential for repeated use of ionic liquid [C2OHmim][Br] as a catalyst for the conversion of epichlorohydrin into cyclic carbonates.
In conclusion, ionic liquid [C2OHmim][Br] showed high epichlorohydrin conversions and selectivity without using any solvent or co-catalyst. Increasing the temperature had a positive effect on the conversion of epichlorohydrin. With a rise in temperature from 50 to 90 °C, the conversion increased from 28 to 97%, and the product yield from 27% to 94%. The selectivity for the formation of the main product remained quite high at 97%. Further increase in temperature had an adverse effect on selectivity, which reduced to 90% at 110 °C. The most effective catalyst concentration was 2 mol.%. Increasing the catalyst concentration from 0.5 mol.% to 2 mol.% led to elevated epichlorohydrin conversion at all temperatures studied, reaching a maximum (85%) at 900C. However, further increasing the catalyst concentration above 2 mol.% did not produce a positive effect. Epichlorohydrin conversion and product yield increased disproportionately, while selectivity for the main product decreased. Increasing the pressure also had a positive effect on the conversion of epichlorohydrin. An increase from 110 kPa to 1300 kPa led to a two-fold rise in conversion from 49% to 89%. When the pressure was raised beyond 650 kPa, the yield of the main product increased by 11 %, specifically from 71 to 82 %. Increasing the pressure did not have a significant effect on selectivity, which averaged 91%. Therefore, increasing the pressure above 650 kPa is not always reasonable, because it does not proportionally affect epichlorohydrin conversion and yield of the chloropropylene carbonate. The pressure can be increased when technologically necessary, and at the same time, the selectivity on the main product will remain high. Based on the results of the experiments, the optimal reaction conditions were determined to be 90 °C and 650 kPa, with a catalyst loading of 2 mol.%. The main product was obtained with a yield of 94% and a selectivity of 97% within 2 hours. Ionic liquid [C2OHmim][Br] showed good catalytic activity both in single and repeated use. After the 5th use of the catalyst, the conversion of epichlorohydrin decreased by less than 10%, from 84% to 76%. Irradiation significantly accelerated the carboxylation reaction of epichlorohydrin but slightly reduced the selectivity for the main product. Ultrasonic exposure turned out to be the most effective method, achieving epichlorohydrin conversion of 83% in 4 hours with reduced selectivity. Meanwhile, the same conversion was achieved only after 10 hours without irradiation. Intensifying the process for obtaining cyclic carbonates can be specifically advisable when the conversion rate is important or when a carbonate and a dihalogen alcohol are required. The simplicity of synthesis, low required pressure, high conversion (99%), and reusability suggest the feasibility of using [C2OHmim][Br] as a catalyst for the carboxylation reaction of epichlorohydrin in industrial applications. The action of ultrasonic irradiation can also significantly accelerate the reaction of CO2 conversion to cyclic carbonates.
The main part and results were funded by the Russian Science Foundation with the project No. 22-79-10302. The study of conversion, main product yield, and selectivity using GC MS analytical support was funded by the Ministry of Science and Higher Education of the Russian Federation within the framework of the scientific project of the laboratory “Laboratory of Electronic Grade Substances Technologies”, project No. FSSM2022-0005.
Author Contributions
Maria Atlaskina: Conceptualization, Project administration, Writing – review and editing; Zakhar Markin: Formal analysis, Investigation; Writing – original draft; Kirill Smorodin: Investigation, Methodology; Sergey Kryuchkov: Investigation, Methodology; Nikita Tsivkovsky: Software; Anton Petukhov: Supervision; Artem Atlaskin: Software, Validation; Olga Kazarina: Data curation, Validation; Andrey Vorotyntsev: Data curation; Ilya Vorotyntsev: Supervision.
Conflict of Interest
The authors declare that there are no conflicts of interest.
| Filename | Description |
|---|---|
| R1-CE-7500-20250306110553.docx | --- |
Akhmetshina, AI, Petukhov, AN, Gumerova, OR, Vorotyntsev, AV, Nyuchev, AV & Vorotyntsev, IV 2019, ‘Solubility of H?S and CO? in imidazolium-based ionic liquids with bis(2-ethylhexyl) sulfosuccinate anion’, Journal of Chemical Thermodynamics, vol. 130, pp. 173-182, https://doi.org/10.1016/j.jct.2018.10.013
Anggerta, LA, Kurniawansyah, F, Tetrisyanda, R & Wibawa, G 2025, ‘Catalytic synthesis of diethyl carbonate from carbon dioxide using catalyst KI/EtONa with propylene oxide as dehydration agent and process optimization based on Box-Behnken design’, International Journal of Technology, vol. 16, pp. 243-254, https://doi.org/10.14716/IJTECH.V16I1.6417
Anwar, MN, Fayyaz, A, Sohail, NF, Khokhar, MF, Baqar, M, Yasar, A, Rasool, K, Nazir, A, Raja, MUF, Rehan, M, Aghbashlo, M, Tabatabaei, M & Nizami, AS 2020, ‘CO? utilization: Turning greenhouse gas into fuels and valuable products’, Journal of Environmental Management, vol. 260, article 110059, https://doi.org/10.1016/j.jenvman.2019.110059
Atlaskina, ME, Kazarina, OV, Petukhov, AN, Atlaskin, AA, Tsivkovsky, NS, Tiuleanu, P, Malysheva, YB, Lin, H, Zhong, GJ, Lukoyanov, AN, Vorotyntsev, AV & Vorotyntsev, IV 2024, ‘Amino acid-based ionic liquid as a promising CO? sorption increasing agent by aqueous MDEA solution’, Journal of Molecular Liquids, vol. 395, article 123635, https://doi.org/10.1016/j.molliq.2023.123635
Aviantara, DB, Suciati, F, Hadiko, G, Indrasti, NS & Yani, M 2024, ‘Microwave-assisted impregnation of zinc metal ions on surface of quenched pulverized shrimp shell waste’, International Journal of Technology, vol. 15, pp. 1946-1958, https://doi.org/10.14716/IJTECH.V15I6.6170
de la Hoz, A, Díaz-Ortiz, À & Moreno, A 2005, ‘Microwaves in organic synthesis: Thermal and non-thermal microwave effects’, Chemical Society Reviews, vol. 34, pp. 164-178, https://doi.org/10.1039/b411438h
Dharman, MM, Choi, HJ, Kim, DW & Park, DW 2011, ‘Synthesis of cyclic carbonate through microwave irradiation using silica-supported ionic liquids: Effect of variation in the silica support’, Catalysis Today, vol. 164, pp. 544-547, https://doi.org/10.1016/j.cattod.2010.11.009
Dmitrenko, M, Liamin, V, Kuzminova, A, Mazur, A, Lahderanta, E, Ermakov, S & Penkova, A 2020, ‘Novel mixed matrix sodium alginate-fullerenol membranes: Development, characterization, and study in pervaporation dehydration of isopropanol’, Polymers (Basel), vol. 12, no. 4, article 864, https://doi.org/10.3390/POLYM12040864
Dmitrenko, ME, Penkova, AV, Missyul, AB, Kuzminova, AI, Markelov, DA, Ermakov, SS & Roizard, D 2017, ‘Development and investigation of mixed-matrix PVA-fullerenol membranes for acetic acid dehydration by pervaporation’, Separation and Purification Technology, vol. 187, pp. 285-293, https://doi.org/10.1016/j.seppur.2017.06.061
Fatah, A, Bennour, Z, Ben Mahmud, H, Gholami, R & Hossain, MM 2020, ‘A review on the influence of CO?/shale interaction on shale properties: Implications of CCS in shales’, Energies (Basel), vol. 13, no. 12, article 3200, https://doi.org/10.3390/en13123200
Ghazali-Esfahani, S, Song, H, P?unescu, E, Bobbink, FD, Liu, H, Fei, Z, Laurenczy, G, Bagherzadeh, M, Yan, N & Dyson, PJ 2013, ‘Cycloaddition of CO? to epoxides catalyzed by imidazolium-based polymeric ionic liquids’, Green Chemistry, vol. 15, pp. 1584-1589, https://doi.org/10.1039/c3gc37085b
Ghorai, S, Nanda, D, Ghosh, A & Dash, PS 2024, ‘Review on the recent advances in catalytic conversion of carbon dioxide for synthesis of cyclic propylene carbonate’, Molecular Catalysis, vol. 553, article 113720, https://doi.org/10.1016/j.mcat.2023.113720
Guo, JX, Huang, C, Wang, JL & Meng, XY 2020, ‘Integrated operation for the planning of CO? capture path in CCS-EOR project’, Journal of Petroleum Science and Engineering, vol. 186, article 106720, https://doi.org/10.1016/j.petrol.2019.106720
Guo, L, Lamb, KJ & North, M 2021, ‘Recent developments in organocatalysed transformations of epoxides and carbon dioxide into cyclic carbonates’, Green Chemistry, vol. 23, pp. 77-118, https://doi.org/10.1039/d0gc03465g
He, H, Zhu, QQ, Zhang, WW, Zhang, HW, Chen, J, Li, CP & Du, M 2020, ‘Metal and co-catalyst free CO? conversion with a bifunctional covalent organic framework (COF)’, ChemCatChem, vol. 12, pp. 5192-5199, https://doi.org/10.1002/CCTC.202000949
Holkar, CR, Jadhav, AJ, Pinjari, DV & Pandit, AB 2019, ‘Cavitationally driven transformations: A technique of process intensification’, Industrial & Engineering Chemistry Research, vol. 58, pp. 5797-5819, https://doi.org/10.1021/acs.iecr.8b04524
Hu, YL, Lu, M & Yang, XL 2015, ‘Highly efficient synthesis of cyclic carbonates from carbon dioxide and epoxides catalyzed by ionic liquid [Heemim][ZrCl5]’, RSC Advances, vol. 5, pp. 67886-67891, https://doi.org/10.1039/c5ra11786k
Ibrahim, H, Silitonga, AS, Rahmawaty, Dharma, S, Sebayang, AH, Khairil, Sumartono, Sutrisno, J & Razak, A 2020, ‘An ultrasound assisted transesterification to optimize biodiesel production from rice bran oil’, International Journal of Technology, vol. 11, pp. 225-234, https://doi.org/10.14716/IJTECH.V11I2.905
Islam, MH, Burheim, OS, Hihn, JY & Pollet, BG 2021, ‘Sonochemical conversion of CO? into hydrocarbons: The Sabatier reaction at ambient conditions’, Ultrasonics Sonochemistry, vol. 73, article 105474 https://doi.org/10.1016/j.ultsonch.2021.105474
Kartohardjono, S, Paramitha, A, Putri, AA & Andriant, R 2017, ‘Effects of absorbent flow rate on CO? absorption through a super hydrophobic hollow fiber membrane contactor’, International Journal of Technology, vol. 8, pp. 1429-1435, https://doi.org/10.14716/IJTECH.V8I8.679
Kinzel, NW, Werlé, C & Leitner, W 2021, ‘Transition metal complexes as catalysts for the electroconversion of CO?: An organometallic perspective’, Angewandte Chemie International Edition, vol. 60, pp. 11628-11686, https://doi.org/10.1002/ANIE.202006988
Koutsoukos, S, Philippi, F, Rauber, D, Pugh, D, Kay, CWM & Welton, T 2022, ‘Effect of the cation structure on the properties of homobaric imidazolium ionic liquids’, Physical Chemistry Chemical Physics, vol. 24, pp. 6453-6468, https://doi.org/10.1039/D1CP05169E
Kryuchkov, S, Smorodin, K, Stepakova, A, Atlaskin, A, Tsivkovsky, N, Atlaskina, M, Tolmacheva, M, Kazarina, O, Petukhov, A, Vorotyntsev, A & Vorotyntsev, I 2024, ‘Membrane air separation process simulation: Insight in modelling approach based on ideal and mixed permeance values’, International Journal of Technology, vol. 15, pp. 1218-1236, https://doi.org/10.14716/IJTECH.V15I5.6987
Lei, Y, Gunaratne, HQN & Jin, L 2022, ‘Design and synthesis of pyridinamide functionalized ionic liquids for efficient conversion of carbon dioxide into cyclic carbonates’, Journal of CO? Utilization, vol. 58, article 101930, https://doi.org/10.1016/j.jcou.2022.101930
Li, ZJ, Sun, JF, Xu, QQ & Yin, JZ 2021, ‘Homogeneous and heterogeneous ionic liquid system: Promising “ideal catalysts” for the fixation of CO? into cyclic carbonates’, ChemCatChem, vol. 13, pp. 1848-1866, https://doi.org/10.1002/cctc.202001572
Lopes, EJC, Ribeiro, APC & Martins, LMDRS 2020, ‘New trends in the conversion of CO? to cyclic carbonates’, Catalysts, vol. 10, no. 5, article 479, https://doi.org/10.3390/catal10050479
Lu, M, Zhang, M, Liu, J, Chen, Y, Liao, J, Yang, M, Cai, Y, Li, S & Lan, Y 2022, ‘Covalent organic framework-based functional materials: Important catalysts for efficient CO? utilization’, Angewandte Chemie, vol. 134, no. 15, article e202200003, https://doi.org/10.1002/ange.202200003
Luo, R, Liu, X, Chen, M, Liu, B & Fang, Y 2020, ‘Recent advances on imidazolium-functionalized organic cationic polymers for CO? adsorption and simultaneous conversion into cyclic carbonates’, ChemSusChem, vol. 13, pp. 3945-3966, https://doi.org/10.1002/cssc.202001079
Maina, JW, Pozo-Gonzalo, C, Kong, L, Schütz, J, Hill, M & Dumée, LF 2017, ‘Metal-organic framework-based catalysts for CO? conversion’, Materials Horizons, vol. 4, pp. 345-361, https://doi.org/10.1039/C6MH00484A
Mujmule, RB & Kim, H 2022, ‘Efficient imidazolium ionic liquid as a tri-functional robust catalyst for chemical fixation of CO? into cyclic carbonates’, Journal of Environmental Management, vol. 314, article 115045, https://doi.org/10.1016/j.jenvman.2022.115045
Mustafa, A, Lougou, BG, Shuai, Y, Wang, Z & Tan, H 2020, ‘Current technology development for CO? utilization into solar fuels and chemicals: A review’, Journal of Energy Chemistry, vol. 49, pp. 96-123, https://doi.org/10.1016/j.jechem.2020.01.023
Nisya, AF, Rochmadi, R & Budiman, A 2024, ‘Mass transfer phenomena during the ultrasound-assisted extraction of algal oil from Spirulina sp.’, International Journal of Technology, vol. 15, pp. 927-936, https://doi.org/10.14716/IJTECH.V15I4.5974
Nunes, LJR 2023, ‘The rising threat of atmospheric CO?: A review on the causes, impacts, and mitigation strategies’, Environments - MDPI, vol. 10, vo. 4, article 66 https://doi.org/10.3390/environments10040066
Olaniyan, B & Saha, B 2020, ‘Comparison of catalytic activity of ZIF-8 and Zr/ZIF-8 for greener synthesis of chloromethyl ethylene carbonate by CO? utilization’, Energies (Basel), vol. 13, no. 3, article 521, https://doi.org/10.3390/en13030521
Orrling, KM 2009, On the versatility of microwave-assisted chemistry: Exemplified by applications in medicinal chemistry, heterocyclic chemistry and biochemistry, Uppsala University
Pal, TK, De, D & Bharadwaj, PK 2020, ‘Metal-organic frameworks for the chemical fixation of CO? into cyclic carbonates’, Coordination Chemistry Reviews, vol. 408, article 213173, https://doi.org/10.1016/j.ccr.2019.213173
Peng, J & Deng, Y 2001, ‘Cycloaddition of carbon dioxide to propylene oxide catalyzed by ionic liquids’, New Journal of Chemistry, vol. 25, pp. 639-641, https://doi.org/10.1039/b008923k
Penkova, AV, Dmitrenko, ME, Ermakov, SS, Toikka, AM & Roizard, D 2018, ‘Novel green PVA-fullerenol mixed matrix supported membranes for separating water-THF mixtures by pervaporation’, Environmental Science and Pollution Research, vol. 25, pp. 20354-20362, https://doi.org/10.1007/s11356-017-9063-9
Petukhov, AN, Atlaskin, AA, Kryuchkov, SS, Smorodin, KA, Zarubin, DM, Petukhova, AN, Atlaskina, ME, Nyuchev, AV, Vorotyntsev, AV, Trubyanov, MM, Vorotyntsev, IV & Vorotyntsev, VM 2021, ‘A highly-efficient hybrid technique - Membrane-assisted gas absorption for ammonia recovery after the Haber-Bosch process’, Chemical Engineering Journal, vol. 421, article 127726, https://doi.org/10.1016/j.cej.2020.127726
Qu, Y, Chen, Y & Sun, J 2022, ‘Conversion of CO? with epoxides to cyclic carbonates catalyzed by amino acid ionic liquids at room temperature’, Journal of CO? Utilization, vol. 56, article 101840, https://doi.org/10.1016/j.jcou.2021.101840
Sediawan, WB, Hartati, I, Sulistyo, H, Azis, MM & Rahma, UA 2023, ‘Microwave-assisted hydrotropic distillation of myrcene-rich essential oil of Cymbopogon Citratus’, International Journal of Technology, vol. 14, pp. 310-319, https://doi.org/10.14716/IJTECH.V14I2.5381
Shah, HUR, Ahmad, K, Bashir, MS, Shah, SSA, Najam, T & Ashfaq, M 2022, ‘Metal-organic frameworks for efficient catalytic conversion of CO? and CO into applied products’, Molecular Catalysis, vol. 517, article 112055, https://doi.org/10.1016/J.MCAT.2021.112055
Sun, J, Zhang, S, Cheng, W & Ren, J 2008, ‘Hydroxyl-functionalized ionic liquid: A novel efficient catalyst for chemical fixation of CO? to cyclic carbonate’, Tetrahedron Letters, vol. 49, pp. 3588-3591, https://doi.org/10.1016/j.tetlet.2008.04.022
Trubyanov, MM, Kirillov, SY, Vorotyntsev, AV, Sazanova, TS, Atlaskin, AA, Petukhov, AN, Kirillov, YP & Vorotyntsev, IV 2019, ‘Dynamic behavior of unsteady-state membrane gas separation: Modelling of a closed-mode operation for a membrane module’, Journal of Membrane Science, vol. 587, article 117173, https://doi.org/10.1016/j.memsci.2019.117173
Valluri, S, Claremboux, V & Kawatra, S 2022, ‘Opportunities and challenges in CO? utilization’, Journal of Environmental Sciences (China), vol. 113, pp. 322-344, https://doi.org/10.1016/j.jes.2021.05.043
Vega, F, Baena-Moreno, FM, Gallego Fernández, LM, Portillo, E, Navarrete, B & Zhang, Z 2020, ‘Current status of CO? chemical absorption research applied to CCS: Towards full deployment at industrial scale’, Applied Energy, vol. 260, article 114313, https://doi.org/10.1016/j.apenergy.2019.114313
Vorotyntsev, AV, Petukhov, AN, Sazanova, TS, Pryakhina, VI, Nyuchev, AV, Otvagina, KV, Markov, AN, Atlaskina, ME, Vorotyntsev, IV & Vorotyntsev, VM 2019, ‘Imidazolium-based SILLPs as organocatalysts in silane production: Synthesis, characterization and catalytic activity’, Journal of Catalysis, vol. 375, pp. 427-440, https://doi.org/10.1016/j.jcat.2019.05.021
Vorotyntsev, IV, Atlaskin, AA, Trubyanov, MM, Petukhov, AN, Gumerova, OR & Akhmetshina, AI 2017, ‘Towards the potential of absorbing pervaporation based on ionic liquids for gas mixture separation’, Desalination and Water Treatment, vol. 75, pp. 305-313
Vorotyntsev, VM, Drozdov, PN, Vorotyntsev, IV & Murav’Ev, DV 2006, ‘Fine gas purification to remove slightly penetrating impurities using a membrane module with a feed reservoir’, Doklady Chemistry, vol. 411, pp. 243-245, https://doi.org/10.1134/S0012500806120068
Wang, B, Feng, X, Zhang, L, Yang, S, Jiang, X, Zhou, J & Gao, G 2013, ‘One-pot reaction of CO?, epichlorohydrin and amine to synthesize 4-(phenylamino)methyl-ethylene carbonate catalyzed by ionic liquids’, Journal of CO? Utilization, vol. 1, pp. 88-91, https://doi.org/10.1016/j.jcou.2013.02.001
Wu, F, Dou, XY, He, LN & Miao, CX 2010, ‘Natural amino acid-based ionic liquids as efficient catalysts for the synthesis of cyclic carbonates from CO? and epoxides under solvent-free conditions’, Letters in Organic Chemistry, vol. 7, pp. 73-78, https://doi.org/10.2174/157017810790534039
Xiao, L, Su, D, Yue, C & Wu, W 2014, ‘Protic ionic liquids: A highly efficient catalyst for synthesis of cyclic carbonate from carbon dioxide and epoxides’, Journal of CO? Utilization, vol. 6, pp. 1-6, https://doi.org/10.1016/j.jcou.2014.01.004
Ye, Y, Qu, Y & Sun, J 2023, ‘Functionalized mesoporous SiO? materials for CO? capture and catalytic conversion to cyclic carbonates’, Catalysis Reviews, vol. 66, no. 5, pp. 2153-2208, https://doi.org/10.1080/01614940.2023.2202528
Zhang, G & Xu, Z 2024, ‘CO? conversion via MOF-based catalysts’, In: Advances in CO2 Utilization, pp. 1-36, https://doi.org/10.1007/978-981-99-8822-8_1
Zhang, Y, Yang, G, Xie, R, Yang, L, Li, B & Wu, G 2020, ‘Scalable, durable, and recyclable metal?free catalysts for highly efficient conversion of CO? to cyclic carbonates’, Angewandte Chemie, vol. 132, pp. 23491-23498, https://doi.org/10.1002/ange.202010651
Ziaee, MA, Tang, Y, Zhong, H, Tian, D & Wang, R 2019, ‘Urea-functionalized imidazolium-based ionic polymer for chemical conversion of CO? into organic carbonates’, ACS Sustainable Chemistry & Engineering, vol. 7, pp. 2380-2387, https://doi.org/10.1021/acssuschemeng.8b05197
