Optimizing Microwave-Assisted Extraction of Carbohydrate from Scenedesmus sp. Cultivated in Domestic Wastewater
Corresponding email: yeonghwang113@gmail.com
Published at : 31 Jan 2025
Volume : IJtech
Vol 16, No 1 (2025)
DOI : https://doi.org/10.14716/ijtech.v16i1.7301
Tan, YH, Chai, MK, Wong, LS, Ong, MY & Rajamani, R 2025, 'Optimizing microwave-assisted extraction of carbohydrate from scenedesmus sp. cultivated in domestic wastewater', International Journal of Technology, vol. 16, no. 1, pp. 255-274
| Yeong Hwang Tan | Institute of Sustainable Energy, Universiti Tenaga Nasional, Jalan Ikram-Uniten, 43000 Kajang, Selangor, Malaysia |
| Mee Kin Chai | Institute of Sustainable Energy, Universiti Tenaga Nasional, Jalan Ikram-Uniten, 43000 Kajang, Selangor, Malaysia |
| Ling Shing Wong | Faculty of Health and Life Sciences, INTI International University, Persiaran Perdana BBN, Putra Nilai, 71800 Nilai, Negeri Sembilan, Malaysia |
| Mei Yin Ong | Institute of Sustainable Energy, Universiti Tenaga Nasional, Jalan Ikram-Uniten, 43000 Kajang, Selangor, Malaysia |
| Ranjithkumar Rajamani | Center for Global Health Research, Saveetha Medical College and Hospital, Saveetha Institute of Medical and Technical Sciences (SIMATS), Saveetha University, Chennai, 602105, Tamil Nadu, India |
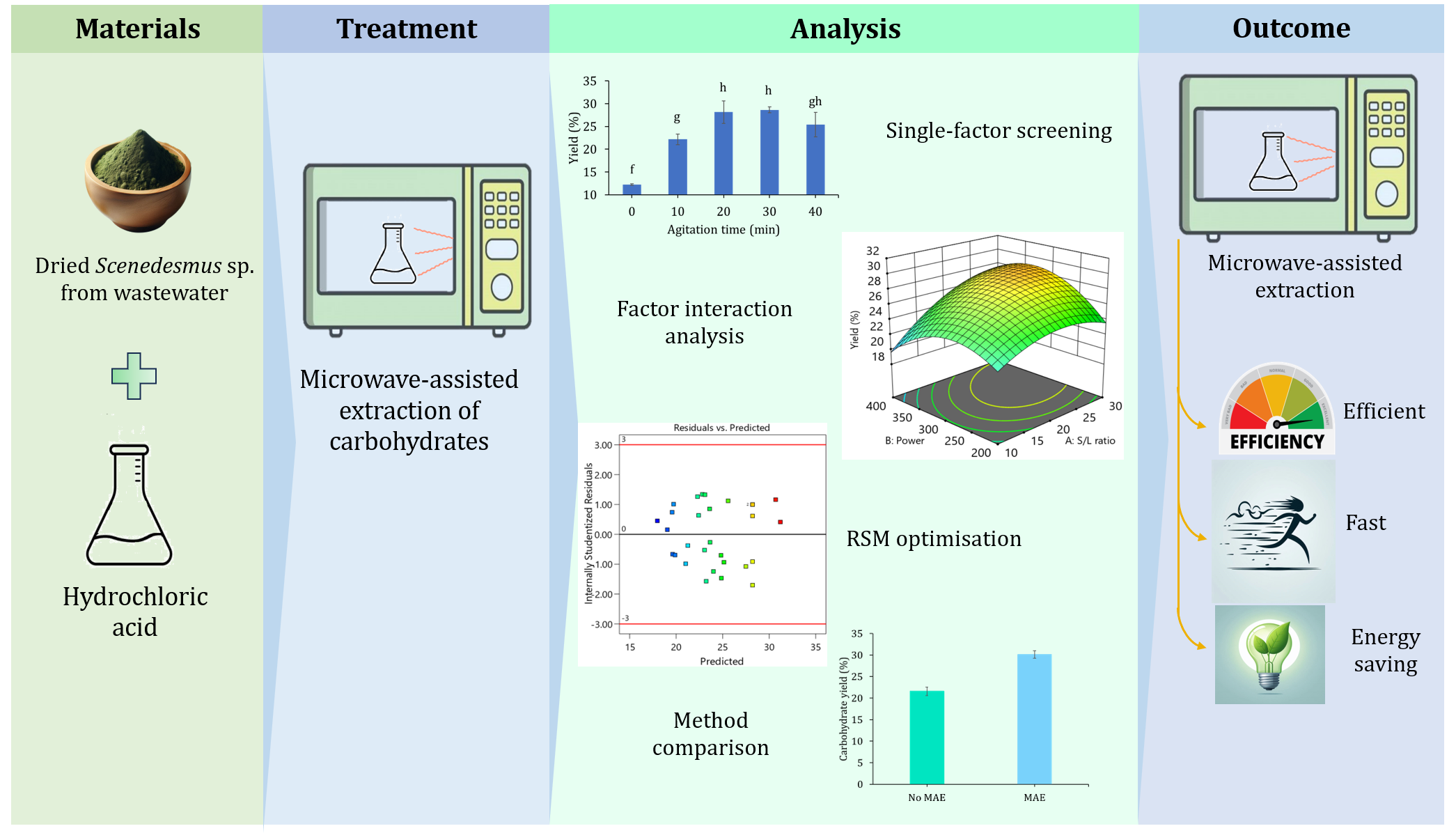
Microalgae including Scenedesmus sp. are gaining significant attention due to the rapid growth conditions, high biomass production, and significant content of valuable biomolecules such as proteins, lipids, and carbohydrates. Among these biomolecules, carbohydrates extracted from Scenedesmus sp. biomass has the potential to be used in biofuel, food, and pharmaceutical industries. Conventional methods of extraction include long energy-intensive heating processes with toxic solvents because carbohydrates are packed inside the microalga cell wall. To address the limitation, microwave-assisted extraction (MAE) has been shown as an efficient, energy-saving, low-environment impact, and rapid extraction method for plant materials. Therefore, this research aimed to optimize MAE through response surface methodology (RSM) coupled with Box-Behnken design (BBD) to extract carbohydrate from Scenedesmus sp. cultivated in local unsterilized domestic wastewater. The experimental factors were examined, including solid-to-liquid ratio, agitation time, microwave irradiation time, and microwave power. The results of different statistical metrics showed that the developed quadratic model was sufficient to predict carbohydrate yield. The sensitivity analysis showed that solid-to-liquid ratio had the most significant impact (55.98%) on carbohydrate yield, followed by microwave irradiation time (24.88%), and agitation time (18.16%). The highest carbohydrate extraction yield (30.11?±?0.88%) was obtained when the solid-to-liquid ratio, agitation time, microwave power, and microwave irradiation time were kept at 29 mg/mL, 28 min, 300 W, and 163s respectively. MAE obtained a 39.46% increase in carbohydrate extraction compared to conventional heating. Furthermore, MAE significantly reduced extraction time by 63 - 96% compared to other conventional extraction methods reported in previous research. The results showed that MAE was a fast and effective method for extracting carbohydrate from Scenedesmus sp., offering potential applications in the biofuel, agricultural, and industrial industries.
Carbohydrate; Microalgae biomass; Microwave-assisted extraction; Response surface methodology; Scenedesmus sp.
Microalgae are gaining significant attention because of their ability to generate various biomolecules used in various industries such as food, medicines, and biofuel (Sardi et al., 2015). The composition of these biomolecules shows substantial variation based on the specific algae species and cultivation conditions (Tibbetts et al., 2015). Among the different microalgae species, Scenedesmus sp. has gained attention due to its rapid growth conditions, high biomass production, and valuable biomolecules such as proteins, and, lipids, carbohydrate (Mirzayanti et al., 2024; Khatoon et al., 2019). Scenedesmus sp. is a member of the Chlorococcales order and Scenedesmaceae family and often flourishes in freshwater environment such as lakes and rivers (Kartika et al., 2023; Phinyo et al., 2017). It is rich in carbohydrate (15-50%), lipid (8-40%), protein (13-40%), essential amino acid (8-20%), vitamins (<1%), pigments (1- 5%) such as chlorophyll, ?-carotene, lutein and astaxanthin (Nisya et al., 2024; Tan et al., 2024; Zhang et al., 2019). Among these biomolecules, carbohydrate is one of the valuable metabolites, mainly comprised of glucose (Verspreet et al., 2021; Larronde-Larretche and Jin, 2017). Carbohydrate has great potential as a renewable resource for biofuel production such as bioethanol, biobutanol, and biogas (de Carvalho Silvello et al., 2022). Furthermore, certain carbohydrate derivatives extracted from Scenedesmus sp. biomass possess pharmaceutical characteristics including antiviral, cytotoxic, antioxidant, and anticholinesterase activities, serving as suitable ingredients for drug development (Singab et al., 2018). Other biological properties are very useful for industrial, agricultural, and food industries (Moreira et al., 2022). This carbohydrate is located inside the complex cell wall, which is made up of different components such as algaenan, amines, cellulose, and glucan (Baudelet et al., 2017). However, the presence of complex cell walls hinders the effective extraction of carbohydrate from microalgae biomass (Alhattab et al., 2019). Due to the complex cellular composition and complexity, there is a need to break down microalgae cell wall effectively to achieve maximum extraction. The conventional methods often include the use of a heated reflux apparatus in a multi-step procedure to extract carbohydrate. These methods also require lengthy treatment processes and hazardous solvents consumption, making negative impacts on the environment and not economically viable for large-scale application (Gharibzahedi et al., 2022). Moreover, continuous heating at high temperature for a long time might degrade carbohydrate, reducing pharmacological properties (Yi et al., 2020). Recently, different advanced extraction methods including pressurized liquid, supercritical fluid, and microwave-assisted extraction (MAE) have been developed (Mena-García et al., 2019). MAE uses non-ionizing radiation in a frequency range between 300 MHz and 300 GHz to penetrate natural materials, generating volumetric heat through molecular friction within an electromagnetic field. This energy input facilitates dipolar rotation of polar solvents and conductive diffusion of dissolved ions, causing a significant increase in the pressure and the temperature inside the cells. The internal thermal stress breaks down the cell wall rupture and enhances solvent penetration, leading to the transfer of carbohydrate into solvent (Mirzadeh et al., 2020). Additionally, the combined influence of electric and magnetic fields can disrupt hydrogen bonds, enhancing carbohydrate solubility and extraction efficiency. This synergistic effect occurs immediately, presenting MAE as a fast extraction method (Chen et al., 2022). Besides short duration, MAE method can achieve high extraction yields using low quantities of solvent and energy (Aparamarta et al., 2019; Yuan and Macquarrie, 2015). Harmful organic solvents can be replaced with safer alternatives such as water or ethanol (Silva et al., 2018). Therefore, MAE is considered an efficient and sustainable method for the efficient extraction of valuable compounds. Microwave has been proven to enhance the anaerobic digestion process of microalgae biomass which is converted into biogas without the presence of oxygen (Zaidi et al., 2019; Feng et al., 2019). MAE has been extensively studied, showing higher extraction efficiency compared to conventional methods (Al-Dhabi and Ponmurugan, 2020; Yuan and Macquarrie, 2015; Seixas et al., 2014). Despite the significant contributions, the application of MAE on microalgae carbohydrate is still very limited. Extraction efficiency of carbohydrate using MAE is significantly affected by various factors, including the solid-to-liquid ratio, microwave power, irradiation temperature, and irradiation time (Soria et al., 2014). Simultaneous optimization of the factors is highly needed to increase carbohydrate yield significantly. Response surface methodology (RSM) is a powerful statistical tool that has been effectively used to analyze the impact of multiple factors and optimize processes. RSM is also used to evaluate the interactions between different factors while reducing the required number of experimental trials (Raissi and Farsani, 2009). Microalgae biomass cultivation can be carried out simultaneously with wastewater treatment. Although chemical and physical treatments effectively remove nutrients from wastewater, the methods are expensive and energy-consuming (Gude, 2016). Exploitation of microalgae for wastewater treatment is more environmentally friendly and sustainable (Prihantini et al., 2021). Based on a sustainability perspective, the use of wastewater can reduce microalgae cultivation cost, freshwater demand, and greenhouse gas emissions (Mat Aron et al., 2021; Batista et al., 2015). Several investigations have been conducted on the lipid extraction from microalgae through MAE (Rokicka et al., 2021; Zhou et al., 2019; de Moura et al., 2018). However, there is limited information for carbohydrate production, particularly from microalgae cultivated in unsterilized domestic wastewater in Malaysia. Previous research showed that microalgae such as Scenedesmus sp. were capable of efficiently removing nutrients, including phosphorus, ammonia, and nitrogen from local unsterilized domestic wastewater with high growth rates and biomass production (Tan et al., 2023). In this research, domestic wastewater was collected from the treatment plant in Kuala Lumpur, Malaysia, and used for the cultivation of Scenedesmus sp. without prior sterilization. Subsequently, MAE was used to extract carbohydrate from Scenedesmus sp. cultivated in local unsterilized domestic wastewater. RSM coupled with Box-Behnken design (BBD) was used to optimize several factors, such as solid-to-liquid ratio, agitation time, microwave power, and microwave irradiation time, affecting MAE process on carbohydrate production. A comparison of extraction yield between optimized MAE with conventional method (heating without microwave) was also analyzed and discussed. By investigating the effects of factors, this research aimed to identify optimal conditions to maximize yield efficiency while minimizing processing time and resource consumption. The results were expected to contribute to a greener and more resource-efficient method in carbohydrate production, with significant implications for industrial applications.
2.1. Microalgae cultivation and harvesting
Scenedesmus sp. was bought from the University of Texas Culture Collection, United States, and was cultivated in Bold Basal Medium (BBM). Local domestic wastewater samples were collected from the same treatment plant, Indah Water Konsortium (IWK) located in Kuala Lumpur, Malaysia. Collected wastewater (3500 mL) was vacuum filtered and used to cultivate Scenedesmus sp. in an aquarium tank. Scenedesmus sp. was maintained at a temperature of 25±2 °C and exposed to a 16-hour light cycle generated by fluorescent lamps (2000 ± 200 lux) that emanate white light. After 14 days of cultivation, microalgae biomass was collected through centrifugation (3 min, 3000 rpm, 24 °C) and rinsed several times with deionized water. Subsequently, biomass was dried in an oven at 50°C until constant weight.
2.2. Chemicals
Concentrated sulfuric acid, D-glucose, phenol, and concentrated hydrochloric acid (HCl) of higher purity (>98%) or analytical grade were procured from R&M Chemicals (Malaysia). A Milli Q system (Millipore, Bedford, MA, USA) was used to produce deionized water.
2.3. MAE of carbohydrate
The experiment process was shown in Figure 1, where dried microalgae powder (250 - 1500 milligrams) was weighed and mixed with HCI (25 mL, 2M). Subsequently, the sample was heated in the household microwave oven operating at a frequency of 2.45 GHz. Extraction factors were examined, including microwave power, agitation time, solid-to-liquid ratio, and microwave irradiation time. After MAE, the mixture was agitated on a hot plate at 80°C and 300 rpm for a certain amount of time. Subsequently, the mixture was cooled to room temperature (25°C) and centrifuged at a speed of 5000 rpm for a duration of 10 minutes. The supernatant was used for carbohydrate extraction.
Figure 1 Illustration scheme for the experiment process in this research.
2.4. Carbohydrate extraction and determination
Phenol sulfuric acid method (Nielsen., 2010) was applied to determine the total carbohydrate content in Scenedesmus sp. Initially, supernatant sample (0.2 mL) was mixed with 5% (w/v) phenol solution (0.2 mL) in a test tube. After shaking for 10 seconds, concentrated sulfuric acid (2 mL) was immediately added. The sample was gently shaken for 10 seconds and incubated in water bath at 90°C for 10 minutes. The spectrophotometer was used to measure the absorbance at a wavelength of 490 nm. The absorbance readings were compared to a standard curve generated using glucose to quantify the total carbohydrate content and all analysis was carried out in triplicate.
2.5. Optimization of Extraction Factors Using RSM-BBD Method
Initially, a screening experiment with a single-factor experiment design was conducted to examine the range of experiment factors. The impact of each factor on the total carbohydrate yield was evaluated across a range of values (microwave power: 100-400 W, agitation time: 0-40 min, solid-to-liquid ratio: 10-60 mg/mL, and microwave irradiation time: 30-180 s).
For the multifactor optimization, Design Expert software (Version 13, Stat-Ease Inc., Minneopolis, MN, USA) was used to carry out the experimental designs, statistical analysis, and regression model. RSM coupled with a BBD was used to optimize the four independent factors including solid-to-liquid ratio (%), agitation time (min), microwave irradiation time (s), and microwave power (W) at three levels (-1, 0, 1), which were symbolized by letter A, B, C and D, respectively. Each experimental run was carried out in triplicate and the response value was an average value of extraction yield. Table 1 showed the levels and ranges of independent factors that were investigated in this research, with experimental design containing 29 trials. A quadratic polynomial model (equation 1) was used to show the functional connection between independent factors and the response (Thawechai et al., 2016). Table 2 shows experimental design and test results of the response.
Y = b0+i=1kbiXi+i=1kbiiXi2+i
where Y is a dependent factor, b0 is a constant coefficient, bi is an ith linear coefficient, bii is a quadratic coefficient, and bij are the two-factor interaction factors.
The response from each experimental design was used to plot response surface graphs through multiple non-linear regressions. The effects and regression coefficients associated with individual linear, quadratic, and interaction terms were analyzed using the analysis of variance (ANOVA) table. Model adequacy was assessed using the R², predicted R2, and adjusted R² values. Subsequently, confirmation experiments were carried out at optimized conditions to validate the statistical design. Statistical significance was interpreted as a p-value below 0.05 (Breig and Luti, 2021).
Table 1 Factors and levels for RSM-BBD.
2.6. Sensitivity Analysis
Sensitivity analysis was calculated using equation 2 to determine the significance of each factor, including solid-to-liquid ratio, microwave power, agitation time, and microwave irradiation time. The analysis was carried out by calculating the total squares obtained from the ANOVA table generated using RSM (Ong and Nomanbhay, 2022; Ginot et al., 2006). The equation is shown below:
Sensitivity index (%) = SSindividualSStotal (2)
where SSindividual and SStotal represent the sum of squares of each factor and the overall sum of squares of all factors, respectively.
Table 2 Experimental conditions and carbohydrate yield.
2.7. Comparison with conventional extraction
To compare the effectiveness of optimized MAE with conventional method, the samples were extracted under the same conditions without microwave treatment. Subsequently, dried biomass powder (0.725 g) was mixed with HCI (25 mL, 2 M), the sample was heated on the hot plate at 80°C and 300 rpm for 28 minutes. The mixture was cooled down to room temperature (25°C) and centrifuged at 5000 rpm for 10 minutes. The supernatant was used for carbohydrate extraction as described in section 2.3.
3.1. Screening Experiment
This section analyzes the effect of solid-to-liquid ratio, agitation time, microwave power, and microwave irradiation time on carbohydrate yield. The screening experiments were conducted by varying a single factor throughout its whole range while holding all other factors unchanged. Figure 2 shows the outcomes of screening experiment research.
Figure 2 Screening experiment results showing (a) effect of solid-to-liquid ratio (at constant agitation time of 20 min, microwave power of 300 W and microwave irradiation time of 150s (b) Agitation time (at constant solid-to-liquid ratio of 20 mg/mL, microwave power of 300 W and microwave irradiation time of 150s), (c) microwave power (at constant solid-to-liquid ratio of 20 mg/mL, agitation time of 20 min and microwave irradiation time of 150s), and (d) microwave irradiation time (at constant solid-to-liquid ratio of 20 mg/mL, agitation time of 20 min and microwave power of 300W) for MAE of carbohydrate from Scenedesmus sp. Mean with identical letters are not statistically different from one another (P<0.05, ANOVA with Tukey test). The error bars show a range of three standard deviations from the mean.
3.1.1. Effect of solid-to-liquid ratio
Several investigations have shown that solid-to-liquid ratio has a substantial impact on carbohydrate extraction yield (Du et al., 2024; Thuy et al., 2020; Chaiklahan et al., 2013). Figure 2a showed that the yield of carbohydrate rose as the solid-to-liquid ratio increased to the ratio of 20 mg/mL. The solid-to-liquid ratio increased alongside the surface area of interaction between carbohydrate particles and solvents. This led to a gradual increase in the concentration gradient of carbohydrate at the boundary between the inside and outside of biomass (Wang et al., 2023). Consequently, the mass transfer driving force increased, promoting the diffusion of carbohydrate particles. The phenomenon strengthened carbohydrate extraction, although the yield decreased when the solid-to-liquid ratio exceeded 20 mg/mL. The decrease was attributed to a high solid-to-liquid ratio, showing a reduced amount of solvent per unit of biomass. This caused inefficient mass transfer of carbohydrate from biomass to the solvent due to saturation, which hindered the ability to efficiently dissolve more carbohydrate (Hanafi et al., 2024). Moreover, a high concentration of biomass could lead to the formation of a compact matrix that was difficult for the solvent to permeate and interact with carbohydrate (Du et al., 2024; Guo et al., 2023).
3.1.2. Effect of agitation time
The impact of agitation time on extraction yield of carbohydrate was investigated. As shown in Figure 2b, the yield had a substantial rise as the agitation time rose from 0 to 20 minutes, reaching the highest extraction at 28.15%. When the mixture was not agitated (0 min), carbohydrate had inadequate interaction with the solvent and biomass, causing a slow mass transfer and reduced extraction yield. Increasing the agitation time after MAE enhanced extraction could be attributed to the significant difference in solute concentration between biomass and the solvent. As time increased, the difference gradually decreased, reaching a constant extraction yield (Zainol et al., 2023; Susanti et al., 2020).
3.1.3. Effect of microwave power
Microwave power is a significant factor in carbohydrate extraction (Le et al., 2019; Arasi et al., 2016). Figure 2c showed that carbohydrate yield increased with greater microwave power, with the highest value obtained at 300 W. This result was obtained due to the appropriate microwave power, which improved heat generation in biomass and carbohydrate solubility, thereby enhancing carbohydrate yield (Kaderides et al., 2019). Increasing microwave power would enhance the dipole rotations, causing a greater level of power to dissipate inside the reaction mixture. This phenomenon quickly generated heat in the reaction mixture and loosened the cell wall, thereby increasing the penetration of solvent into biomass and carbohydrate yield (Arasi et al., 2016). When power surpassed 300 W, the yield was decreased, suggesting that excessive energy generated by the high irradiation power disrupted molecular connections in the solvent and biomass. The disrupted molecular connection structure coupled with increased microwave power. This phenomenon caused thermal breakdown of carbohydrate, leading to a drop in carbohydrate yield (Al-Dhabi and Ponmurugan, 2020).
3.1.4. Effect of microwave irradiation time
The results in Figure 2d showed that increasing the duration of microwave irradiation exposure had a positive impact on carbohydrate yield. The increase was attributed to the sufficient time needed for microwave energy to penetrate evenly throughout the entire sample (Cheong et al., 2016). The heat required duration to be generated and dissipated uniformly through dipole rotations and ionic conduction (Maran et al., 2015). After 150 s, extending microwave irradiation time led to a decrease in yield due to the thermal breakdown of carbohydrate (Arasi et al., 2016). Based on the results of screening experiments, a solid-to-liquid ratio of 10-30 mg/mL, agitation time of 0-30 min, microwave power of 200-400W, and microwave irradiation time of 120-180 s were selected for further optimization investigation using RSM.
3.2. Model fitting
A total of 29 tests were conducted to determine the impact of solid-to-liquid ratio, microwave power, agitation time, and microwave irradiation time, on carbohydrate yield. The operating conditions and their results are shown in Table 2. Based on observation, run-order 29 with solid-to-liquid ratio of 30 mg/mL, microwave power of 300W, agitation time of 30 min, and microwave irradiation time of 150 s, produced the highest level of carbohydrate yield (31.33 ± 1.51%). Meanwhile, run-order 17 with a solid-to-liquid ratio of 20 mg/mL, microwave power of 300W, agitation time of 10 min, and microwave irradiation time of 120 s, showed the least amount of carbohydrate yield (18.11 ± 1.10%). These results were used to optimize the four individual factors using RSM coupled with BDD. The Design-Expert 13 software was used to carry out multiple regression analysis of the data. As shown by the results, a polynomial equation was obtained to express the relationship between each of the investigated factors and carbohydrate yield. The coded form of the constructed equation is stated below:
The obtained data was statistically analyzed and an ANOVA table was created to evaluate the impact of independent factors on the result (Table S1). The ANOVA confirmed that equation 3 accurately represented the correlation between the result and the independent factors. F- and p-values were used to define the significance of the coefficient term, as shown in Table 3. A higher F-value with a lower p-value showed a greater level of importance. The model was statistically significant with a p-value less than 0.05 (Yi et al., 2010). The ANOVA results in this research showed that the F-value for the model was 118.30. This suggested that there was a very small (only 0.01%) probability for a “model F-value” to occur due to noise. Moreover, the model's significance was confirmed by the probability p?<?0.0001, with the lack of fit on the F-value showing 0.749. This showed that lack of fit was insignificant relative to the pure error, suggesting no significant improvement (Breig and Luti, 2021).
Y = 28.213 + 2.292A + 0.304B + 1.306C + 1.528D + 1.644AB + 3.783AC + 3.193AD - 1.165BC - 1.12BD - 2.275CD - 1.897A² - 3.064B² - 2.495C² - 2.633D² (3)
The p-values were less than 0.05 for the linear factors, namely solid-to-liquid ratio, microwave power, agitation time, and microwave irradiation time, showing high significance on carbohydrate yield. Similar results were obtained for quadratic terms (B, AC, AD, BC, BD, A2, B2, C2, and D2), which yielded a p-value less than 0.05 and showed a significant effect on carbohydrate yield. Therefore, estimating the optimized conditions using multiple factor design was more suitable than single factor that did not consider the interaction effect causing false results (Humbird and Fei, 2016; Silva et al., 2011).
Further statistical metrics, such as the coefficient of determination (R2), adjusted R2, predicted R2, and coefficient of variation (CV), were used to verify the adequacy of the developed model (Breig and Luti, 2021). As shown in Table 3, R2 value was 0.992, showing that 99.2% of the total variations were well explained by the model. Furthermore, the model could predict the response for future observations effectively with the difference between the predicted R2 (0.964) and adjusted R2 value (0.983) less than 0.2 (Rezaee et al., 2014). The high predicted R2 and adjusted R2 showed a strong relationship between the experimental and predicted values. The standard deviation for the model was found to be low, which was 0.469. Model with R2 values close to one and a low standard deviation showed a greater degree of accuracy in predicting the response factor (Hamidu et al., 2021). Meanwhile, the adequacy precision quantifying the ratio of signal to noise of the model was 39.254, showing a satisfactory signal (Yi et al., 2010). A low value of the CV (1.95%) showed a high level of accuracy and reproducibility in the experimental results (Liyana-Pathirana and Shahidi, 2005). These results suggested that the selected quadratic model was sufficient in predicting the response factors for the experimental data.
Table 3 ANOVA and fit statistics for designed model.
Generating a normal probability plot of the residuals can facilitate the determination of normal data distribution (Ositadinma et al., 2019). As shown in Figure 3a, the data points on the normal residue plot spread somewhat near a straight line, suggesting that data were normally distributed. The plot in Figure 3b also showed the data points were on a straight line, suggesting a very close correlation between the experimental carbohydrate yield and expected results (Joseph et al., 2018). These validations further confirmed the model prediction.
Figure 3 Diagnostic plots for the model: (a) normal % probability versus externally studentized residuals and (b) Predicted versus Actual Plots.
3.3. Perturbation plot and sensitivity analysis
Perturbation plot shows the impact of all factors on a single graph. For carbohydrate extraction using MAE, perturbation plot was showed to assess the effect of each factor on the yield. The response was graphed by varying a single factor throughout the whole range while keeping all others constant. A curve with a high curvature showed the factor had a significant impact on the response while a linear curve suggested minimal effect (Borode and Olubambi, 2023). From Figure 4, the curvature was observed in solid-to-liquid ratio (A), microwave power (B), agitation time (C), and irradiation time (D), showing the response carbohydrate yield was very sensitive to all four factors. Moreover, a curve with a positive slope would improve the response value, while a curve negative slope showed low response (Yan et al., 2022; Ipakchi et al., 2020). Based on the results, all factors had a synergistic effect on carbohydrate yield at low level. Specifically, microwave power (B), agitation time (C), and irradiation time (D)) have a negative effect at the high level except solid-to-liquid ratio (A).
Figure 4 Perturbation plots for carbohydrate yield
A sensitivity analysis using equation 2 was performed to further determine the most significant factors for carbohydrate yield. Figure 5 showed that solid-to-liquid ratio had the greatest influence (55.98%) on carbohydrate yield, followed by the irradiation time (24.88%), and agitation time (18.16%). Carbohydrate yield was minimally affected by microwave power (0.98%).
Figure 5 Results of sensitivity analysis for RSM model
3.4. Combined effect of independent factors on carbohydrate yield
In this section, 3D-surface and contour plot were used to provide a more comprehensive explication of the impacts of each factor and their combined effect on carbohydrate yield. Figures 5a-e showed 3D response surface graphs and their related contour plots, which explained the relationship between carbohydrate yield and the changes in the factors. The plots were acquired for a specific set of factors while holding the values of other constants at the zero level. A circular shape of response surfaces suggested that the interaction between the respective factors was insignificant. However, an elliptical or saddle-shaped contour plot suggested that there was a substantial interaction between the factors (Wang et al., 2017; Si and Cui, 2013). Figures 6a-e suggested that the interaction between all independent factors was significant.
Figure 6a shows the relationship between microwave power and solid-to-liquid ratio for carbohydrate yield. The data showed that to maximize carbohydrate yield, microwave power should be controlled. The low carbohydrate yield at reduced microwave power could be explained by the incomplete breakdown of microalgae cell walls (Onumaegbu et al., 2019). When microwave power was kept at a high level (+1), there was a decrease in carbohydrate yield obtained at a low level (-1) and high level (+1) of solid-to-liquid ratio.
The enhanced breakdown of biomass at elevated levels of microwave power could be attributed to the strong polarity of HCI molecules, which caused widespread absorption of microwave. After effectively absorbing greater microwave radiation, biomass was exposed to a significant amount of electromagnetic energy, leading to strong contact between dipoles and collisions between molecules. Subsequently, the electromagnetic energy quickly transformed into heat energy,1 facilitating the breakdown of biomass. High temperature induced by high microwave power also produced higher solubility of extractant due to the lower solvent viscosity and surface tension (Mandal et al., 2007). However, excessively high temperature could lead to the degradation of biomass. These results were in accordance with Maran et al. (2015) and Song et al. (2009) who found that during MAE, middle microwave power enhanced the yields of carbohydrate extraction from higher biomass, followed by a significant decrease at greater power.
Figure 6 Effect of two-factor interaction on carbohydrate yield
The response surface plot for agitation time and solid-to-liquid ratio for carbohydrate yield is shown in Figure 6b. The result showed that agitation time at a low level was not sufficient to induce high carbohydrate yield. This was in line with the result obtained in the single-factor design (screening experiment). The efficiency of carbohydrate extraction enhanced significantly as agitation time progressively increased for all solid-to-liquid ratio. An optimal carbohydrate yield was observed when agitation time was increased to 20 min. Prolonged agitation could cause more extensive cell wall damage, thereby increasing the cell wall permeability to carbohydrate. This high permeability was proven to be effective in facilitating the diffusion of carbohydrate from the intracellular structure into the surrounding solvent, leading to higher extraction. As agitation time increased, the yield decreased significantly for the low level of solid-to-liquid ratio. This decrease could be attributed to the degradation of carbohydrate at high temperature during long-time agitation.
Figure 6c shows the response surface plots of the impact of microwave irradiation time and solid-to-liquid ratio on carbohydrate yield in the conditions of microwave power of 300W and agitation time of 20 min. The results showed that microwave irradiation time at a low factor level with a low factor level of solid-to-liquid ratio was not sufficient to produce a high carbohydrate yield. Increasing microwave irradiation time and solid-to-liquid ratio were required to obtain a high carbohydrate yield. Similarly, Gao et al. (2023) and Alexander et al. (2020) observed a consistent elevation in carbohydrate extraction from higher lignocellulosic biomass concentration as microwave irradiation time increased. This phenomenon could be attributed to the elevated energy requirements for larger biomass amount. As biomass quantity increased, a greater amount of microwave energy was needed to ensure all parts of the material received adequate penetration and thermal conversion, leading to effective cell wall disruption.
Figures 6d, 6e, and 6f showed the interaction between agitation time and microwave power, microwave irradiation time and microwave power, as well as agitation time and microwave irradiation time. A gradual increase in carbohydrate yield was observed as the two independent factors increased. When the independent factor increased to an optimum level, there was a corresponding rise in carbohydrate yield. At ranges beyond optimum level, carbohydrate yield showed a small reduction because of prolonged exposure to microwave radiation or heat causing the deterioration of carbohydrate (Ren et al., 2017; Zhang et al., 2015).
3.5. Verification of the models
The adequacy of the model equation in estimating the highest carbohydrate yield using MAE was determined through the optimal conditions. These included solid-to-liquid ratio of 29 mg/mL, 28 min agitation time, 300 W microwave power, and 163 s microwave irradiation time. In this research, the predicted carbohydrate yield was 31.63%. The verification tests were conducted in triplicates using the optimal extraction conditions. The actual yield of carbohydrate was 30.11?±?0.88%, which was not statistically different from the predicted value of 31.63%. The small difference observed between these results validated the suitability of the model in predicting carbohydrate yield.
3.6. Comparison with other extraction methods
To compare the effectiveness of MAE, the same experimental conditions of 29 mg/mL solid-to-liquid ratio and 28 min agitation time were carried out using conventional heating. The results showed that carbohydrate yield was 21.59?±?0.98%. In comparison, the yield obtained from optimized MAE was 39.46% higher than the conventional method. This suggested that a longer agitation time might be required for extraction without microwave to increase carbohydrate yield. Extraction with conventional heating depends on the transmission of heat by convection and conduction (Bundhoo, 2018). The heat might be lost to the environment and medium during transmission. Meanwhile, the oscillating electric field in microwave induces molecular vibrations causing both inter- and intra-molecular friction, which leads to rapid heating (Guzik et al., 2021). The rapid increase in temperature within biomass leads to pressure accumulation inside the cells. This phenomenon causes the cell membranes to burst, leading to electroporation which triggers the increase in the cell membrane permeability (Balasubramanian et al., 2011). Microwave can also generate the heat inside microalgae cells through volumetric heating. Therefore, there is a rapid rise in temperature and pressure during microwave heating, which causes the rupture of the cell walls and release of molecules (Sharifvaghefi and Zheng, 2022; Wahidin et al., 2014).
Table 4 summarizes carbohydrate yield obtained from various microalgae using MAE and other extraction methods, with varying results.
Table 4 Comparison of extraction method from this research and other investigations.
Silva et al. (2018) showed that the optimal conditions to extract carbohydrate from Spirulina platensis using MAE were extraction time of 20 min, microwave power of 434 W, solid-to-liquid ratio of 1:30 (w/v). Fathimah et al. (2021) discovered the optimal conditions were 30°C temperature, 50% ethanol in water, 1:30 solid-to-liquid ratio, and 15 min extraction time. Dobrin?i? et al. (2021) identified the optimal conditions for carbohydrate extraction from macroalgae, Fucus virsoides, and Cystoseira barbata, using MAE at a temperature of 80°C for 10 min.
Carbohydrate yield was influenced by microalgae growth environment and extraction factors. The growth environment such as light intensity, type of medium, concentration of nutrients, and microalgae could significantly affect the yield (Samiee-Zafarghandi et al., 2018; Cheng et al., 2017; Razaghi et al., 2014). Meanwhile, microwave factors including power, time, solvent type, and solid-to-liquid ratio were essential to carbohydrate extraction.
The results showed that MAE possessed several advantages in terms of environmental impact and time consumption compared to other extraction methods. The total extraction time using MAE in this research was significantly lower than others. Silva et al. (2018) stated that under optimal conditions of MAE, carbohydrate yield obtained from Spirulina platensis was 15.89% lower than solid-liquid extraction. However, energy and time consumption were reduced by 99.70% and 99.17% respectively. Sebastian et al. (2019) also showed that MAE consumed lower energy and shorter processing time compared to autoclave commonly used in acid extraction of carbohydrate. Moreover, there is tendency to use non-toxic solvents such as water for carbohydrate extraction (Silva et al., 2018). Peng et al. (2023) showed that the antioxidant activities of carbohydrate extracted from Chlorella sp. were enhanced after MAE. Based on these results, MAE could be considered a highly efficient, energy-saving, low-environment impact, and rapid method for carbohydrate extraction. This method showed a potential for large-scale applications in the biofuel, agricultural, and industrial sectors, which is currently not available. Due to the sustainability and efficiency of microwave technology, future research should explore the potential of MAE for scaling up carbohydrate extraction from microalgae.
In conclusion, this research examined the effectiveness of carbohydrate extraction from Scenedesmus sp. cultivated in local unsterilized domestic wastewater using MAE. The optimization of four factors, namely solid-to-liquid ratio, agitation duration, microwave irradiation time, and microwave power, was carried out using RSM-BBD. The results of different statistical metrics showed that the selected quadratic model was sufficient to predict carbohydrate yield. Based on the sensitivity analysis, solid-to-liquid ratio had the most significant impact on carbohydrate yield, followed by the irradiation, and agitation time. The optimal conditions were 29 mg/mL, 28 min, 300W, and 163 s for solid-to-liquid ratio, agitation time, microwave power, and irradiation time, respectively. The verification results discovered that there were no significant variations between the predicted (31.63%) and the actual experimental yield (30.11?±?0.88%) under optimal conditions. When compared to extraction with conventional heating, carbohydrate yield obtained from optimized MAE was 39.46% higher. Furthermore, MAE significantly reduced extraction time by 63 - 96% compared to other conventional methods reported in previous research. The results showed that microwave was efficient extraction method for extracting carbohydrate from Scenedesmus sp., offering potential applications in the biofuel, agricultural, and industrial industries. Due to the sustainability and efficiency of microwave technology, future research should explore the potential of MAE for scaling up carbohydrate extraction from microalgae. Additionally, a detailed analysis of the major carbohydrate constituents after extraction would provide valuable insights into potential biomass applications.
The authors are grateful to the Ministry of Higher Education Malaysia (Grant no: FRGS/1/2020/STG02/UNITEN/02/1), Indah Water Konsortium, and Universiti Tenaga Nasional UNITEN-YCU postgraduate scholarship 2021 for the support in this research. Furthermore, the authors (O.M.Y.) are grateful to AAIBE Chair of Renewable Energy at UNITEN for their support through grant number 201801KETTHA and to UNITEN for providing research facilities.
Author Contributions
Conceptualization, Y.H.T., M.K.C. and L.S.W.; methodology, Y.H.T.; validation, Y.H.T., O.M.Y. and R.R.; formal analysis, Y.H.T., O.M.Y. and R.R.; investigation, Y.H.T.; resources, M.K.C. and L.S.W.; data curation, Y.H.T. and O.M.Y.; writing—original draft preparation, Y.H.T and O.M.Y.; writing—review and editing, M.K.C., L.S.W. and R.R.; supervision, M.K.C. and L.S.W.; project administration, M.K.C. and L.S.W.; funding acquisition, M.K.C. and L.S.W.
Conflict of Interest
The authors declare no conflicts of interest.
| Filename | Description |
|---|---|
| R2-CE-7301-20241129081106.pdf | --- |
Al-Dhabi, NA & Ponmurugan, K 2020, ‘Microwave assisted extraction and characterization of polysaccharide from waste jamun fruit seeds’, International Journal of Biological Macromolecules, vol. 152, pp. 1157-1163, https://doi.org/10.1016/j.ijbiomac.2019.10.204
Alexander, RA, Innasimuthu, GM, Rajaram, SK, Jeganathan, PM & Somasundarar, CS 2020, ‘Process optimization of microwave-assisted alkali pretreatment for enhanced delignification of Prosopis juliflora biomass’, Environmental Progress and Sustainable Energy, vol. 39, no. 1, pp. 1-11, https://doi.org/10.1002/ep.13289
Alhattab, M, Kermanshahi-Pour, A & Brooks, MSL 2019, ‘Microalgae disruption techniques for product recovery: Influence of cell wall composition’, Journal of Applied Phycology, vol. 31, no. 1, pp. 61-88, https://doi.org/10.1007/s10811-018-1560-9
Aparamarta, HW, Gunawan, S, Azhar, B, Aditya, HT, Widjaja, A & Ju, YH 2019, ‘Comparative study of batchwise solvent extraction and the microwave-assisted extraction method for the purification of triglyceride for biodiesel feedstock from crude Calophyllum inophyllum oil (CCIO)’, International Journal of Technology, vol. 10, no. 3, pp. 551-560, https://doi.org/10.14716/ijtech.v10i3.2920
Arasi, MASG, Rao, MG & Bagyalakshmi, J 2016, ‘Optimization of microwave-assisted extraction of polysaccharide from Psidium guajava L. fruits’, International Journal of Biological Macromolecules, vol. 91, pp. 227-232, https://doi.org/10.1016/j.ijbiomac.2016.05.039
Balasubramanian, S, Allen, JD, Kanitkar, A & Boldor, D 2011, ‘Oil extraction from Scenedesmus obliquus using a continuous microwave system - design, optimization, and quality characterization’, Bioresource Technology, vol. 102, no. 3, pp. 3396-3403, https://doi.org/10.1016/j.biortech.2010.09.119
Batista, AP, Ambrosano, L, Graça, S, Sousa, C, Marques, PASS, Ribeiro, B, Botrel, EP, Neto, PC & Gouveia L 2015, ‘Combining urban wastewater treatment with biohydrogen production - An integrated microalgae-based approach’, Bioresource Technology, vol. 184, pp. 230-235, https://doi.org/10.1016/j.biortech.2014.10.064
Baudelet, PH, Ricochon, G, Linder, M & Muniglia, L 2017, ‘A new insight into cell walls of Chlorophyta’, Algal Research, vol. 25, pp. 333-371, https://doi.org/10.1016/j.algal.2017.04.008
Beigbeder, JB & Lavoie, JM 2022, ‘Effect of photoperiods and CO2 concentrations on the cultivation of carbohydrate-rich P. kessleri microalgae for the sustainable production of bioethanol’, Journal of CO2 Utilization, vol. 58, pp. 101934, https://doi.org/10.1016/j.jcou.2022.101934
Borode, A & Olubambi, P 2023, ‘Modelling the effects of mixing ratio and temperature on the thermal conductivity of GNP-Alumina hybrid nanofluids: A comparison of ANN, RSM, and linear regression methods’, Heliyon, vol. 9, no. 8, article e19228, https://doi.org/10.1016/j.heliyon.2023.e19228
Breig, SJM & Luti, KJK 2021, ‘Response surface methodology: A review on its applications and challenges in microbial cultures’, Materials Today: Proceedings, vol. 42, pp. 2277-2284, https://doi.org/10.1016/j.matpr.2020.12.316
Bundhoo, ZMA 2018, ‘Microwave-assisted conversion of biomass and waste materials to biofuels’, Renewable and Sustainable Energy Reviews, vol. 82, pp. 1149-1177, https://doi.org/10.1016/j.rser.2017.09.066
Chaiklahan, R, Chirasuwan, N, Triratana, P, Loha, V, Tia, S & Bunnag B 2013, ‘Polysaccharide extraction from Spirulina sp. and its antioxidant capacity’, International Journal of Biological Macromolecules, vol. 58, pp. 73-78, https://doi.org/10.1016/j.ijbiomac.2013.03.046
Chen, X, Yang, J, Shen, M, Chen, Y, Yu, Q & Xie, J 2022, ‘Structure, function and advance application of microwave-treated polysaccharide: A review’, Trends in Food Science & Technology, vol. 123, pp. 198-209, https://doi.org/10.1016/j.tifs.2022.03.016
Cheng, D, Li, D, Yuan, Y, Zhou, L, Li, X, Wu, T, Wang, L, Zhao, Q, Wei, W & Sun, Y 2017, ‘Improving carbohydrate and starch accumulation in Chlorella sp. AE10 by a novel two-stage process with cell dilution’, Biotechnology for Biofuels, vol. 10, pp. 75, https://doi.org/10.1186/s13068-017-0753-9
Cheong, KL, Wang, LY, Wu, DT, Hu, DJ, Zhao, J & Li, SP 2016, ‘Microwave-assisted extraction, chemical structures, and chain conformation of polysaccharides from a novel Cordyceps sinensis Fungus UM01’, Journal of Food Science, vol. 81, no. 9, pp. C2167-C2174, https://doi.org/10.1111/1750-3841.13407
de Carvalho Silvello, MA, Gonçalves, IS, Azambuja, SPH, Costa, SS, Silva, PGP, Santos, LO & Goldbeck, R 2022, ‘Microalgae-based carbohydrates: A green innovative source of bioenergy’, Bioresource Technology, vol. 344, pp. 126304, https://doi.org/10.1016/j.biortech.2021.126304
de Moura, RR, Etges, BJ, dos Santos, EO, Martins, TG, Roselet, F, Abreu, PC, Primel, EG & D’Oca, MG 2018, ‘Microwave-assisted extraction of lipids from wet microalgae paste: A quick and efficient method’, European Journal of Lipid Science and Technology, vol. 120, no. 7, pp. 1-7, https://doi.org/10.1002/ejlt.201700419
Dobrin?i?, A, Pedisi?, S, Zori?, Z, Jurin, M, Roje, M, ?ož-Rakovac, R & Dragovi?-Uzelac, V 2021, ‘Microwave-assisted extraction and pressurized liquid extraction of sulfated polysaccharides from Fucus virsoides and Cystoseira barbata’, Foods, vol. 10, no. 7, article 1481, https://doi.org/10.3390/foods10071481
Du, C, Liu, X, Algadi, H, Hou, Y, Fu, X, Li, H, Fan, J, Singh, MV, Li, Y, Zhang, X, Xu, J & Guo, Z 2024, ‘Polysaccharide extraction optimization, monosaccharide composition, and antioxidant activity analysis of different varieties of Gastrodia elata Bl aerial parts’, Biomass Conversion and Biorefinery, vol. 14, pp. 29353-29365, https://doi.org/10.1007/s13399-023-05014-x
El-Naggar, NEA, Hussein, MH, Shaaban-Dessuuki, SA & Dalal, SR 2020, ‘Production, extraction and characterization of Chlorella vulgaris soluble polysaccharides and their applications in AgNPs biosynthesis and biostimulation of plant growth’, Scientific Reports, vol. 10, no. 1, pp. 3011, https://doi.org/10.1038/s41598-020-59945-w
Fathimah, RN, Setyaningsih, W, Carrera, C, Astari, AD, Masithoh, RE, Suryaningtyas, IT & Palma, M 2021, ‘A microwave-based technique to determine saccharides and polyols contents in Spirulina (Arthrospira platensis)’, Arabian Journal of Chemistry, vol. 14, no. 4, pp. 103094, https://doi.org/10.1016/j.arabjc.2021.103094
Feng, RZ, Zaidi, AA, Zhang, K & Shi, Y 2019, ‘Optimisation of microwave pretreatment for biogas enhancement through anaerobic digestion of microalgal biomass’, Periodica Polytechnica Chemical Engineering, vol. 63, no. 1, pp. 65-72, https://doi.org/10.3311/PPch.12334
Gao, P, Shen, X, Guo, Y, Yue, D, Jin, M, Li, D, Liu, C & Liu, X 2023, ‘RSM-optimization of microwave-assisted extraction of R. laevigata polysaccharides with bioactivities’, Emirates Journal of Food and Agriculture, vol. 35, no. 12, pp. 1-10, https://doi.org/10.9755/ejfa.2023.3159
Garcia-Vaquero, M, Ummat, V, Tiwari, B & Rajauria, G 2020, ‘Exploring ultrasound, microwave and ultrasound-microwave assisted extraction technologies to increase the extraction of bioactive compounds and antioxidants from brown macroalgae’, Marine Drugs, vol. 18, no. 3, pp. 172-187, https://doi.org/10.3390/md18030172
Gharibzahedi, SMT, Marti-Quijal, FJ, Barba, FJ & Altintas, Z 2022, ‘Current emerging trends in antitumor activities of polysaccharides extracted by microwave- and ultrasound-assisted methods’, International Journal of Biological Macromolecules, vol. 202, pp. 494-507, https://doi.org/10.1016/j.ijbiomac.2022.01.088
Ginot, V, Gaba, S, Beaudouin, R, Faivre, AF & Monod, H 2006, ‘Combined use of local and ANOVA-based global sensitivity analyses for the investigation of a stochastic dynamic model: Application to the case study of an individual-based model of a fish population’, Ecological Modelling, vol. 193, pp. 479-491, https://doi.org/10.1016/j.ecolmodel.2005.08.025
Gude, VG 2016, ‘Wastewater treatment in microbial fuel cells - An overview’, Journal of Cleaner Production, vol. 122, pp. 287-307, https://doi.org/10.1016/j.jclepro.2016.02.022
Guo, P, Chen, H, Ma, J, Zhang, Y, Chen, H, Wei, T, Gao, D & Li, J 2023, ‘Enzyme-assisted extraction, characterization, and in vitro antioxidant activity of polysaccharides from Potentilla anserina L.’, Frontiers in Nutrition, vol. 10, pp. 1216572, https://doi.org/10.3389/fnut.2023.1216572
Guzik, P, Kulawik, P, Zaj?c, M & Migda?, W 2021, ‘Microwave applications in the food industry: An overview of recent developments’, Critical Reviews in Food Science and Nutrition, vol. 62, no. 29, pp. 7989-8008, https://doi.org/10.1080/10408398.2021.1922871
Hamidu, LAJ, Aroke, UO, Osha, OA & Muhammad, IM 2021, ‘D-Optimal response mixture design modeling of polystyrene waste adhesive formulations’, International Journal of Engineering Technologies and Management Research, vol. 8, no. 3, pp. 7-17, https://doi.org/10.29121/ijetmr.v8.i3.2021.867
Hanafi, MA, Anwar, F & Saari, N 2024, ‘Valorization of biomass for food protein via deep eutectic solvent extraction: Understanding the extraction mechanism and impact on protein structure and properties’, Food Frontiers, vol. 5, no. 3, pp. 1265-1301, https://doi.org/10.1002/fft2.389
Humbird, D & Fei, Q 2016, ‘Scale-up considerations for biofuels’, in Eckert, CA & Trinh, CT (eds), Biotechnology for Biofuel Production and Optimization, Elsevier BV, Amsterdam, Netherlands, pp. 513-537, https://doi.org/10.1016/B978-0-444-63475-7.00020-0
Ipakchi, H, Rezadoust, AM, Esfandeh, M & Mirshekar, H 2020, ‘Modeling and optimization of electrospinning conditions of PVB nanofiber by RSM and PSO-LSSVM models for improved interlaminar fracture toughness of laminated composites’, Journal of Composite Materials, vol. 54, no. 3, pp. 363-378, https://doi.org/10.1177/002199831986312
Joseph, T, Chinonye, O, Oluchukwu, A & Elijah, O 2018, ‘Application of Response Surface Methodology in Phenol Red Adsorption Using Kola Nut (Cola acuminata) Shell Activated Carbon’, International Research Journal of Pure and Applied Chemistry, vol. 15, no. 4, pp. 1-14, https://doi.org/10.9734/IRJPAC/2017/39421
Kaderides, K, Papaoikonomou, L, Serafim, M & Goula, AM 2019, ‘Microwave-assisted extraction of phenolics from pomegranate peels: Optimization, kinetics, and comparison with ultrasounds extraction’, Chemical Engineering and Processing - Process Intensification, vol. 137, pp. 1-11, https://doi.org/10.1016/j.cep.2019.01.006
Kartika, R, Ritonga, AH, Sulastri, L, Nurliana, S, Irawan, D & Simanjuntak, P 2023, ‘Biosorption of hexavalent chromium Cr(VI) using microalgae scenedesmus sp as environmental bioindicator’, International Journal of Technology, vol 14, no. 4, pp. 791-799, https://doi.org/10.14716/ijtech.v14i4.5188
Khatoon, H, Rahman, NA, Suleiman, SS, Banerjee, S & Abol-Munafi, AB 2019, ‘Growth and proximate composition of Scenedesmus obliquus and Selenastrum bibraianum cultured in different media and condition’, Proceedings of the National Academy of Sciences India Section B - Biological Sciences, vol. 89, no. 1, pp. 251-257, https://doi.org/10.1007/s40011-017-0938-9
Larronde-Larretche, M & Jin, X 2017, ‘Microalgal biomass dewatering using forward osmosis membrane: Influence of microalgae species and carbohydrates composition’, Algal Research, vol. 23, pp. 12-19, https://doi.org/10.1016/j.algal.2016.12.020
Laurens, LML, Nagle, N, Davis, R, Sweeney, N, Van Wychen, S, Lowell, A & Pienkos, PT 2015, ‘Acid-catalyzed algal biomass pretreatment for integrated lipid and carbohydrate-based biofuels production’, Green Chemistry, vol. 17, no. 2, pp. 1145-1158, https://doi.org/10.1039/C4GC01612B
Le, B, Golokhvast, KS, Yang, SH & Sun, S 2019, ‘Optimization of microwave-assisted extraction of polysaccharides from Ulva pertusa and evaluation of their antioxidant activity’, Antioxidants, vol. 8, no. 5, pp. 129, https://doi.org/10.3390/antiox8050129
Liyana-Pathirana, C & Shahidi, F 2005, ‘Optimization of extraction of phenolic compounds from wheat using response surface methodology’, Food Chemistry, vol. 93, no. 1, pp. 47-56, https://doi.org/10.1016/j.foodchem.2004.08.050
Lupatini, AL, de Oliveira Bispo, L, Colla, LM, Costa, JAV, Canan, C & Colla, E 2017, ‘Protein and carbohydrate extraction from Spirulina platensis biomass by ultrasound and mechanical agitation’, Food Research International, vol. 99, pp. 1028-1035, https://doi.org/10.1016/j.foodres.2016.11.036
Mandal, V, Mohan, Y & Hemalatha, S 2007, ‘Microwave-assisted extraction - An innovative and promising extraction tool for medicinal plant research’, Pharmacognosy Review, vol. 1, no. 1, pp. 7-18
Maran, JP, Swathi, K, Jeevitha, P, Jayalakshmi, J & Ashvini, G 2015, ‘Microwave-assisted extraction of pectic polysaccharide from waste mango peel’, Carbohydrate Polymers, vol. 123, pp. 67-71, https://doi.org/10.1016/j.carbpol.2014.11.072
Mat Aron, NS, Khoo, KS, Chew, KW, Veeramuthu, A, Chang, JS & Show, PL 2021, ‘Microalgae cultivation in wastewater and potential processing strategies using solvent and membrane separation technologies’, Journal of Water Process Engineering, vol. 39, pp. 101701, https://doi.org/10.1016/j.jwpe.2020.101701
Mena-García, A, Ruiz-Matute, AI, Soria, AC & Sanz, ML 2019, ‘Green techniques for extraction of bioactive carbohydrates’, TrAC - Trends in Analytical Chemistry, vol. 119, pp. 115612, https://doi.org/10.1016/j.trac.2019.07.023
Mirzadeh, M, Arianejad, MR & Khedmat, L 2020, ‘Antioxidant, antiradical, and antimicrobial activities of polysaccharides obtained by microwave-assisted extraction method: A review’, Carbohydrate Polymers, vol. 229, pp. 115421, https://doi.org/10.1016/j.carbpol.2019.115421
Mirzayanti, YW, Marlinda, L, Irawan, H, Al Muttaqii, M, Ma'sum, Z, Asri, NP & Chern, JM 2024, ‘Performance of in-situ stirring batch reactor transesterification of Nannochloropsis sp. microalgae into biodiesel’, International Journal of Technology, vol. 15, no. 4, pp. 859-869, https://doi.org/10.14716/ijtech.v15i4.6678
Moreira, JB, Vaz, BS, Cardias, BB, Cruz, CG, Almeida, ACA, Costa, JAV & Morais, MG 2022, ‘Microalgae polysaccharides: An alternative source for food production and sustainable agriculture’, Polysaccharides, vol. 3, no. 2, pp. 441-457, https://doi.org/10.3390/polysaccharides3020027
Nielsen, SS 2010, Phenol-sulfuric acid method for total carbohydrates. in: food analysis laboratory manual. food science texts series, Nielsen, SS, (edn), Springer, Boston, Massachusetts, United States, pp. 48-53, https://doi.org/10.1007/978-1-4419-1463-7_6
Nisya, AF, Rochmadi, R & Budiman, A 2024, ‘Mass transfer phenomena during the ultrasound-assisted extraction of algal oil from Spirulina sp.’, International Journal of Technology, vol. 15, no. 4, pp. 927-936, https://doi.org/10.14716/ijtech.v15i4.5974
Ong, MY & Nomanbhay, S 2022, ‘Optimization study on microwave-assisted hydrothermal liquefaction of Malaysian macroalgae Chaetomorpha sp. for phenolic-rich bio-oil production’, Energies, vol. 15, no. 11, pp. 3974, https://doi.org/10.3390/en15113974
Onumaegbu, C, Alaswad, A, Rodriguez, C & Olabi, A 2019, ‘Modelling and optimization of wet microalgae Scenedesmus quadricauda lipid extraction using microwave pre-treatment method and response surface methodology’, Renewable Energy, vol. 132, pp. 1323-1331, https://doi.org/10.1016/j.renene.2018.09.008
Ositadinma, IC, Tagbo, NJ & Elijah, OC 2019, ‘Optimum process parameters for activated carbon production from rice husk for phenol adsorption’, Current Journal of Applied Science and Technology, vol. 36, no. 6, pp. 1-11, https://doi.org/10.9734/cjast/2019/v36i630264
Peng, H, Xv, X, Cui, X, Fu, Y, Zhang, S, Wang, G, Chen, X & Song, W 2023, ‘Physicochemical characterization and antioxidant activity of polysaccharides from Chlorella sp. by microwave-assisted enzymatic extraction’, Frontiers in Bioengineering and Biotechnology, vol. 11, pp. 1-12, https://doi.org/10.3389/fbioe.2023.1264641
Phinyo, K, Pekkoh, J & Peerapornpisal, Y 2017, ‘Distribution and ecological habitat of Scenedesmus and related genera in some freshwater resources of Northern and North-Eastern Thailand’, Biodiversitas, vol. 18, no. 3, pp. 1092-1099, https://doi.org/10.13057/biodiv/d180329
Prihantini, NB, Maulana, F, Wardhana, W, Takarina, ND, Nurdin, E, Handayani, S, Nasruddin, H & Haryani, GS 2021, ‘Wild mixed culture microalgae biomass from UI Agathis Small Lake harvested directly using ultrasound harvesting module as biofuel raw material’, International Journal of Technology, vol. 12, no. 5, pp. 1081-1090, https://doi.org/10.14716/ijtech.v12i5.5226
Raissi, S & Farsani, RE 2009, ‘Statistical process optimization through multi-response surface methodology’, World Academy of Science, Engineering and Technology, vol. 39, pp. 280-284
Razaghi, A, Godhe, A & Albers, E 2014, ‘Effects of nitrogen on growth and carbohydrate formation in Porphyridium cruentum’, Open Life Sciences, vol. 9, no. 2, pp. 156-162, https://doi.org/10.2478/s11535-013-0248-z
Ren, B, Chen, C, Li, C, Fu, X, You, L & Liu, RH 2017, ‘Optimization of microwave-assisted extraction of Sargassum thunbergii polysaccharides and its antioxidant and hypoglycemic activities’, Carbohydrate Polymers, vol. 173, pp. 192-201, https://doi.org/10.1016/j.carbpol.2017.05.094
Rezaee, R, Maleki, A, Jafari, A, Mazloomi, S, Zandsalimi, Y & Mahvi, AH 2014, ‘Application of response surface methodology for optimization of natural organic matter degradation by UV/H2O2 advanced oxidation process’, Journal of Environmental Health Science and Engineering, vol. 12, no. 1, pp. 1-8, https://doi.org/10.1186/2052-336X-12-67
Rokicka, M, Zieli?ski, M, Dudek, M & D?bowski, M 2021, ‘Effects of ultrasonic and microwave pretreatment on lipid extraction of microalgae and methane production from the residual extracted biomass’, Bioenergy Research, vol. 14, pp. 752-760, https://doi.org/10.1007/s12155-020-10202-y
Samiee-Zafarghandi, R, Karimi-Sabet, J, Abdoli, MA & Karbassi, A 2018, ‘Increasing microalgal carbohydrate content for hydrothermal gasification purposes’, Renewable Energy, vol. 116, pp. 710-719, https://doi.org/10.1016/j.renene.2017.10.020
Sardi, B, Ningrum, RF, Ardianyah, VA, Qadariyah, L & Mahfud, M 2022, ‘Production of liquid biofuels from microalgae chlorella sp. via catalytic slow pyrolysis’, International Journal of Technology, vol. 13, no. 1, pp. 147-156, https://doi.org/10.14716/ijtech.v13i1.4358
Sebastian, J, Rouissi, T, Brar, SK, Hegde, K & Verma, M 2019, ‘Microwave-assisted extraction of chitosan from Rhizopus oryzae NRRL 1526 biomass’, Carbohydrate Polymers, vol. 219, pp. 431-440, https://doi.org/10.1016/j.carbpol.2019.05.047
Seixas, FL, Fukuda, DL, Turbiani, FRB, Garcia, PS, Petkowicz, CLO & Gimenes, ML 2014, ‘Extraction of pectin from passion fruit peel (Passiflora edulis f. flavicarpa) by microwave-induced heating’, Food Hydrocolloids, vol. 38, pp. 189-192, https://doi.org/10.1016/j.foodhyd.2013.12.001
Sharifvaghefi, S & Zheng, Y 2022, ‘Microwave vs conventional heating in hydrogen production via catalytic dry reforming of methane’, Resources Chemicals and Materials, vol. 1, no. 3-4, pp. 290-307, https://doi.org/10.1016/j.recm.2022.08.003
Si, J & Cui, B 2013, ‘Dye Congo Red adsorptive decolorization by adsorbents obtained from Trametes pubescens pellets’, Desalination and Water Treatment, vol. 51, no. 37-39, pp. 7088-7100, https://doi.org/10.1080/19443994.2013.792445
Silva, AS, de Magalhães, WT, Moreira, LM, Rocha, MVP & Bastos, AKP 2018, ‘Microwave-assisted extraction of polysaccharides from Arthrospira platensis using the concept of green chemistry’, Algal Research, vol. 35, pp. 178-184, https://doi.org/10.1016/j.algal.2018.08.015
Silva, GF, Camargo, FL & Ferreira, ALO 2011, ‘Application of response surface methodology for optimization of biodiesel production by transesterification of soybean oil with ethanol’, Fuel Processing Technology, vol. 92, no. 3, pp. 407-413, https://doi.org/10.1016/j.fuproc.2010.10.002
Singab, AN, Ibrahim, N, Elsayed, AE, El-Senousy, W, Aly, H, Abd Elsamiae, AS & Matloub, A 2018, ‘Antiviral, cytotoxic, antioxidant and anti-cholinesterase activities of polysaccharides isolated from microalgae Spirulina platensis, Scenedesmus obliquus and Dunaliella salina’, Archives of Pharmaceutical Sciences Ain Shams University, vol. 2, no. 2, pp. 121-137, https://doi.org/10.21608/aps.2018.18740
Song, JF, Li, DJ & Liu, CQ 2009, ‘Response surface analysis of microwave-assisted extraction of polysaccharides from cultured Cordyceps militaris’, Journal of Chemical Technology and Biotechnology, vol. 84, no. 11, pp. 1669-1673, https://doi.org/10.1002/jctb.2227
Soria, AC, Ruiz-Aceituno, L, Ramos, L & Sanz, LM 2014, ‘Microwave-assisted extraction of polysaccharides’, in Ramawat, KG & Mérillon, JM (eds), Polysaccharides, Springer, Cham, Switzerland, pp. 1-18, https://doi.org/10.1007/978-3-319-03751-6_43-1
Susanti, DY, Sediawan, WB, Fahrurrozi, M & Hidayat, M 2020, ‘Optimization of agitation and kinetic studies on proanthocyanidin compound extraction from red sorghum grains in agitated vessel’, IOP Conference Series: Materials Science and Engineering, vol. 778, no. 1, p. 012085, https://doi.org/10.1088/1757-899X/778/1/012085
Tan, XB, Zhao, ZY, Gong, H, Jiang, T, Liu, XP & Liao, JY 2024, ‘Growth of Scenedesmus obliquus in anaerobically digested swine wastewater from different cleaning processes for pollutants removal and biomass production’, Chemosphere, vol. 352, p. 141515, https://doi.org/10.1016/j.chemosphere.2024.141515
Tan, YH, Chai, MK, Na, JY & Wong, LS 2023, ‘Microalgal growth and nutrient removal efficiency in non-sterilised primary domestic wastewater’, Sustainability (Switzerland), vol. 15, no. 8, pp. 1-13, https://doi.org/10.3390/su15086601
Thawechai, T, Cheirsilp, B, Louhasakul, Y, Boonsawang, P & Prasertsan, P 2016, ‘Mitigation of carbon dioxide by oleaginous microalgae for lipids and pigments production: Effect of light illumination and carbon dioxide feeding strategies’, Bioresource Technology, vol. 219, pp. 139-149, https://doi.org/10.1016/j.biortech.2016.07.109
Thuy, NTT, Giang, DH, Linh, PK & Dat, NT 2020, ‘Extracting conditions optimization and bioactivity of polysaccharides from the pods of haricot vert’, Chemistry Journal of Moldova, vol. 15, no. 2, pp. 45-53, https://doi.org/10.19261/cjm.2020.795
Tibbetts, SM, Milley, JE & Lall, SP 2015, ‘Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors’, Journal of Applied Phycology, vol. 27, no. 3, pp. 1109-1119, https://doi.org/10.1007/s10811-014-0428-x
Verspreet, J, Soetemans, L, Gargan, C, Hayes, M & Bastiaens, L 2021, ‘Nutritional profiling and preliminary bioactivity screening of five microalgae strains cultivated in northwest Europe’, Foods, vol. 10, no. 7, pp. 1-17, https://doi.org/10.3390/foods10071516
Vieira, BB, Soares, J, Amorim, ML, Bittencourt, PVQ, de Cássia Superbi, R, de Oliveira, EB, dos Reis Coimbra, JS & Martins, MA 2021, ‘Optimized extraction of neutral carbohydrates, crude lipids and photosynthetic pigments from the wet biomass of the microalga Scenedesmus obliquus BR003’, Separation and Purification Technology, vol. 269, p. 118711, https://doi.org/10.1016/j.seppur.2021.118711
Wahidin, S, Idris, A & Shaleh, SRM 2014, ‘Rapid biodiesel production using wet microalgae via microwave irradiation’, Energy Conversion and Management, vol. 84, pp. 227-233, https://doi.org/10.1016/j.enconman.2014.04.034
Wang, N, Dai, LM, Chen, ZS, Li, T, Wu, JY, Wu, HB, Wu, HL & Xiang, WZ 2022, ‘Extraction optimization, physicochemical characterization, and antioxidant activity of polysaccharides from Rhodosorus sp. SCSIO-45730’, Journal of Applied Phycology, vol. 34, pp. 285-299, https://doi.org/10.1007/s10811-021-02646-2
Wang, Y, Wang, C, Xue, H, Jin, Y, Yang, M & Leng, F 2017, ‘Statistical optimization for the production of recombinant cold-adapted superoxide dismutase in E. coli using response surface methodology’, Bioengineered, vol. 8, no. 6, pp. 693-699, https://doi.org/10.1080/21655979.2017.1303589
Wang, Y, Wang, C, Xue, H, Jin, Y, Yang, M & Leng, F 2023, ‘Comparative analysis of three kinds of extraction kinetic models of crude polysaccharides from Codonopsis pilosula and evaluate the characteristics of crude polysaccharides’, Biomass Conversion and Biorefinery, vol. 13, no. 14, pp. 12917-12933, https://doi.org/10.1007/s13399-022-02518-w
Yan, W, Meng, X, Cui, X, Liu, Y, Chen, Q & Jin, L 2022, ‘Evaporative cooling performance prediction and multi-objective optimization for hollow fiber membrane module using response sur face methodology’, Applied Energy, vol. 325, p. 119855, https://doi.org/10.1016/j.apenergy.2022.119855
Yi, S, Su, Y, Qi, B, Su, Z & Wan, Y 2010, ‘Application of response surface methodology and central composite rotatable design in optimizing the preparation conditions of vinyltriethoxysilane modified silicalite/polydimethylsiloxane hybrid pervaporation membranes’, Separation and Purification Technology, vol. 71, pp. 252-262, https://doi.org/10.1016/j.seppur.2009.12.005
Yi, Y, Xu, W, Wang, HX, Huang, F & Wang, LM 2020, ‘Natural polysaccharides experience physiochemical and functional changes during preparation: A review’, Carbohydrate Polymers, vol. 234, p. 115896, https://doi.org/10.1016/j.carbpol.2020.115896
Yirgu, Z, Leta, S, Hussen, A, Khan, M & Aragaw, T 2021, ‘Optimization of microwave-assisted carbohydrate extraction from indigenous Scenedesmus sp. grown in brewery effluent using response surface methodology’, Heliyon, vol. 7, no. 5, p. e07115, https://doi.org/10.1016/j.heliyon.2021.e07115
Yuan, Y & Macquarrie, D 2015, ‘Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity’, Carbohydrate Polymers, vol. 129, pp. 101-107, https://doi.org/10.1016/j.carbpol.2015.04.057
Zaidi, AA, Feng, RZ, Malik, A, Khan, SZ, Bhutta, AJ, Shi, Y & Mushtaq, K 2019, ‘Conjoint effect of microwave irradiation and metal nanoparticles on biogas augmentation from anaerobic digestion of green algae’, International Journal of Hydrogen Energy, vol. 44, no. 29, pp. 14661-14670, https://doi.org/10.1016/j.ijhydene.2019.02.245
Zainol, N, Aziz, NH & Baharudin, AS 2023, ‘Influence of agitation and solvent percentage on the extraction of phytochemical compound from Asystasia gangetica’, Food Chemistry Advances, vol. 3, article 100538, https://doi.org/10.1016/j.focha.2023.100538
Zhang, L, Tu, Z, Wang, H, Kou, Y, Wen, Q, Fu, Z & Chang, H 2015, ‘Response surface optimization and physicochemical properties of polysaccharides from Nelumbo nucifera leaves’, International Journal of Biological Macromolecules, vol. 74, pp. 103-110, https://doi.org/10.1016/j.ijbiomac.2014.11.020
Zhang, Y, Wu, H, Yuan, C, Li, T & Li, A 2019, ‘Growth, biochemical composition, and photosynthetic performance of Scenedesmus acuminatus during nitrogen starvation and resupply’, Journal of Applied Phycology, vol. 31, no. 5, pp. 2797-2809, https://doi.org/10.1007/s10811-019-01783-z
Zhao, G, Chen, X, Wang, L, Zhou, S, Feng, H, Chen, WN & Lau, R 2013, ‘Ultrasound assisted extraction of carbohydrates from microalgae as feedstock for yeast fermentation’, Bioresource Technology, vol. 128, pp. 337-344, https://doi.org/10.1016/j.biortech.2012.10.038
Zhao, Y, Han, C, Wu, Y, Sun, Q, Ma, M, Xie, Z, Sun, R & Pei, H 2024, ‘Extraction, structural characterization, and antioxidant activity of polysaccharides from three microalgae’, Science of the Total Environment, vol. 931, p. 172567, https://doi.org/10.1016/j.scitotenv.2024.172567
Zhou, X, Jin, W, Tu, R, Guo, Q, Han, S, Chen, C, Wang, Q, Liu, W, Jensen, PD & Wang, Q 2019, ‘Optimization of microwave assisted lipid extraction from microalga Scenedesmus obliquus grown on municipal wastewater’, Journal of Cleaner Production, vol. 221, pp. 502-508, https://doi.org/10.1016/j.jclepro.2019.02.260
