Deep Eutectic Solvents and Natural Deep Eutectic Solvents for Extraction and Purification of Proteins from Animal and Botanical Sources - A Review
Corresponding email: andreyheriedzal@gmail.com
Published at : 18 Sep 2024
Volume : IJtech
Vol 15, No 5 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i5.6925
Kumoro, A.C., Wardhani, D.H., Kusworo, T.D., Djaeni, M., Ping, T.C., Alhanif, M., 2024. Deep Eutectic Solvents and Natural Deep Eutectic Solvents for Extraction and Purification of Proteins from Animal and Botanical Sources - A Review. International Journal of Technology. Volume 15(5), pp. 1420-1437
| Andri Cahyo Kumoro | 1. Department of Chemical Engineering, Faculty of Engineering, Universitas Diponegoro, Semarang, 50275, Indonesia 2. Institute of Food and Remedies Biomaterials (INFARMA), Faculty of Engineering, Uni |
| Dyah Hesti Wardhani | 1. Department of Chemical Engineering, Faculty of Engineering, Universitas Diponegoro, Semarang, 50275, Indonesia 2. Institute of Food and Remedies Biomaterials (INFARMA), Faculty of Engineering, Uni |
| Tutuk Djoko Kusworo | Department of Chemical Engineering, Faculty of Engineering, Universitas Diponegoro, Semarang, 50275, Indonesia |
| Mohamad Djaeni | Department of Chemical Engineering, Faculty of Engineering, Universitas Diponegoro, Semarang, 50275, Indonesia |
| Tan Chin Ping | Department of Food Technology, Faculty of Food Science and Technology, Universiti Putra Malaysia, Serdang, 43400, Malaysia |
| Misbahudin Alhanif | 1. Department of Chemical Engineering, Faculty of Engineering, Universitas Diponegoro, Semarang, 50275, Indonesia 2. Institute of Food and Remedies Biomaterials (INFARMA), Faculty of Engineering, Uni |
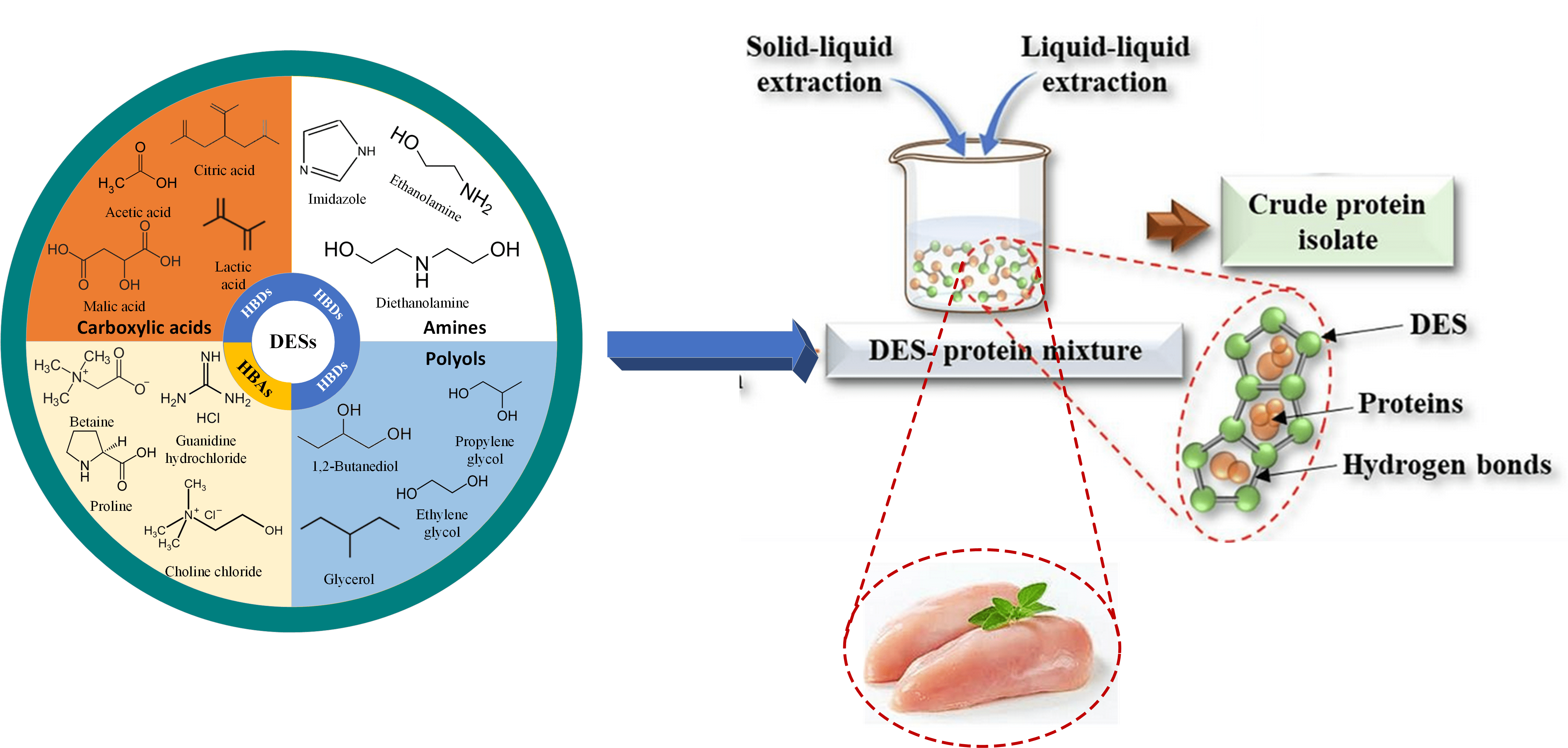
The
rising global population has led to an escalating demand for affordable,
high-quality proteins intended for human consumption. Different efforts have
been made to develop efficient and greener solvents for protein production.
Most proteins are produced from raw plant and animal parts, but the use of the
residues in commercial-scale protein production is very scarce. Therefore, this
research aims to collect an overview of deep eutectic solvents (DESs) and
natural deep eutectic solvents (NADESs) for protein extraction and
purification. In this context, solvent type and operating parameters should
also be selected to modify DESs and NADESs physicochemical characteristics for
the achievement of high protein yield with preserved functional properties. The
results show that appropriate implementation of combined DESs and NADESs with
advanced extraction and purification techniques can improve protein yield and
prevent detrimental effects on the extracted protein and environment. The
application of DESs and NADESs can increase the efficiency of protein
extraction and recovery in various parts of plants and animals by 55.72% and
98.16%, respectively, with purity reaching 99.82%. This research also reviews
safety, environmental impacts, and drawbacks as well as shows feasible future
recommendations for commercial-scale protein production processes. NADESs are safer than petroleum-based solvents but
have higher toxicity than DESs. Magnetic adsorbent and magnetic solid-phase
extraction methods have been shown to reduce labor-intensive steps, resulting
in shorter operating times and superior protein recovery while maintaining
functional properties. Meanwhile, protein producers' knowledge and motivation
to use DES and NADES are strengthened.
Animal and plant parts; Deep eutectic solvent; Extraction; Operating parameter; Protein
Protein is the basic unit of living organisms
acquired from sustainable origins, namely animals, plants, and microorganisms. Since
the world’s population is forecasted to reach 9 billion by 2050, the demand for
affordable high-quality protein is expected to increase (Grudniewska et al., 2018). Therefore, effcient, greener and
sustainable methods for protein production receives a growing interest. The underused
sources have also attracted attention for the preparation of affordable pure
proteins. In this context, the food industries may also adopt
underused plant and animal residues by extracting and refining proteins as
valuable biomacromolecules showing numerous health benefits to reduce
environmental problems and support sustainable development goals.
The
crucial step in using protein is to adopt suitable extraction technologies to
acquire protein from animal or plant matrices. Conventional procedures are techniques
regularly used, such as chemical methods. However, the methods can lead to
reduced extraction yields due to protein degradation (Kumar et al., 2021b). Numerous industries also continue to depend
on traditional methods because of financial viability. Chemical methods are
categorized according to petroleum-based solvents, water, alkalis, organic
solvents, and acids (Bowen et al., 2022; Kumar et al., 2021b). Meanwhile, chemical methods are used
to enhance the retrieval of proteins (Kumar et al., 2021a; Bose et al., 2019). Protein purification methods include
precipitation through the addition of an agent, electrophoresis, ion exchange,
and affnity chromatography (Lin et al., 2021). Conventional acid-based extraction is less
promising due to lower quality of protein (Kumar et al., 2021b). Petroleum-based and solvent-based chemical
methods have been used in the food and pharmaceutical industries for centuries.
However, this solvent has serious problems, those related to low extract yield
and coextraction of unwanted substances, long extraction time, operational
complexity, expensive, toxicity, biodegradability, fammability, safety and
environmental sustainability, denaturation or reduced biological activities (Ling and Hadinoto, 2022; Fuad, Nadzir, and Harun, 2021; de Jesus and Filho, 2020).
Organic
solvents are crucial in protein extraction and precipitation processes, leading
to the production of pure products. Water-based extraction is a widely used
method for obtaining proteins from different sources (Chen et al., 2019a). The process is commonly adopted because of
the elevated solubility and stability of isolated protein, which is mostly
attributed to high content. Protein with the capacity of attaching to lipids
have non-polar or polar side chains and contain aromatic amino acids that
readily dissolve in organic solvents such as ethanol, butanol, and acetone (Qiaoyun et al., 2017). Aqueous two-phase systems, created by
combining polyethylene glycol (PEG) with salts can be used efficiently for
purification and separation of proteins (Asenjo and Andrews, 2011).
Alkalis
such as NaOH and KOH are frequently used to maintain a basic pH and enhance the
yield compared to organic extraction. Altering the pH to a basic level caused
the disulphide linkages to dissolve, leading to an enhancement in recovery and
yield (Contreras et al., 2019). The solubility is enhanced by an elevation
in the pH of solvent due to the ionization of acidic and neutral amino acids at
high pH levels. Therefore, extracting protein in an alkaline environment results
in greater protein yields. In this context, a yield above 90% can be obtained
from soybeans, rapeseed, and other seed commodities through alkaline extraction
at high pH. Alkaline enhances protein extraction and disrupts the structure of
amino acids, lysine and cysteine. This significantly degrades the digestibility
and acceptability of the extracted protein (Kumar et al., 2021b).
To
address this limitation, the food industries have initiated the use of greener
solvents as substitutes for petroleum-based solvents, alkalis, organic
solvents, and acids to manufacture products (Ling
and Hadinoto, 2022; Kumar et al., 2021a; Kumoro
et al., 2019). In addition, ionic liquids (ILs),
deep eutectic solvents (DESs) and analogues from natural sources (sugars,
acids, and amino acids) known as natural deep eutectic solvents (NADESs) are
progressing as advanced agents to decrease the adverse impacts of petroleum-based
solvents (Ling and Hadinoto, 2022). DESs are composed of a quaternary ammonium
salt (QAS) and a hydrogen bond donor (HBD), such as a carboxylic acid or
alcohol. These components are mixed in specific molar ratios to form a eutectic
mixture with a lower melting point (Abbott et al., 2003). Meanwhile, NADESs consist of natural
compounds such as sugar (glucose), organic acids (citric acid), and amino acids
(choline). This compound forms a eutectic mixture with properties similar to
DES but derived from renewable resources (Dai et al., 2013b).
Despite
having comparable physical characteristics to ILs, such as high viscosity, low
volatility, chemical and thermal stability, as well as non-flammability, DESs
are not made of ionic compounds. Non-ionic chemicals can produce DESs (Ling and Hadinoto, 2022), which are more cost-effective with higher
biodegradability (Kudlak, Owczarek, and Namiesnik, 2015). These solvents are environmentally friendly,
easy to synthesize, cheap, biodegradable, and possess low toxicity (Chen et al., 2018).
The
use of DESs and NADESs as an environmentally friendly solvent for extracting
valuable compounds has gained significant interest in recent years. This is due
to the distinct characteristics, including customizable properties,
versatility, ease of preparation, unique super-molecular structure that shows
strong attraction to different compounds, high solubility, and stabilizing
capability (Zannou and Koca, 2022; Dai et al., 2013a). The majority of investigations on extraction
have mostly focused on bioactive small compounds (Pratiwi et al., 2020; de Faria et al., 2017). Several reviews have been published
on the latest developments in extraction of bioactive small compounds using
DESs. Bonacci et al. (2020) gave an example showing the ability to
extract DESs from tiny molecules such as phenolic compounds. The use of choline
chloride glycerol DESs resulted in a significantly higher recovery yield of
oleuropein (~88,287 ppm) from olive oil processing wastes. This was achieved in
10 minutes, which is twice the usual water extraction method requiring 30
minutes. The research reported by de Faria et
al. (2017) also observed similar performance of DES. In
this context, higher recovery of polyphenols and flavonoid compounds was
obtained from saffron processing wastes compared to conventional solvents such
as aqueous methanol, aqueous ethanol, and water. Mulia
et al. (2015) have also succeeded in extracting mangostin
with the highest yield of 2.6% (w/w) from dried mangosteen skin using NADESs.
This was obtained using a mixture of choline chloride and 1,2-propanediol with
a mole ratio of 1:3.
Even
though DESs and NADESs extraction of small bioactive compounds is highly practical,
the investigation of biological macromolecules, such as proteins, oils and
carbohydrates, has gained attention (Ling and Hadinoto, 2022; Mulia et al., 2018). Considering the distinct physicochemical
characteristics of proteins in comparison to other small or large molecules,
the function of DES and NADESs should be reported in extracting proteins
derived from animals and plants. The selection of solvents depends on factors
such as the solubility of protein, extraction efficiency, and desired
properties of solvents. There are differences in protein from animals and
plants due to variations in biochemical composition, cellular structure, and
properties. Animal protein extraction includes methods such as aqueous, organic
solvent, or enzymatic digestion, depending on the tissue type and desired
protein (Malva et al., 2018). Meanwhile, plant protein extraction requires
additional steps to break down cell walls, such as mechanical disruption or
enzymatic treatment, followed by aqueous or buffer solutions (Wang, Liu, and Lu, 2013). Extraction from animals and plants with
greener and environmentally friendly solvents is interesting for further
analysis.
In
this research, extraction of DES and NADESs from animals and plants is
presented. The review commenced with a discussion of the fundamental and
physicochemical characteristics of DESs and NADESs suitable for protein
extraction processes. The main results and trends observed in solvent-based
protein extraction were discussed with the safety and environmental aspects.
Ionic
liquids (ILs) are used as solvents for reaction or extraction processes and
provide numerous advantageous characteristics, such as high boiling point,
broad liquid range, selective dissolving capacity, excellent thermal stability,
non-flammable and molecular structure variety (Morais et al., 2020). However, the preparation is very
complicated, requiring high equipment, manufacturing costs, and laborious
recovery from the mixture with the target analyte (Ling and Hadinoto, 2022). To overcome the limitation, DESs are developed
as a new generation of advanced greener solvents.
As a
eutectic mixture, DES can be synthesized partially or completely from non-ionic
compounds, which function as hydrogen bond donors (HBDs) such as betaine,
choline chloride, guanidine hydrochloride and proline, as well as hydrogen bond
acceptors (HBA) including amine groups (ethanolamine, dimethylamine, and
imidazole), polyols (ethylene glycol, propylene glycol, and glycerol), and
carboxylic acids (acetic, citric, maleic, and lactic acids) (Saini et al., 2022). Meanwhile, NADESs consist of natural
compounds such as sugar (glucose), organic acids (citric acid), or amino acids
(choline). This compound forms a eutectic mixture with properties similar to
DESs but derived from renewable resources (Dai et al., 2013b). The preparation comprises sequential
procedures, such as size reduction, heating, evaporation, and low-temperature
drying (Saini et al., 2022). After careful selection of the prerequisite
substances, homogenization is carried out at ideal conditions (i.e., <100°C)
to attain a eutectic mixture and equilibrate the resulting solution with
ambient conditions (Mišan et al., 2020). Therefore, the physicochemical
characteristics of DESs are equivalent to ILs as a perfect substitute. The
macromolecular structures are appropriate for protein extraction concerning
solubility, affnity, and stability (Landa-Castro et al., 2020). The influence of the composition of HBAs and
HBDs on the characteristics is explained in Section 4.
DESs
and NADESs can exist as binary or ternary mixtures. In addition, NADESs possess simpler preparation procedures, better
efficiency, selectivity, biodegradability, thermal stability, and
sustainability (Ijardar, Singh, and Gardas, 2022). Based on chemical compositions and formulas,
DESs are grouped into four classes (Abbott et al., 2004).
3. DESs and
NADESs Physicochemical Characteristics Related to Extraction Process
Even though the physical
characteristics of DESs are closely similar to the conventional ILs, the
chemical characteristics are significantly different. The influential
physicochemical characteristics are melting point, freezing temperature,
polarity, solubility, miscibility, pH, density, viscosity, interfacial tension,
refraction index, and ionic conductivity (Omar and Sadeghi, 2022b). The anion size, equilibrium molar ratio of
HBA/HBD, alkyl chain length, and molecular mass of HBA/HBD at the melting point
determine the physicochemical characteristics (Omar and Sadeghi,
2022a). However, viscosity and polarity possess
significant effects on extraction performance (Tolmachev et al.,
2022). Interfacial tension also has a remarkable
effect on liquid-liquid and ultrasound-assisted extraction processes (Kumoro et al.,
2022).
The fluidity of DESs is mainly
characterized by viscosity (Omar and Sadeghi, 2022b), influencing the solubility of target
samples. In this context, the biomacromolecules are more soluble in
less-viscous DESs. Temperature, type of HBAs and HBDs, HBA/HBD molar ratio, and
molecular mass greatly affect viscosity. Meanwhile, an increase in temperature
appreciably decreases viscosity due to hydrogen bond network cleavage between
HBA and HBD as well as the reduction of internal resistance of molecules (Ling and Hadinoto,
2022). DESs possess higher viscosity when sugar,
carboxylic acid and metallic compounds are selected as the HBD, while less
viscous type can be prepared using ethylene glycol, glycerol, and phenol (Ling and Hadinoto,
2022). After water addition, the viscosity
decreases, facilitating better mixing and mass transfer leading to a higher
extraction performance. An extreme amount of water ceases the advantageous
characteristics of DES. Therefore, an appropriate water quantity should be selected
to prevent the disruption of hydrogen bond interactions among DES-forming
substances (Ling et al.,
2020).
Polarity, which is closely related to
Hansen’s solubility parameter (HSP) is a theoretical method used as an
introductory tool to estimate the solubility of target biomacromolecules in
different solvents and select the most appropriate DESs and NADESs for
extraction from respective natural sources (El-Kantar et al.,
2019). Since DESs are mostly polar substances, the
superior ability to dissolve the target analytes can improve extraction yield (Gullón et al.,
2020). Eutectic solvent polarity is attributed to
the HBDs used for the synthesis and the highest are usually synthesized from
carboxylic acids. In contrast, polyols and sugars result in eutectic solvents
with the lowest polarity (Xu et al.,
2019a). The dilution of DESs with water beyond 50%
causes more hydrogen bond destruction and leads to a decrease in extraction
ability (Dai et al.,
2015).
The density of DESs is higher than
pure water (Omar and Sadeghi,
2022b), with the value ranging from 1.0 to 1.35 g/cm3
at 25oC. However, the density of metallic salts containing DESs lies
between 1.3 to 1.6 g/cm3 (Zhang et al.,
2012), depending on the molecular arrangement,
temperature, HBA/HBD molar ratio, as well as the presence of cavities and
voids. Temperature greatly decreases the density, refraction index, and
acoustic velocity due to the enhancement of ionic movement and unoccupied
volume (Omar and Sadeghi,
2022b).
Surface tension is an important
characteristic of DESs utilization in the interface and colloid system (Kumoro et al.,
2022). In this context, HBDs and HBAs have an
appreciable influence on the surface tension of DESs. Since DESs are highly
hydrophobic, the surface tension is enhanced greatly when the water mole
fraction is higher than 0.9 (Chen et al.,
2019b). However, the surface tension decreases as a
function of solvent concentration when petroleum-based solvents or crystal
water in the salt component are added with an increasing temperature from 20 to
60°C (Chen et al.,
2019b).
The majority of DESs are highly
viscous liquids with large ion sizes and possess low ionic conductivity (below
1 mS/cm at ambient temperature) which limits the application in
isoelectric-based protein precipitation (Omar and Sadeghi,
2022b). The ionic conductivity is influenced by
alkyl chain length of cation, the characteristics of organic salt, HBDs and
HBAs ionic ratio, temperature, and water content (Dai et al.,
2015). Therefore, the conductivity can be improved
by increasing the free volume through the reduction of cation size and the
substitution of HBD with fluorinated substances to reduce viscosity (Abbott, Capper, and Gray, 2006). Another strategy is to increase the
temperature which helps to break the hydrogen bond network and increase ionic
motility (Omar and Sadeghi,
2022b). The tunable physical characteristics,
specifically density, viscosity, ionic conductivity, and freezing point
obtained by a careful selection of biodegradable HBD and HBA couplings make
DESs and NADESs more attractive for specific applications (Zhang et al.,
2012).
4. DESs and
NADESs as Extracting Media for Protein Extraction and Purification
DESs,
formed by the combination of hydrogen bond donors and acceptors, have been
extensively studied for the versatile applications. For instance, Abbott et al. (2003) showed the exceptional solvent properties of
DESs, particularly in extraction of bioactive compounds from natural sources.
The results showed that DESs, such as those composed of choline chloride and
urea, reported superior extraction yields and selectivity compared to conventional
solvents. Similarly, Dai et al. (2013a) suggested the efficiency in catalyzing
various chemical reactions, showing the potential as green reaction media.
Conversely, NADESs are derived from naturally occurring compounds such as
sugars, organic acids, and amino acids, and the efficiency has been reported in
various processes. For example, Socas-Rodríguez et
al. (2021) investigated the use as extraction solvents
for bioactive compounds from different matrices. The research showed that
NADESs offered a greener alternative to conventional solvents, with comparable
or improved extraction efficiency. Generally, DESs and NADESs offer promising
prospects as efficient and greener solvents across various industries. The
unique properties, coupled with the renewable and sustainable nature, position
solvents as viable alternatives, contributing to the development of more
sustainable processes.
Efficiency
and yield when using DES or NADES can vary depending on the specific
application and the characteristics of solvent system. These eco-friendly
solvents offer several advantages contributing to improved efficiency and yield
in various processes, including extraction, synthesis, and catalysis. According
to Smith, Abbott, and Ryder (2014), solvents possess unique properties in
enhancing the efficiency and yield of chemical processes. The properties
include the ability to enhance solubility, provide selective extraction,
operate under mild reaction conditions, and offer recyclability. By leveraging
the characteristics, DES and NADES have shown potential to facilitate higher
yields and improved efficiency compared to conventional solvents (Smith, Abbott, and Ryder, 2014).
To
elaborate on a specific example, extraction of bioactive compounds is
considered from plant material using NADESs. Choi et
al. (2011) reported the use in understanding cellular
metabolism and physiology, emphasizing the potential for efficient extraction
of target compounds from natural sources. The selected method includes
preparing the NADESs by mixing components such as choline chloride and organic
acids in specific ratios, followed by extraction of bioactive compounds from
plant material at an appropriate temperature. The resulting extract can be
analyzed using analytical techniques to quantify yield and purity. Moreover, Dai et al. (2015) discussed the tailoring of properties,
including the addition of water, to optimize performance in various
applications. This optimization process enhances efficiency and yield by
fine-tuning solvent characteristics to suit specific extraction or synthesis
requirements.
In
the case of protein extractions, the most used DESs belong to type III and
comprise ChCl as HBA and amines, amide, carboxylic acids, sugars, and polyols
as HBD (Zhang et al., 2012). Ling and Hadinoto (2022) showed that there were considerable
variations in melting points when the HBA:HBD ratios in ChCl:urea were changed.
A 1:2 ratio resulted in a significantly lower melting temperature of 12oC,
while a 1:1 ratio generated NADESs with a high melting point of 50oC.
Therefore, the choice of HBD component has a major impact on the melting point
of the resulting DES as well as the effect of the molar ratio of HBA and HBD.
For example, the use of ethylene glycol, citric acid, malonic acid, oxalic
acid, xylitol, and glycerol as HBD produced DESs with melting temperatures
ranging from 69 °C to room temperature. In this context, the type of HBD
affects the melting point of the synthesized DESs (Zhang et al.,
2012). An appropriate HBA/HBD molar ratio
in the mixture plays a crucial role in ensuring the suitability of the
resulting DESs to selectively dissolve the targeted proteins and easy solvent
recovery.
4.1. DESs and NADESs for Solid–Liquid
Extraction of Protein from Plant and Animal Parts
The mechanism of DESs and NADESs in
solid-liquid extraction of proteins from plant and animal parts includes a
series of steps. These solvents penetrate the cell structures of plant
materials or animal tissues, disrupting hydrogen bonds and hydrophobic
interactions (Morais et al.,
2020). Within plant or animal material, DESs and
NADESs solubilize proteins through interactions such as hydrogen bonding and
electrostatic interactions (Bowen et al.,
2022). Meanwhile, extraction methods such as shaking or stirring enhance
protein leaching from the solid matrix into solvent phase (Zhou, Fakayode and Li, 2023). Subsequent phase separation techniques, such
as centrifugation or filtration, isolate protein-containing solvent phase from
the solid debris (García et al.,
2015). Protein recovery from solvent phase is also
achieved through precipitation or purification methods, with the possibility of
solvent regeneration for further use (Abbott et al.,
2004). This mechanism shows the potential of DESs
and NADESs as sustainable alternatives for protein extraction, offering
applications in various industries including food, pharmaceuticals, and biotechnology.
Different investigations have also
focused on the usage of biomass residues as tabulated in Table 1. These greener
solvents have proven the ability to achieve higher extraction performance in
terms of product yield and purity. Liu et al.
(2017) prepared DESs by mixing several HBAs, such as
ChCl, glycine, betaine, alanine chloride, acetylcholine chloride, and nicotinic
acid with PEG200 to extract pumpkin seeds protein. The PEG200-based DESs were
mixed with a four-fold volume of ethanol as well as 1 M hydrochloric acid to
control the pH at 4.5 and recover 93.8% of the extracted protein (Table 1).
Currently, extraction of proteins using PEG-based DESs is growing more rapidly.
This is because the extracted proteins are more stable and accepted by Food and
Drug Administration (FDA), which opens wider opportunities for more sustainable
developments in the agricultural, food and pharmaceutical sectors (Morgenstern et
al., 2017). Even though PEG-based DESs show excellent
affnity to protein as well as a remarkable capacity to ease precipitation, more
research is required to ensure the scale-up and practical application at a
commercial scale.
Hernández-Corroto et al. (2020) used highly polar and hydrophilic DESs derived from ChCl and
acetic acid to extract pomegranate peel protein. The resulting extract
contained 19.2 mg protein/g with high antihypertensive activity, which was
stronger than petroleum-based extract. After selecting nine ChCl – diol
mixtures as DESs, Yue et al. (2021) found that ChCl–1,4-butanediol/water mixture
was the perfect solvent for oat protein extraction with an effciency of 55.72%
as well as better stability and foaming capacity (Table 1). DESs derived from multicomponent
mixtures could precipitate protein more rapidly than binary mixtures caused by
high partitioning capacity and polarity.
Chen et al. (2021) prepared DESs by mixing ChCl with glycerol to
extract soy protein with a yield of 10% higher than the acid-based
precipitation method, which reported enhanced heat resistance and
hydrophobicity. An equivalent observation was stated by Lin et al. (2021) when acidic DESs based on ChCl and levulinic acid were used to
extract protein from bamboo shoots. The research observed a more profound
protein yield enhancement (60%) compared to extraction using sodium hydroxide
solution.
With the intention of marine
by-product valorization, Rodrigues et al. (2021) prepared DES by blending betaine and
propylene glycol in a ratio of 1:3 to recover proteins from sardine fish heads
and entrails. A yield of 162.2 mg protein/g fish parts greater than the common
aqueous extraction was obtained. This product contained numerous hydrophobic
amino acids, namely alanine, isoleucine, leucine, and valine that are
applicable to produce less polar DESs. In addition, the resulting extracts
showed stronger antioxidant and antimicrobial capacities. The existence of
hydrophobic DESs components promotes more intensive interactions between the
respective proteins with the cell membrane of the assayed microorganisms.
Rodrigues et al. (2021) extracted protein from sardine processing
residue using betaine–propylene glycol and obtained a yield of 162.2 mg
protein/g fish with higher antioxidant and antimicrobial activities than the
water extract. DESs made from lactic acid and L-cysteine are capable of
recovering keratin from coarse wool residue without altering the polypeptide
structure (Okoro et al.,
2022). Based on those reviewed facts, DESs and
NADESs are capable of extracting valuable proteins and preserving desirable
functional characteristics.
Table 1 Extraction
of proteins from sustainable sources using various DESs and NADESs
|
NADESs/DESs |
Protein Sources |
Process Parameters |
Yields |
References |
|
Solid-liquid extraction (SLE) | ||||
|
ChCl–based NADES with ethylene glycol |
orange peel |
Temp.: 4°C, time: 15 min |
7.7 mg/g FW |
(Panic et al.,
2021) |
|
ChCl–levulinic acid |
basal bamboo shoot (BBS), sheath, tip bamboo shoot (TBS) |
Temp.: 80°C, time: 50 min, solid/liquid ratio (30 mg/mL) |
Protein: 15.46 ± 0.30 mg/g DW, 9.54 ± 0.17 mg/g DW and 39.16 ± 1.22
mg/g DW for BBS, sheath and TBS. |
(Lin et al.,
2021) |
|
ChCl–butanediol |
oat |
Temp.: 80°C, time: 1.5 h |
Total protein yield: 55.72%, extracted protein has high solubility,
foaming capacity, and stability. |
(Yue et al.,
2021) |
|
ChCl–glycerol |
soybean |
Temp.:60°C, time: 3.9 h, liquid/solid ratio: 10.3, stirring speed: 873
rpm, water content: <15 wt.% |
Total protein yield: 34.62%, extracted protein has good heat
resistance and is highly hydrophobic. |
(Chen et al.,
2021) |
|
ChCl–acetic acid
|
pomegranate peels |
Time: 15 min, ChCl:AA molar ratio 1:2 |
Protein yield: 19.2 mg/g of protein with excellent antihypertensive
capacity |
(Hernández-Corroto
et al., 2020) |
|
ChCl–PEG |
pumpkin seed |
Temp.: 43oC, liquid/solid ratio: 28 mL/g, microwave power:
140 W, DES concentration: 28% w/w, precipitation time: 4 min |
Protein yield: 93.95% (w/w) of protein |
(Liu et al.,
2017) |
|
Betaine–propylene glycol (B:PG) |
Sardine processing residues |
Temp.:80°C, time: 18 h, B:PG molar ratio: 1:3, liquid/solid ratio: 80 |
Total protein yield: 162.2 mg/g protein. |
(Rodrigues et
al., 2021) |
|
Liquid-liquid extraction (LLE) | ||||
|
ChCl–glycerol |
BSA |
Temp.: 30oC, DES: 1.3 g, salt solution concentration: 0.9
g/mL |
98.16% of BSA was transferred into the DES-rich phase of ATPS,
back-extraction effciency:32.96% |
(Xu et al., 2016) |
|
Tetrabutylammonium bromide–glycolic acid |
Lysozyme from chicken egg white |
Temp.: 35oC, DES <1.0 g, salt solution concentration
< 0.25 g/mL |
98.16% of lysozyme was transferred into the DES-rich phase of ATPS,
91.73% of the initial activity of lysozyme was retained |
(Xu et al.,
2019b) |
|
[TBAC][PPG400]/ [Pro][Xyl] |
Chymotrypsin |
Temp.: 35oC, [Pro][Xyl]: 1.6 g, Protein: 8 mg,
[TBAC][PPG400]: 1.0 g |
extraction effciency: 97.30% |
(Meng et al.,
2019) |
There are many parameters,
which provide essential roles during biomacromolecules extraction, specifically
proteins using DESs and NADESs, such as temperature, duration, solvent-to-solid
ratio, solid particle size, and pH (Kumoro et al.,
2022). An appropriate selection of temperature,
water content, and pH will alter polarity, solubility, interfacial tension, and
viscosity supporting the achievement of high extraction performance (Huang et al.,
2017).
4.2. DESs for
Liquid-Liquid Extraction of Protein from Plant and Animal Parts
Aqueous two-phase systems (ATPS) are
used to facilitate selective liquid-liquid extraction of proteins by
homogenizing a water-soluble polymer with another inorganic salt, such as
PEG-salt-water mixture and ethylene oxide–propylene oxide or
copolymer–polyoxyethylene detergent at a concentration higher than the critical
value (Xu et al.,
2016). Xu et al.
(2016) prepared an ATPS by mixing ChCl and glycerol
with a salt solution and successfully extracted 98.16% of bovine serum albumin
(BSA) from the DESs phase without any protein conformation changes. Hydrogen
bonding, salting out, and hydrophobic interactions are possible characteristics
facilitating protein uptake (Xu et al.,
2016).
Xu et al.
(2019b) recovered lysozyme (Lyz) from chicken egg
white using DESs-based ATPS derived from tetrabutylammonium bromide (TBAB), glycolic
acid (Gly) and Na2SO4 salt. More than 98% of the lysozyme
was transferred to the DESs-phase with 91.73% of the initial activity
preserved. Similarly, Meng et al. (2019) selected a mixture of tetrabutylammonium
chloride (TBAC), L-proline – xylitol (Pro– Xyl) of 1:6 and polypropylene glycol
400 as DESs and ATPS to extract chymotrypsin from the mixture with BSA and
lysozyme. The result also showed that 97.30% chymotrypsin was accumulated in
the [Pro][Xyl]-rich phase under optimum conditions (pH 7.0, 35oC, 12
minutes shaking). The phase separation capacity of DESs can be improved by
enhancing the alkyl side chain length of carboxylic acids and the addition of a
benzyl group.
Liquid-liquid extraction of proteins
using DESs and NADESs in combination with ATPS includes a sophisticated
mechanism. Solvents penetrate the cellular structures of
plant materials or animal tissues, disrupting intermolecular forces and
solubilizing proteins (Abbott et al.,
2003). Subsequently, the mixture is combined with
an aqueous solution to form a biphasic system, where protein partitions between
the two phases based on the physicochemical properties (Singh and Tavana,
2018). Liquid-liquid extraction methods such as
stirring or shaking facilitate the transfer of protein into solvent-rich phase,
while the remaining sample matrix remains in the aqueous phase. Subsequent
separation methods, such as centrifugation or inversion isolate
protein-enriched phase from the aqueous phase (Mendes et al.,
2023). Protein recovery can be achieved through
precipitation or purification methods, with the potential for solvent
regeneration for reuse. This integrated method obtains the advantages of DESs
and NADESs in combination with ATPS to offer an efficient and sustainable
method for protein extraction from diverse biological sources.
4.3. Protein Purifcation Using DESs and NADESs
The extracted
proteins must be purified to allow food, pharmaceutical, and nutraceutical
industries to commercialize safe products for actual human and animal
consumption. The well-established methods to purify protein are alkali,
ammonium sulphate or acetone precipitation, salting-out, ion exchange,
electrophoresis, and affnity chromatography (Sindhu et al., 2012). However, the established techniques suffer
from limitations, such as protein activity loss, denaturation or complexation,
higher operating costs, and equipment operation difficulty. For example, an
enzyme's activity can be distorted by excessive interaction with a polar
solvent. In this context, a smart purifcation strategy using ATPS can be a
potential alternative method due to shorter purification and phase separation
time, excellent capacity to preserve biological activity, great
biocompatibility, low toxicity, and lower requirement of water (Gai et al., 2011). DESs have also been used as a part of ATPS
to purify protein (Table 2) (Zeng et al., 2016).
Purification process using ATPS offers a
versatile method for separating biomolecules based on differential partitioning
between two immiscible aqueous phases (Albertsson, 1970). This strategy includes the design of
phase-forming components such as polyethylene glycol (PEG) and dextran to
optimize protein partitioning behavior (Singh and Tavana, 2018). After mixing the sample with ATPS, the target
protein preferentially partitions into a phase while impurities remain in the
other, facilitating efficient separation (Hatti-Kaul, 2000). Smart technologies integrated into purification
process enable real-time monitoring of protein, allowing for precise adjustment
of conditions to enhance separation efficiency (Bernau et al., 2022). Meanwhile, separation techniques such as
centrifugation are used to isolate the phases containing the purified protein,
followed by further purification steps when necessary (Du et al., 2022). By obtaining automation and data-driven
optimization, this ATPS-based strategy ensures high yields and purity levels in
biotechnology and pharmaceutical applications.
Table 2
Utilization of DES-based ATPS for protein purification
|
|
ATPS |
Proteins |
Purification rate (%) |
References |
|
Associated with DES |
Betaine: glycerol: H2O (1: 2: 1)
-K2HPO4 |
BSA |
99.82 |
(Li et al., 2016) |
|
ChCl: glycerol (1: 1)-K2HPO4 |
BSA |
98.71 |
(Xu et al., 2016) | |
|
ChCl: glycerol (1: 1)-K2HPO4 |
Trypsin |
94.36 |
(Xu, Wang, and Hou, 2020) | |
|
ChCl: urea (1: 2)-K2HPO4 |
R-phycoerythrin |
92.60 |
Xu, Wang, and Hou, 2020) | |
|
Protein purification by MSPE |
PEG4000–MgSO4 |
BSA |
82.68 |
(Saravanan et al., 2008) |
|
Betaine–K2HPO4 |
BSA |
90 |
(Zeng et al., 2016) |
To
achieve the
objective, Li et al. (2016) adopted betaine as the HBA to compose six
types of DESs using urea, methyl urea, glucose,
sorbitol, glycerol, and ethylene glycol as HBD to extract and purify BSA
from protein mixture. In this context, betaine – urea mixture was found as the
most favorable DESs in combination with ATPS for BSA extraction and
purification from the complex systems where an effciency of 99.82% was
achieved. Moreover, Xu et al. (2015) used DESs derived from ChCl as HBA and
ethylene glycol, glycerol, glucose, and sorbitol as HBD to extract and purify
BSA from the mixture. The result confirmed that ChCl–glycerol mixture at a 1:1
molar ratio was the most favorable DESs. Under optimum pH, temperature, and
time, BSA and trypsin recoveries were 98.71% and 94.36%, respectively.
Meanwhile, Xu, Wang, and Hou (2020) observed that ChCl–urea (1:2) mixture
was the most preferred DESs to extract R-phycoerythrin from red algae. A
ternary mixture of ChCl–urea–K2HPO4 was the suitable ATPS
to purify R-phycoerythrin with a separation effciency of 92.60% (Xu, Wang, and Hou, 2020).
5. Limitations of DESs and
NADESs as Proteins Extraction and Purification Media
Even though many chemicals comply with
the functions of HBD and HBA to form DESs, some of solvents are not appropriate
for protein extraction from natural sources. Therefore, a careful selection
should be carried out to determine the appropriate DESs, specifically those related
to recovery and isolation from the phase (Smith, Abbott, and Ryder, 2014). DESs viscosities and densities decline with
the increase in temperature but the ionic conductivity rises (Lores et al.,
2017). These opposite features can be the critical
problems of viability and adaptability potentially affecting the utilizations
by the industry.
Efficient recovery and isolation are essential
for protein separation from DESs to enable recycling. Xu et al. (2015) observed that recovery
from this solvent was slow since the process occurred under a mass transfer
regime due to high interfacial resistance. Even though salt concentration is modified by mixing DESs with a
freshly ethanolic saline solution mixture, only 32.9% of protein is recovered. Therefore,
more advanced improvements should be addressed to the existing protein back
extraction and DESs recovery techniques to attract more interest from
enterprises (Li et al.,
2016).
6. Safety and
Environmental Aspects Associated with The Utilization of DESs and NADESs
NADESs
are less toxic than petroleum-based solvents, and the adverse effects on human
health remain unclear but solvents are regarded as safe (GRAS).
A previous in vivo research on mice and Wistar rats confirmed that NADESs reported
higher toxicity than DESs, which was rooted in high viscosity values. At high concentrations, solvent becomes very viscous
limiting the circulation and inducing acute effects, liver failure, and
mortality (Benlebna et al., 2018). Ecotoxicity research of DESs reported
disparate sensitivities to the tested ecosystem models, which mainly varied
with the composition of DESs. The results showed that DESs and NADESs toxicity was
affected by some parameters, namely viscosity, HBD/HBA molar ratio, organic
acids content, pH, type of cells or organisms, and synergistic effects.
Previous safety analysis is required before using DESs and NADESs for food and medicinal
applications. Since the characteristics largely depend on the
combinations of the components, a compendious database of toxicological properties of DESs should be set up which supports
the numerous component variations of the mixtures with computational predictive
approximations. In addition, most NADESs
combinations and applications are patented limiting the industrial applications.
7. New Methods for
Solid-Phase Extraction Using DESs and NADESs
The
direct utilization of DESs and NADESs using traditional extraction methods can collaborate
with ultrasound-assisted (UAE) and microwave-assisted extraction (MAE) to
achieve higher protein yield and purity. The application can be expected to
extensively break cell wall structure and ease the release of intracellular
protein from plant and animal matrices (Chemat et al., 2019). Correspondingly, DESs can also be developed
in MAE for the recovery of proteins and show greater performances than
conventional techniques (Bubalo et al., 2016). This pilot strategy possesses various
advantages mainly related to higher efficiency, shorter operation time, and
lower solvent requirement than conventional extraction methods using
petroleum-based solvents.
To enhance extraction performance, magnetic adsorbents can be incorporated into ATPS, which function to adsorb proteins for pure protein recovery (Liu et al., 2012). In magnetic solid-phase extraction (MSPE), nanoparticles are dispersed into extraction medium to adsorb proteins. The analytes are immediately segregated from the magnetic adsorbents with the aid of an external magnetic eld enabling nearly complete recovery of protein molecules and recycling of nano adsorbing particles (Table 3) (Wen et al., 2016). This innovative method eliminates some lengthy and laborious stages, namely centrifugation and filtration, that result in a shorter operating time, and exceptional protein recovery with preserved functional properties and offers an important function in purification procedures (Huang et al., 2015).
Table 3 Application of magnetic
particle-modified DESs for protein purification
|
Target Protein |
Magnetic particle |
DESs/ NADESs |
Extraction capacity (mg.g-1) |
References |
|
chymotrypsin |
Fe3O4@TiO2 |
[ChCl][Xyl](1: 1) |
347.8 |
(Li et al., 2021) |
|
R-phycoerythrin |
MB-NH2@CD |
[BeCh][Tri](1:
2) |
549.87 |
(Xu, Wang, and Hou, 2020) |
|
BSA |
Fe3O4-NH2@GO |
[ChCl] [glycerol] (1: 1) |
44.59 |
(Xu et al., 2015) |
|
BSA |
M-CNT@ |
N-[APTMAC][Xyl](1: 1) |
225.15 |
(Ni et al., 2020) |
|
BSA |
Fe@GO @Amino functional dicationic
ionic liquid |
|
89.7 |
(Wen et al., 2016) |
|
BSA |
Fe@GO |
|
6.7 |
In conclusion, DESs and NADESs were reported to
show higher extraction efficiency, improved bioactivity, better recyclability,
reusability and biodegradability potential, as well as less toxicity than conventional
organic solvents. The collaboration of solvents with green chemistry-based
industrial processes to reclaim valuable substances from plant and animal
sources reported more favourable results compared to the available conventional
solvent extraction processes. In addition, some important process parameters,
namely solid and solvent ratio, temperature, mixing, pH, and duration,
significantly affected the effectiveness of DESs during extraction processes.
From a scale-up and industrial application perspective, the use of DESs
required further research concerning thermal stability, analyte purification,
solvent recovery, operational cost, toxicity, and environmental impacts.
The authors acknowledge Universitas Diponegoro for its financial
assistance under the third-year term of World Class Research Universitas
Diponegoro (Kategori A) with contract No.: 118-17/UN7.6.1/PP/2021.
Abbott,
A.P., Boothby, D., Capper, G., Davies, D.L., Rasheed, R.K., 2004. Deep Eutectic
Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile
Alternatives to Ionic Liquids. Journal of the American Chemical Society,
Volume 126(29), pp. 9142–9147
Abbott,
A.P., Capper, G., Davies, D.L., Rasheed, R.K., Tambyrajah, V., 2003. Novel
Solvent Properties of Choline Chloride/Urea Mixtures. Chemical
Communications, Volume 1, pp. 70–71
Abbott,
A.P., Capper, G., Gray, S., 2006. Design of Improved Deep Eutectic Solvents
Using Hole Theory. Chemphyschem: a European Journal of Chemical Physics and
Physical Chemistry, Volume 7(4), pp. 803–806
Albertsson,
P.-Å., 1970. Partition of Cell Particles and Macromolecules in Polymer
Two-Phase Systems. Advances in Protein Chemistry, Volume 24, pp. 309–341
Asenjo,
J.A., Andrews, B.A., 2011. Aqueous Two-Phase Systems for Protein Separation: A
Perspective. Journal of Chromatography A, Volume 1218(49), pp. 8826–8835
Benlebna,
M., Ruesgas-Ramón, M., Bonafos, B., Fouret, G., Casas, F., Coudray, C., Durand,
E., Cruz Figueroa-Espinoza, M., Feillet-Coudray, C., 2018. Toxicity of Natural
Deep Eutectic Solvent Betaine:Glycerol in Rats. Journal of Agricultural and
Food Chemistry, Volume 66(24), pp. 6205–6212
Bernau,
C.R., Knödler, M., Emonts, J., Jäpel, R.C., Buyel, J.F., 2022. The Use of
Predictive Models to Develop Chromatography-Based Purification Processes. Frontiers
in Bioengineering and Biotechnology, Volume 10, pp. 1–24
Bonacci,
S., Di Gioia, M.L., Costanzo, P., Maiuolo, L., Tallarico, S., Nardi, M., 2020.
Natural Deep Eutectic Solvent as Extraction Media for The Main Phenolic
Compounds from Olive Oil Processing Wastes. Antioxidants, Volume 9(6), p. 513
Bose,
U., Broadbent, J.A., Byrne, K., Hasan, S., Howitt, C.A., Colgrave, M.L., 2019.
Optimisation of Protein Extraction for In-Depth Profiling of The Cereal Grain
Proteome. Journal of Proteomics, Volume 197, pp. 23–33
Bowen,
H., Durrani, R., Delavault, A., Durand, E., Chenyu, J., Yiyang, L., Lili, S.,
Jian, S., Weiwei, H., Fei, G., 2022. Application of Deep Eutectic Solvents in
Protein Extraction and Purification. Frontiers in Chemistry, Volume 10,
pp. 1–10
Bubalo,
M.C., Curko, N., Tomaševic, M., Kovacevic-Ganic, K., Radojcic-Redovnikovic, I.,
2016. Green Extraction of Grape Skin Phenolics by Using Deep Eutectic Solvents.
Food Chemistry, Volume 200, pp. 159–166
Chemat, F., Abert-Vian, M., Ravi, H.K., Khadhraoui,
B., Hilali, S., Perino, S., Tixier, A.-S.F., 2019. Review of
Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and
Prospects. Molecules (Basel, Switzerland), Volume 24(16), p. 3007
Chen,
J., Jiang, X., Yang, G., Bi, Y., Liu, W., 2018. Green and Efficient Extraction
of Resveratrol from Peanut Roots Using Deep Eutectic Solvents. Journal of
Chemistry, Volume 2018(1), p. 4091930
Chen,
Q., Chaihu, L., Yao, X., Cao, X., Bi, W., Lin, J., Chen, D.D.Y., 2021.
Molecular Property-Tailored Soy Protein Extraction Process Using a Deep
Eutectic Solvent. ACS Sustainable Chemistry & Engineering, Volume 9(30),
pp. 10083–10092
Chen,
R., Wang, X.-J., Zhang, Y.-Y., Xing, Y., Yang, L., Ni, H., Li, H.-H., 2019a.
Simultaneous Extraction and Separation of Oil, Proteins, And Glucosinolates
from Moringa Oleifera Seeds. Food Chemistry, Volume 300, p. 125162
Chen,
Y., Chen, W., Fu, L., Yang, Y., Wang, Y., Hu, X., Wang, F., Mu, T., 2019b.
Surface Tension of 50 Deep Eutectic Solvents: Effect of Hydrogen-Bonding
Donors, Hydrogen-Bonding Acceptors, Other Solvents, and Temperature. Industrial
& Engineering Chemistry Research, Volume 58(28), pp. 12741–12750
Choi,
Y. H., van Spronsen, J., Dai, Y., Verberne, M., Hollmann, F., Arends, I.W.C.E.,
Witkamp, G.J., Verpoorte, R., 2011. Are Natural Deep Eutectic Solvents the
Missing Link in Understanding Cellular Metabolism and Physiology? Plant
Physiology, Volume 156(4), pp. 1701–1705
Contreras,
M. del M., Lama-Muñoz, A., Manuel Gutiérrez-Pérez, J., Espínola, F., Moya, M., Castro,
E., 2019. Protein Extraction from Agri-Food Residues for Integration in
Biorefinery: Potential Techniques and Current Status. Bioresource
Technology, Volume 280, pp. 459–477
Dai,
Y., van Spronsen, J., Witkamp, G.-J., Verpoorte, R., Choi, Y.H., 2013a. Natural
Deep Eutectic Solvents as New Potential Media for Green Technology. Analytica
Chimica Acta, Volume 766, pp. 61–68
Dai,
Y., Witkamp, G.-J., Verpoorte, R., Choi, Y.H., 2013b. Natural Deep Eutectic
Solvents as a New Extraction Media for Phenolic Metabolites in Carthamus
Tinctorius L. Analytical Chemistry, Volume 85(13), pp. 6272–6278
Dai,
Y., Witkamp, G.-J., Verpoorte, R., Choi, Y.H., 2015. Tailoring Properties of
Natural Deep Eutectic Solvents with Water to Facilitate Their Applications. Food
Chemistry, Volume 187, pp. 14–19
de
Faria, E.L.P., do Carmo, R.S., Cláudio, A.F.M., Freire, C.S.R., Freire, M.G.,
Silvestre, A.J.D., 2017. Deep Eutectic Solvents as Efficient Media for the
Extraction and Recovery of Cynaropicrin
from Cynara Cardunculus L. Leaves. International Journal of Molecular
Sciences, Volume 18(11), p. 2276
De
Jesus, S.S., Filho, R.M., 2020. Recent Advances in Lipid Extraction Using Green
Solvents. Renewable and Sustainable Energy Reviews, Volume 133, p.
110289
Du,
M., Hou, Z., Liu, L., Xuan, Y., Chen, X., Fan, L., Li, Z., Xu, B., 2022.
Progress, Applications, Challenges and Prospects Of Protein Purification
Technology. Frontiers in Bioengineering and Biotechnology, Volume 10,
pp. 1–26
El-Kantar,
S., Rajha, H. N., Boussetta, N., Vorobiev, E., Maroun, R. G., Louka, N., 2019.
Green Extraction of Polyphenols from Grapefruit Peels Using High Voltage
Electrical Discharges, Deep Eutectic Solvents and Aqueous Glycerol. Food
Chemistry, Volume 295, pp. 165–171
Fuad,
F.M., Nadzir, M.M., Harun, A., 2021. Hydrophilic Natural Deep Eutectic Solvent:
A Review on Physicochemical Properties and Extractability of Bioactive
Compounds. Journal of Molecular Liquids, Volume 339, p. 116923
Gai,
Q., Qu, F., Zhang, T., Zhang, Y., 2011. Integration of Carboxyl Modified
Magnetic Particles and Aqueous Two-Phase Extraction for Selective Separation of
Proteins. Talanta, Volume 85(1), pp. 304–309
García, G., Aparicio, S., Ullah, R., Atilhan, M.,
2015. Deep Eutectic Solvents: Physicochemical Properties and
Gas Separation Applications. Energy & Fuels, Volume 29(4), pp.
2616–2644
Grudniewska,
A., de Melo, E.M., Chan, A., Gnilka, R., Boratynski, F., Matharu, A.S., 2018.
Enhanced Protein Extraction from Oilseed Cakes Using Glycerol–Choline Chloride
Deep Eutectic Solvents: A Biorefinery Approach. ACS Sustainable Chemistry
& Engineering, Volume 6(11), pp. 15791–15800
Gullón,
P., Gullón, B., Romaní, A., Rocchetti, G., Lorenzo, J.M., 2020. Smart Advanced
Solvents for Bioactive Compounds Recovery from Agri-Food By-Products: A Review.
Trends in Food Science & Technology, Volume 101, pp. 182–197
Hatti-Kaul,
R., 2000. Methods and Protocols: Methods and Protocols. Humana Press, pp. 1–10
Hernández-Corroto,
E., Plaza, M., Marina, M.L., García, M.C., 2020. Sustainable Extraction of
Proteins and Bioactive Substances from Pomegranate Peel (Punica Granatum L.)
Using Pressurized Liquids and Deep Eutectic Solvents. Innovative Food
Science & Emerging Technologies, Volume 60, p. 102314
Huang,
Y., Feng, F., Jiang, J., Qiao, Y., Wu, T., Voglmeir, J., Chen, Z.-G., 2017.
Green and Efficient Extraction of Rutin from Tartary Buckwheat Hull by Using
Natural Deep Eutectic Solvents. Food Chemistry, Volume 221, pp.
1400–1405
Huang,
Y., Wang, Y., Pan, Q., Wang, Y., Ding, X., Xu, K., Li, N., Wen, Q., 2015.
Magnetic Graphene Oxide Modified with Choline Chloride-Based Deep Eutectic
Solvent for The Solid-Phase Extraction of Protein. Analytica Chimica Acta,
Volume 877, pp. 90–99
Ijardar,
S.P., Singh, V., Gardas, R.L., 2022. Revisiting the Physicochemical Properties
and Applications of Deep Eutectic
Solvents. Molecules, Volume 27(4), p. 1368
Kudlak,
B., Owczarek, K., Namiesnik, J., 2015. Selected Issues Related to The Toxicity
of Ionic Liquids and Deep Eutectic Solvents--A Review. Environmental Science
and Pollution Research International, Volume 22(16), pp. 11975–11992
Kumar,
M., Potkule, J., Patil, S., Saxena, S., Patil, P.G., Mageshwaran, V., Punia,
S., Varghese, E., Mahapatra, A., Ashtaputre, N., Souza, C.D., Kennedy, J.F., 2021a.
Extraction of Ultra-Low Gossypol Protein from Cottonseed: Characterization
Based on Antioxidant Activity, Structural Morphology and Functional Group
Analysis. LWT, Volume 140, p. 110692
Kumar,
M., Tomar, M., Potkule, J., Verma, R., Punia, S., Mahapatra, A., Belwal, T.,
Dahuja, A., Joshi, S., Berwal, M.K., Satankar, V., Bhoite, A.G., Amarowicz, R.,
Kaur, C., Kennedy, J.F., 2021b. Advances in The Plant Protein Extraction:
Mechanism and Recommendations. Food Hydrocolloids, Volume 115, p. 106595
Kumoro, A.C., Hasan, M., Singh, H., 2019. Extraction
of Andrographolide from Andrographis paniculata Dried Leaves Using
Supercritical CO2 and Ethanol Mixture. Industrial & Engineering
Chemistry Research, Volume 58(2), pp. 742–751
Kumoro,
A.C., Wardhani, D.H., Kusworo, T.D., Djaeni, M., Ping, T.C., Azis, Y.M.R.F.,
2022. Fish Protein Concentrate for Human Consumption: A Review of Its
Preparation By Solvent Extraction Methods And Potential For Food Applications. Annals
of Agricultural Sciences, Volume 67(1), pp. 42–59
Landa-Castro,
M., Sebastián, P., Giannotti, M.I., Serrà, A., Gómez, E., 2020.
Electrodeposition of Nanostructured Cobalt Films From a Deep Eutectic Solvent:
Influence Of The Substrate And Deposition Potential Range. Electrochimica
Acta, Volume 359, p. 136928
Li,
H., Wang, Y., He, X., Chen, J., Xu, F., Liu, Z., Zhou, Y., 2021. A Green Deep
Eutectic Solvent Modified Magnetic Titanium Dioxide Nanoparticles for The
Solid-Phase Extraction of Chymotrypsin. Talanta, Volume 230, p. 122341
Li,
N., Wang, Y., Xu, K., Huang, Y., Wen, Q., Ding, X., 2016. Development of Green
Betaine-Based Deep Eutectic Solvent Aqueous Two-Phase System for The Extraction
of Protein. Talanta, Volume 152, pp. 23–32
Lin,
Z., Jiao, G., Zhang, J., Celli, G.B., Brooks, M.S.-L., 2021. Optimization of
Protein Extraction From Bamboo Shoots and Processing Wastes Using Deep Eutectic
Solvents in A Biorefinery Approach. Biomass Conversion and Biorefinery,
Volume 11(6), pp. 2763–2774
Ling,
J.K., Hadinoto, K., 2022. Deep Eutectic Solvent as Green Solvent in Extraction
of Biological Macromolecules: A Review. International Journal of Molecular
Sciences, Volume 23(6). p. 3381
Ling,
J.K.U., Chan, Y.S., Nandong, J., Chin, S.F., Ho, B.K., 2020. Formulation of
Choline Chloride/Ascorbic Acid Natural Deep Eutectic Solvent: Characterization,
Solubilization Capacity and Antioxidant Property. LWT, Volume 133, p.
110096
Liu,
Q., Shi, J., Cheng, M., Li, G., Cao, D., Jiang, G., 2012. Preparation of
Graphene-Encapsulated Magnetic Microspheres for Protein/Peptide Enrichment and
MALDI-TOF MS analysis. Chemical Communications, Volume 48(13), pp.
1874–1876
Liu,
R.-L., Yu, P., Ge, X.-L., Bai, X.-F., Li, X.-Q., Fu, Q., 2017. Establishment of
an Aqueous PEG 200-Based Deep Eutectic Solvent Extraction and Enrichment Method
for Pumpkin (Cucurbita moschata) Seed Protein. Food Analytical Methods,
Volume 10(6), pp. 1669–1680
Lores,
H., Romero, V., Costas, I., Bendicho, C., Lavilla, I., 2017. Natural Deep
Eutectic Solvents in Combination with Ultrasonic Energy As A Green Approach for
Solubilisation of Proteins: Application To Gluten Determination By Immunoassay.
Talanta, Volume 162, pp. 453–459
Malva,
A. Della, Albenzio, M., Santillo, A., Russo, D., Figliola, L., Caroprese, M.,
Marino, R., Díaz-Cruz, J.M., 2018. Methods for Extraction of Muscle Proteins
from Meat and Fish Using Denaturing and Nondenaturing Solutions. Journal of
Food Quality, Volume 2018, p. 8478471
Mendes,
M.S.M., Rosa, M.E., Ramalho, F., Freire, M.G., e Silva, F.A., 2023. Aqueous
Two-Phase Systems as Multipurpose Tools to Improve Biomarker Analysis. Separation
and Purification Technology, Volume 317, p. 123875
Meng,
J., Wang, Y., Zhou, Y., Chen, J., Wei, X., Ni, R., Liu, Z., Xu, F., 2019.
Development of Different Deep Eutectic Solvent Aqueous Biphasic Systems for The
Separation Of Proteins. RSC Advances, Volume 9(25), pp. 14116–14125
Mišan,
A., Nadpal, J., Stupar, A., Pojic, M., Mandic, A., Verpoorte, R., Choi, Y.H.,
2020. The Perspectives of Natural Deep Eutectic Solvents in Agri-Food Sector.
Critical Reviews in Food Science and Nutrition, Volume 60(15), pp.
2564–2592
Morais,
E.S., Lopes, A.M.D.C., Freire, M.G., Freire, C.S., Coutinho, J.A., Silvestre,
A.J., 2020. Use of Ionic Liquids and Deep Eutectic Solvents In Polysaccharides
Dissolution And Extraction Processes Towards Sustainable Biomass Valorization. Molecules,
Volume 25(16), p. 3652
Morgenstern,
J., Baumann, P., Brunner, C., Hubbuch, J., 2017. Effect of PEG Molecular Weight
and PEGylation Degree on the Physical Stability of PEGylated Lysozyme. International
Journal of Pharmaceutics, Volume 519(1–2), pp. 408–417
Mulia, K., Adam, D., Zahrina, I., Krisanti, E.A.,
2018. Green Extraction of Palmitic Acid from Palm Oil using Betaine-based
Natural Deep Eutectic Solvents. International Journal of Technology.
Volume 9(2), pp. 335–-344
Mulia, K., Krisanti, E., Terahadi, F., Putri, S.,
2015. Selected Natural Deep Eutectic Solvents for the
Extraction of mangostin from Mangosteen (Garcinia mangostana L.) Pericarp. International
Journal of Technology, Volume 7(1), pp. 22–30
Ni,
R., Wang, Y., Wei, X., Chen, J., Meng, J., Xu, F., Liu, Z., Zhou, Y., 2020.
Magnetic Carbon Nanotube Modified with Polymeric Deep Eutectic Solvent for The
Solid Phase Extraction of Bovine Serum Albumin. Talanta, Volume 206, p. 120215
Okoro, O. V., Jafari, H., Hobbi, P., Nie, L., Alimoradi,
H., Shavandi, A., 2022. Enhanced keratin Extraction from
Wool Waste Using A Deep Eutectic Solvent. Chemical Papers, Volume 76(5),
pp. 2637–2648
Omar,
K.A., Sadeghi, R., 2022a. Novel Lacmoid-Based Deep Eutectic Solvent Dye as
Writing Ink. Chemical Engineering Research and Design, Volume 180, pp.
50–54
Omar,
K.A., Sadeghi, R., 2022b. Physicochemical Properties of Deep Eutectic Solvents:
A Review. Journal of Molecular Liquids, Volume 360, p. 119524
Panic,
M., Andlar, M., Tišma, M., Rezic, T., Šibalic, D., Cvjetko Bubalo, M., Radojcic Redovnikovic, I., 2021. Natural Deep Eutectic Solvent as A Unique Solvent for
Valorisation of Orange Peel Waste by The Integrated Biorefinery Approach. Waste
Management, Volume 120, pp. 340–350
Pratiwi,
F.A., Utami, T.S., Arbianti, R., 2020. Using Ultrasonic Assisted Extraction to
Produce a Bioinsecticide from Cigarette Butt Waste and Green Solvent to Control
Armyworm Infestation. International Journal of Technology, Volume 11(7),
pp. 1329–1336
Qiaoyun,
C., Xinghong, N., Liang, Z., Zheng, T., Jin, L., Kang, S., Xuan, C., Xinghui,
L., 2017. Optimization of Protein Extraction and Decoloration Conditions for
Tea Residues. Horticultural Plant Journal, Volume 3(4), pp. 172–176
Rodrigues,
L.A., Leonardo, I.C., Gaspar, F.B., Roseiro, L.C., Duarte, A.R.C., Matias,
A.A., Paiva, A., 2021. Unveiling the Potential Of Betaine/Polyol-Based Deep
Eutectic Systems For The Recovery of Bioactive Protein Derivative-Rich Extracts
From Sardine Processing Residues. Separation and Purification Technology,
Volume 276, p. 119267
Saini,
A., Kumar, A., Panesar, P.S., Thakur, A., 2022. Potential of Deep Eutectic
Solvents in The Extraction of Value-Added Compounds from Agro-Industrial
By-Products.
Applied Food Research, Volume 2(2), p. 100211
Saravanan, S., Rao, J.R., Nair, B.U., Ramasami, T., 2008.
Aqueous Two-Phase Poly(Ethylene Glycol)–Poly(Acrylic
Acid) System For Protein Partitioning: Influence of Molecular Weight, pH and
Temperature. Process Biochemistry, Volume 43(9), pp. 905–911
Sindhu,
R., Binod, P., Janu, K.U., Sukumaran, R.K., Pandey, A., 2012. Organosolvent
Pretreatment and Enzymatic Hydrolysis of Rice Straw for The Production of
Bioethanol. World Journal of Microbiology & Biotechnology, Volume
28(2), pp. 473–483
Singh,
S., Tavana, H., 2018. Collagen Partition in Polymeric Aqueous Two-Phase Systems
for Tissue Engineering. Frontiers in Chemistry, Volume 6, pp. 4–10
Smith,
E.L., Abbott, A.P., Ryder, K.S., 2014. Deep Eutectic Solvents (DESs) and Their Applications.
Chemical Reviews, Volume 114(21), pp. 11060–11082
Socas-Rodríguez,
B., Torres-Cornejo, M.V., Álvarez-Rivera, G., Mendiola, J.A., 2021. Deep
Eutectic Solvents for The Extraction of Bioactive Compounds from Natural
Sources and Agricultural By-Products. Applied Sciences, Volume 11(11),
p. 4897
Tolmachev,
D., Lukasheva, N., Ramazanov, R., Nazarychev, V., Borzdun, N., Volgin, I.,
Andreeva, M., Glova, A., Melnikova, S., Dobrovskiy, A., Silber, S. A., Larin,
S., de Souza, R. M., Ribeiro, M. C., Lyulin, S., Karttunen, M., 2022. Computer
Simulations of Deep Eutectic Solvents: Challenges, Solutions, and Perspectives.
International Journal of Molecular Sciences, Volume 23(2), p. 645
Wang,
W., Liu, X., Lu, X., 2013. Engineering Cyanobacteria to Improve Photosynthetic
Production of Alka(E)Nes. Biotechnology for Biofuels, Volume 6(1), p. 69
Wen,
Q., Wang, Y., Xu, K., Li, N., Zhang, H., Yang, Q., Zhou, Y., 2016. Magnetic
Solid-Phase Extraction of Protein by Ionic Liquid-Coated Fe@Graphene Oxide. Talanta,
Volume 160, pp. 481–488
Xu,
K., Wang, Y., Huang, Y., Li, N., Wen, Q., 2015. A Green Deep Eutectic
Solvent-Based Aqueous Two-Phase System for Protein Extracting. Analytica
Chimica Acta, Volume 864, pp. 9–20
Xu,
M., Ran, L., Chen, N., Fan, X., Ren, D., Yi, L., 2019a. Polarity-Dependent
Extraction of Flavonoids from Citrus Peel Waste Using A Tailor-Made Deep
Eutectic Solvent. Food Chemistry, Volume 297, p. 124970
Xu,
P., Wang, Y., Chen, J., Wei, X., Xu, W., Ni, R., Meng, J., Zhou, Y., 2019b.
Development of Deep Eutectic Solvent-Based Aqueous Biphasic System for The
Extraction of Lysozyme. Talanta, Volume 202, pp. 1–10
Xu,
P., Zheng, G.-W., Du, P.-X., Zong, M.-H., Lou, W.-Y., 2016. Whole-Cell
Biocatalytic Processes with Ionic Liquids. ACS Sustainable Chemistry &
Engineering, Volume 4(2), pp. 371–386
Xu,
Y., Wang, Q., Hou, Y., 2020. Efficient Purification of R-phycoerythrin from
Marine Algae (Porphyra yezoensis) Based
on a Deep Eutectic Solvents Aqueous Two-Phase System. Marine Drugs,
Volume 18(12), p. 618
Yue,
J., Zhu, Z., Yi, J., Lan, Y., Chen, B., Rao, J., 2021. Structure and
Functionality of Oat Protein Extracted by Choline Chloride-Dihydric Alcohol
Deep Eutectic Solvent and Its Water Binary Mixtures. Food Hydrocolloids,
Volume 112, p. 106330
Zannou,
O., Koca, I., 2022. Greener Extraction of Anthocyanins and Antioxidant Activity
from Blackberry (Rubus spp) Using Natural Deep Eutectic Solvents. LWT, Volume
158, p. 113184
Zeng,
C.-X., Xin, R.-P., Qi, S.-J., Yang, B., Wang, Y.-H., 2016. Aqueous Two-Phase
System Based on Natural Quaternary Ammonium Compounds for The Extraction of
Proteins. Journal of Separation Science, Volume 39(4), pp. 648–654
Zhang,
Q., De Oliveira Vigier, K., Royer, S., Jérôme, F., 2012. Deep Eutectic
Solvents: Syntheses, Properties and Applications. Chemical Society Reviews,
Volume 41(21), pp. 7108–7146
Zhou,
M., Fakayode, O.A., Li, H., 2023. Green Extraction of Polyphenols via Deep
Eutectic Solvents and Assisted Technologies from Agri-Food By-Products. Molecules,
Volume 28(19), p. 6852
