Enhancing Viability of Probiotic by Microencapsulation: A Case Study in Ice Cream
Corresponding email: shofi1988@itb.ac.id
Published at : 17 Jul 2025
Volume : IJtech
Vol 16, No 4 (2025)
DOI : https://doi.org/10.14716/ijtech.v16i4.6886
Shofinita, D, Harimawan, A, Almaishya, N, Dewi, AM, Thamleonard, J & Achmadi AB 2025, ‘Enhancing viability of probiotic by microencapsulation: a case study in ice cream’, International Journal of Technology, vol. 16, no. 4, pp. 1337-1347
| Dian Shofinita | 1. Department of Food Engineering, Institut Teknologi Bandung, Bandung, Indonesia Jl. Let. Jen. Purn. Dr. (HC). Mashudi No. 1/ Jalan Raya Jatinangor KM 20,75, Sumedang 45363, Indonesia. 2. Department |
| Ardiyan Harimawan | 1. Department of Food Engineering, Institut Teknologi Bandung, Bandung, Indonesia Jl. Let. Jen. Purn. Dr. (HC). Mashudi No. 1/ Jalan Raya Jatinangor KM 20,75, Sumedang 45363, Indonesia. 2. Department |
| Nicolaus Almaishya | Department of Food Engineering, Institut Teknologi Bandung, Bandung, Indonesia Jl. Let. Jen. Purn. Dr. (HC). Mashudi No. 1/ Jalan Raya Jatinangor KM 20,75, Sumedang 45363, Indonesia |
| Aurelia Maghfira Dewi | Department of Food Engineering, Institut Teknologi Bandung, Bandung, Indonesia Jl. Let. Jen. Purn. Dr. (HC). Mashudi No. 1/ Jalan Raya Jatinangor KM 20,75, Sumedang 45363, Indonesia |
| Jason Thamleonard | Department of Food Engineering, Institut Teknologi Bandung, Bandung, Indonesia Jl. Let. Jen. Purn. Dr. (HC). Mashudi No. 1/ Jalan Raya Jatinangor KM 20,75, Sumedang 45363, Indonesia |
| Amarthya Benigna Achmadi | Department of Chemical Engineering, Institut Teknologi Bandung, Bandung, Indonesia Jl. Ganesa No. 10, Bandung 40132, Indonesia |
Public awareness of digestive disorders is increasing the interest in consuming nutritious foods with probiotic. To ensure the effectiveness of probiotic bacteria, maintaining resistance during processing and storage is essential. In this context, microencapsulation by spray drying protects probiotic cells and maintains viability. Therefore, this study aimed to investigate the impact of spray dryer temperature and coating material on microencapsulated probiotic powder production. Viability of microencapsulated probiotic cells when added to ice cream was also evaluated. Spray dryer temperature was varied at 130oC, 140oC, and 150oC while coating agent ratios (maltodextrin (MD): whey protein concentrate (WPC)) included 5:1 and 5:3. The results showed that the temperature and coating composition did not affect yield but influenced powder hygroscopicity and glass temperature, respectively. Probiotic microcapsule powder yield ranged from 16.77±0.32% to 21.65±3.34%. Furthermore, encapsulated probiotic cells maintained high viability during 14-day storage in ice cream at a value of 80.56±0.80%, compared to 44.63±0.21% for non-encapsulated cells.
maltodextrin; microencapsulation; probiotic; spray drying; whey protein concentrate
Unhealthy eating habits are the leading cause of various health problems, particularly digestive issues in the modern era. The rise in digestive system diseases can be attributed to the shift in modern lifestyles, which often include the consumption of instant, high-fat, or low-protein foods (Heidarzadeh-Esfahani et al., 2021). These dietary choices have detrimental effects on the delicate balance of microflora in the small intestines (Aritonang et al., 2019). An effective method to restore disturbed microflora is by incorporating probiotic into diets, which actively suppress the growth of harmful bacteria in the digestive system (Yadav et al., 2022). By consuming food and drinks rich in probiotic, the balance of digestive microbiome can be restored (Cunningham et al., 2021), leading to improved digestive health, enhanced immune function, gut maturation, enhanced energy metabolism, and nutrition uptake (Rusch et al., 2023). In this context, Lactobacillus casei has been proven to help prevent diarrhea and constipation, provide relief from the symptoms of irritable bowel syndrome, gingivitis, as well as assist in anti-inflammatory response (Maftei et al., 2024). For optimal benefits, probiotic must be consumed in a viable state since viability is proportional to effectiveness. Therefore, preserving viability of probiotic bacteria is crucial during processing and storage. To achieve this, a preservation method, such as encapsulation, is required (Rajam and Subramanian, 2022).
Microencapsulation entails trapping probiotic cells in a protective capsule made of matrix material. This encapsulation process enhances viability and prolongs the shelf life of probiotic bacteria (Paula et al., 2019). The encapsulated probiotic is stored inside the coating walls and can be utilized for a specific duration, ensuring viability when needed. Furthermore, coating materials with low viscosity at high solid content are desired due to the ability to yield higher-concentration powders (Siccama et al., 2021). Several coating materials have been reported, including maltodextrin (MD), which is preferred due to the ability to dissolve in cold water (Sahlan et al., 2019), and whey protein concentrate (WPC) (de Araújo Etchepare, 2020), a byproduct of cheese production (Dinika et al., 2019).
Considering nutritious foods often have unfavorable taste, it is necessary to innovate and develop healthy options that contain probiotic. An example of innovation is probiotic ice cream, a frozen treat made from cream, sugar, and fat with various flavors (Gozan et al., 2020). By using microencapsulation, viability of probiotic in ice cream can be preserved in ice cream-making process, storage, and consumption. Previous efforts in probiotic ice cream formulation have been carried out by Afzaal et al. (2019) using sodium alginate and carrageenan, but no study has specifically used WPC, MD, or spray drying as the method of encapsulation. Spray drying method is used to process food materials because of the fast, flexible, and straightforward nature (Shofinita et al., 2023a). Therefore, this study aimed to formulate microencapsulated probiotic powder that could be used as an additive in the production of probiotic ice cream.
2.1. Materials
Probiotic culture was obtained from the fermentation drink “Yakult” (PT Yakult, Indonesia). Materials used in this study include MD of dextrose equivalent (DE) value 11 (Lihua Starch, China) and WPC (Davisco, USA).
2.2. Preparation of Probiotic Cultures
Lactobacillus casei was inoculated from a fermentation drink, while MRS broth and nutrient agar were used as media for inoculation. Probiotic inoculation process started by growing the bacteria in a petri dish and then transferring to an agar slant for the next step. The presence of probiotic was checked with the bacteria staining test method using crystal violet, which gave L. casei a deep blue color. Finally, probiotic was transferred into MRS broth before being mixed with the feed solution.
2.3. Preparation of Feed Solutions
Feed solutions were made by mixing coating material solutions with probiotic. The coating material solution was prepared by mixing MD, WPC, and water at varied ratios. The solution was heated at 100oC and stirred continuously until homogeneous. Subsequently, 270 mL of coating material solution was added to 30 mL MRS broth at room temperature. The number of probiotic cells was calculated using the counting chamber method and the data was used as the initial number of probiotic cells.
2.4. Microencapsulation by Spray Drying
The feed solution was fed into spray dryer (Procept 4M8-TriX Spray Dryer, Belgium) with varying inlet temperatures (130-150oC). Specifically, the feed and air flow rates used were 330 mL/hour and 0.3 m3/min, respectively, with a nozzle size of 1 mm. Microcapsule powder in the collecting vessel, as shown in Figure 1, was then weighed, and compared with the solid content in the liquid feed to determine spray drying yield. The yield was calculated using Equation 1.
Figure 1 Illustration of a probiotic microcapsule
2.5. Probiotic Cell Viability Analysis of Microcapsule Powder
Viability was analyzed according to Arepally and Goswami (2019), where the number of probiotic cells in microcapsule powder was calculated by dissolving 1 g of the powder into a 10 mL volumetric flask using distilled water. The number of probiotic cells was calculated using the counting chamber method. Cell viability was calculated using Equation 2.
2.6. Moisture Content Analysis of Microcapsule Powder
Moisture content was analyzed according to Wardani (2007). Several grams of each sample were dried in an oven for 1 hour at 105. After heating, the samples were desiccated for 30 minutes and weighed using an analytical balance. Moisture content was calculated using Equation 3.
2.7. Hygroscopicity Analysis of Microcapsule Powder
Hygroscopicity was analyzed by desiccating 1 g of powder sample at 25oC with saturated NaCl solution. Subsequently, weighing was carried out until equilibrium was reached. Hygroscopicity (%) was expressed in 1 g of adsorbed moisture per 100 g of dry solid (g/100 g) using Equation 4.
Where m is the increase in mass after equilibrium (g), M is moisture content (%), and Mi is the initial moisture content (g).
2.8. Color Analysis of Microcapsule Powder
L*, a*, and b* color parameters were measured with CIE system using CHN Spec Colorimeter. Total color difference was calculated by comparing spray-dried and commercial probiotic powder using Equation 5.
Where E is total color difference, L* is brightness value, a* is red/green color value, and b* is blue/yellow color value.
2.9. Powder Morphology
Morphology analysis was conducted using a scanning electron microscope (JEOL JSM-6510A, Japan). Probiotic powder was sputtered with gold and analyzed at magnifications of 400x, 1000x, 2000x, dan 5000x.
2.10. Production of Probiotic Ice Cream and Probiotic Cell Viability Analysis
Instant vanilla-flavored ice cream dough was added with the best variety of encapsulated probiotic powder. Ice cream powder was weighed and mixed with 150 mL of cold water. Subsequently, the dough was shaken for 5-10 minutes at high speed and probiotic powder was added before freezing. Probiotic ice cream without encapsulation was also made with the addition of bacteria by mixing the dough with liquid bacteria culture to compare cell viability. All handling and processes followed food safety standards and were conducted in a sterile, non-contaminated food engineering laboratory in accordance with FSSC 22000 standards. Storage was carried out for 14 days, and then the number of cells in probiotic ice cream with and without encapsulation was counted to determine viability (Arepally and Goswami, 2019). The schematic diagram in Figure 2 shows the whole probiotic ice cream production process.
Figure 2 Schematic diagram of probiotic ice cream production
2.11. Statistical Analysis
Data study were obtained from two replicates for each experiment and presented as mean ± standard deviation. Differences were tested for significance by ANOVA (p-value 0.05).
3.1. Effect of Coating Materials on the Number of Probiotic Cells in Feed Solution
The effect of different coating material concentrations on the number of cells in the feed solution was compared and shown in Figure 3. Based on the results, there was no significant difference in probiotic concentration observed between the use of 5:1 MD:WPC ratio versus 5:3 (p-value > 0.05). MD forms a viscous glass, which can protect probiotic from damage. It also enhances stability by trapping probiotic in an amorphous microstructure. Moreover, MD serves as a coating material containing nutrients that modify the intestinal microflora and aid in the growth of probiotic (Yadav et al., 2022). When combined with MD, WPC acts as a film-forming emulsifier and increases the mechanical stability of the capsule. It can be concluded that a higher concentration of MD in the coating material solution leads to a greater number of probiotic being trapped in MD microstructure. Probiotic cell counts in the feed solution, with variations in MD to WPC ratio of 5:1 and 5:3, were measured to be 7.562±0.05 and 7.53±0.03 log cells/mL, respectively. These results indicated that the initial cell count exceeded 7 log cfu/mL cells, meeting the standards for probiotic products.
Figure 3 The amount of probiotic in the sample solution (log cell/mL) before spray drying with variations in the ratio of the coating material MD:WPC
3.2. Effect of Operating Conditions on the Yield of Probiotic Microcapsule Powder
The yield of powder presented in Figure 4 ranged from 16.77±0.32% - 21.65±3.34%. The observation showed that no blocking phenomenon was observed in spray dryer nozzle, suggesting the powder particles did not stick together. Langrish and Premarajah (2013) showed that elevated temperature in the spray dryer inlet may increase yield. However, yield can also be affected by glass temperature (Tg) and moisture content. The use of MD and WPC coating materials influenced Tg of the feed solution mixture. The elevated temperature of the inlet increased Tg of the coating material, accelerating water evaporation and reducing moisture content, thereby improving powder production.
Figure 4 Yield (%) results from spray drying varied by spray dryer inlet temperature and the ratio of coating materials MD:WPC
The addition of probiotic in the feed solution might also increase powder solubility, thereby enhancing powder yield during spray drying (Sundararajan et al., 2023). Furthermore, increasing WPC concentration in the feed solution improved powder yield due to higher solids content. The addition of WPC in the feed solution also influenced the ability of the particles to stick together due to the formation of protein networks (Chen et al., 2020).
3.3. Effect of Operating Conditions on the Color of Probiotic Microcapsule Powder
The L* index of all variations presented in Figure 5a ranged from 93.48±1.19 to 96.49±0.49. Generally, the L* index is a color parameter where L*=0 indicates dark and L*=100 represents white. A higher L* value indicates a whiter color (Kang, 2011), which is desired in the production of food additives as it does not tamper significantly with the original color of the product. Based on the data, all variations have an index in the range of 90-100, showing that the powder produced has a near-perfect white color.
Figure 5 L* Index results (a) and E Index results (b) from spray drying varied by spray dryer inlet temperature and the ratio of coating materials MD:WPC
Increasing spray dryer inlet temperature decreased the whiteness of the powder due to non-enzymatic Maillard browning caused by heating (Ghosal et al., 2023). In addition, the presence of a larger amount of MD coating material often leads to a higher L* index for the powder due to the significant composition of MD with white color. However, in this study, the difference in the amount of MD did not cause any significant color deviation (p-value > 0.05). The total color difference (E) was also calculated to determine the difference between spray-dried and commercial probiotic powder. As shown in Figure 5b,
E index for the sample with spray dryer inlet temperature of 130oC and MD:WPC ratio of 5:3 ranged from 1-2, indicating color similarity with the commercial powder.
3.4. Effect of Operating Conditions on Moisture Content of Probiotic Microcapsule Powder
Moisture content percentage of all variations presented in Figure 6a ranged from 3.60±0.48% to 5.26±0.80%, falling within the acceptable limits of 2-6% (Jin et al., 2019). Figure 6a shows that an increase in inlet temperature led to a decreased moisture content of the powder (p-value < 0.05) due to rapid evaporation of water. In addition, the presence of a larger amount of WPC coating material can decrease moisture content due to the ability to form films that prevent moisture from entering the powder. WPC also improves thermal stability and film strength, thereby extending shelf life and powder quality (Sun et al., 2020).
Figure 6 Moisture content (a) and hygroscopicity (b) results from spray drying varied by spray dryer inlet temperature and the ratio of coating materials MD:WPC
3.5. Effect of Operating Conditions on Hygroscopicity of Probiotic Microcapsule Powder
Hygroscopicity percentage of all variations presented in Figure 6b ranged from 6.20±0.73% to 9.82±0.40%. Increasing the spray dryer inlet temperature decreased hygroscopicity and lowered the moisture left in the powder. In addition, the presence of a larger amount of WPC coating material increased hygroscopicity due to the ability to form a thin film that protects the chemical and physical stability of the powder. WPC also has a high protein content, thereby increasing the affinity of water on the powder surface and hygroscopicity (Wang et al., 2019).
3.6. Scanning Electron Microscopy (SEM) of Probiotic Microcapsule Powder
The surface morphological structure of the powder was analyzed for the sample with a spray dryer inlet temperature of 140oC and WPC ratio of 5:3. The result of SEM is shown in Figure 7.
Figure 7 Surface morphological structure of probiotic microcapsule powders with 5000x magnification
Based on SEM imaging, the powder tends to be spherical in shape with smooth surfaces connected, forming a water bridge phenomenon which indicates the presence of water molecules between the particles (Teixeira et al., 2021). A water bridge predisposes the morphology of the powder to forces between adjacent particles, leading to agglomeration (Shofinita et al., 2021). In general, MD has hygroscopic properties causing the powder to agglomerate and stick when exposed to hot air, while WPC has adhesive properties that make particles stick together. The presence of protein in WPC can also form a gel or network on the particle surface, leading to agglomeration. As shown in Figure 8, there are no visible cracks on the surface of the powder due to spray drying process, resulting in a dry and structurally strong outer layer. Hydrolyzed starch such as MD maintain powder stability and avoid cracking by inducing faster skin formation and increasing glass temperature (Siccama et al., 2021), while WPC helps bind particles, making agglomeration more stable (Mishra et al., 2019).
3.7. Effect of Operating Conditions on Viability Cell of Probiotic Microcapsule Powder
The analysis of probiotic cell viability in the powder was carried out by comparing the number of viable cells before and after spray drying process. The results in Figure 8a show that probiotic cell viability ranged from 44.58±0.33 - 82.92±4.30 %. Increasing spray dryer inlet temperature led to decreased cell viability, while a higher ratio of WPC increased viability (p-value < 0.05).
Figure 8 Cell viability (a) results from spray drying varied by spray dryer inlet temperature and the ratio of coating materials MD:WPC; and the probiotic viability in ice cream (b)
The best protection for microencapsulation was achieved with a 5:3 ratio of MD:WPC. The formulation of probiotic microcapsule powder by complexing MD and WPC led to higher viability and emulsification ability of whey proteins (Meena et al., 2021). WPC, which contains milk fat, also contributed to the support of cell viability. A higher ratio of WPC in the coating material provided better protection for MD matrix and increased heat resistance. Viability of probiotic cells was affected by spray dryer inlet temperature, with higher levels leading to reduced viability due to thermal inactivation and dehydration. On the other hand, higher temperatures increased water evaporation and improved yield. Moisture content played a role in cell viability, with low moisture causing dehydration and high level promoting undesired microorganism growth. Spray dryer inlet temperature of 140oC produced the best viability and stable moisture content. Additionally, all variations exceeded the required 7 log cells/mL, indicating successful protection of probiotic during spray drying.
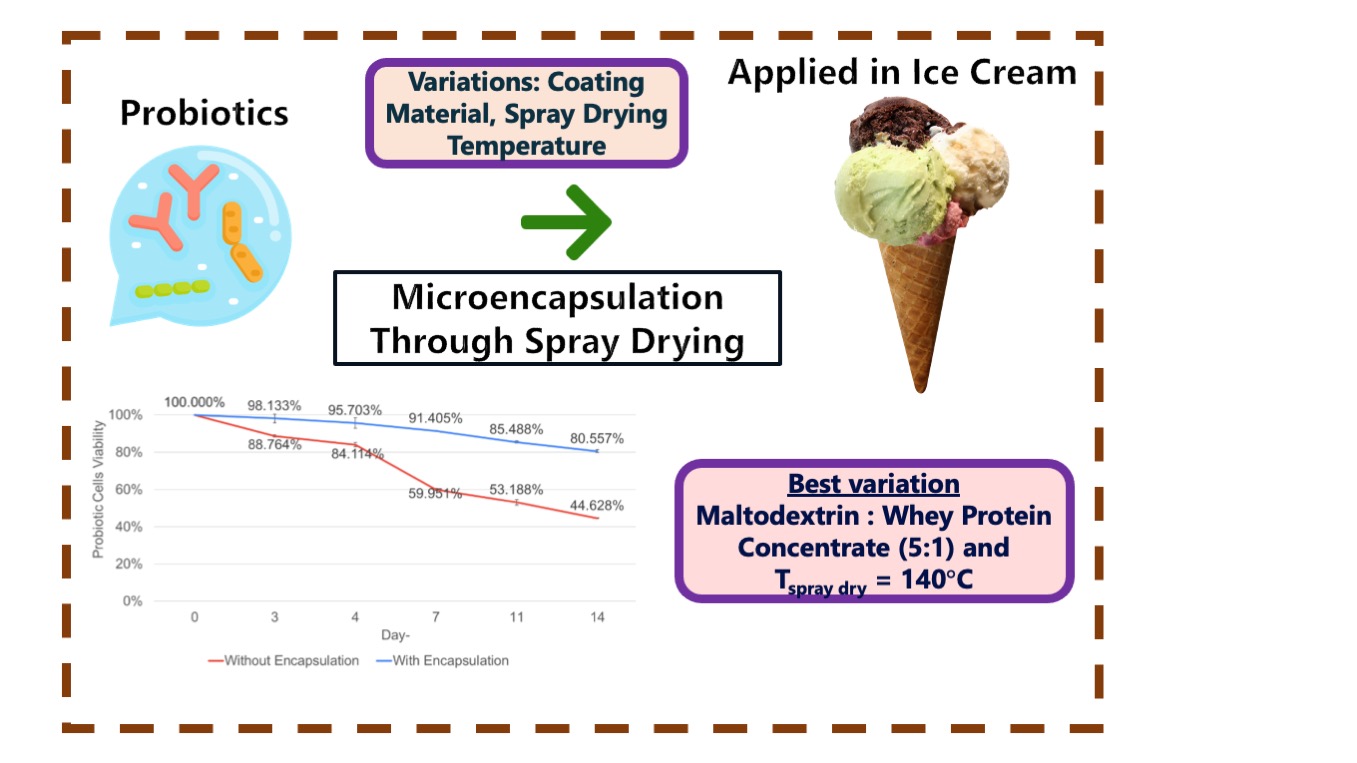
3.8. Effect of Probiotic Microcapsule Powder Coating on Cell Viability During Ice Cream Storage Period
Viability of probiotic cells was tested by incorporating spray-dried powder as an ice cream additive. The analysis focused on the best variety of the powder (MD:WPC=5:1 and 1400C) evaluated over 14 days, with testing conducted twice per week. For comparison, probiotic ice cream without encapsulation was also prepared. Viability of probiotic cells without encapsulation was 44.63±0.21%, while that of encapsulated cells reached 80.56±0.80%. For non-encapsulated probiotic cells, viability significantly decreased during the 14-day storage period. On the other hand, encapsulated probiotic cells maintained a relatively constant viability level despite a slight decrease, as shown in Figure 8b.
Viability of probiotic cells in ice cream was higher when encapsulated because encapsulation provided a protective wall that stabilized cellular structures and reduced environmental stress by limiting molecular movement, significantly increasing stability and survivability (Rodrigues et al., 2020). Furthermore, viability of probiotic cells during storage is greatly influenced by temperature (Jannah et al., 2022). Encapsulated probiotic microcapsule powder stored at lower temperatures had better viability and stability as adverse chemical reactions occurred at a slower rate. Storage below the glass transition temperature improves cell survival due to limited probiotic proliferation and chemical reactions in the cells, lowering internal mobility and oxygen diffusion. The low permeability of the coating material below glass temperature prevents oxygen ingress and preserves the core material. Below the glass transition temperature, food is in a stable state for a long time (glassy state). Meanwhile, above the glass transition temperature, the viscosity of the matrix decreases making the glass and organic polymers become soft (rubbery state) (Shofinita et al., 2020; 2016). Given that probiotic ice cream was stored in a freezer with temperatures below the glass transition of MD and WPC, the results were consistent with data suggesting encapsulated probiotic ice cream had prolonged shelf life, achieving excellent viability even after 14 days of storage. A comparison of probiotic cell viability in various studies is presented in Table 1.
Table 1 Comparison of encapsulation method, encapsulant, probiotic, and viability in various studies
For example, Olivares et al. (2019) reported a substantially higher percentage of probiotic viability after storage with Na-alginate as an encapsulating agent compared to the results. This may be due to the use of vibration which created a thicker layer of encapsulating agent around probiotic, preserving viability. However, vibration encapsulation was unable to produce powder in large quantities and using a viscous encapsulating agent (Pisani et al., 2020; Whelehan and Marison, 2011), compared to spray drying. For industrial purposes, spray drying is more favorable and effective with minimal viability trade-off.
In conclusion, this study successfully formulated a stable microencapsulated probiotic powder to be used as ice cream additive. All operating variations had no detrimental effect on probiotic powder yield, physical properties, and viability. Microencapsulation using MD and WPC achieved about two-fold increase in probiotic viability during storage compared to no encapsulation, proving feasibility in application and potential for further development.
This study was supported by the Faculty of Industrial Technology, Institut Teknologi Bandung (ITB), Indonesia, through the Research, Community Service, and Innovation Program (Program Penelitian, Pengabdian Masyarakat, and Inovasi ITB).
Afzaal, M, Saeed, F, Arshad, MU, Nadeem, MT, Saeed, M & Tufail, T 2019, 'The effect of encapsulation on the stability of probiotic bacteria in ice cream and simulated gastrointestinal conditions', Probiotic Antimicrob Proteins, vol. 11, no. 4, pp. 1348-1354, https://doi.org/10.1007/s12602-018-9485-9
Arepally, D & Goswami, TK 2019, 'Effect of inlet air temperature and gum Arabic concentration on encapsulation of probiotic by spray drying', LWT-Food Science Technology, vol. 99, pp. 583-593, https://doi.org/10.1016/j.lwt.2018.10.022
Aritonang, SN, Roza, E & Rossi, E 2019, Probiotik dan prebiotik dari kedelai untuk pangan fungsional (Probiotics and prebiotics from soybeans for functional foods), Indomedia Pustaka, Sidoarjo
Chen, J, Li, B, Li, H, Liang, R & Li, J 2020, 'Effect of whey protein concentrate on the physical properties of spray-dried cabbage powder', Journal of Food Processing and Preservation, vol. 44, no. 10, article 14953
Cunningham, M, Azcarate-Peril, MA, Barnard, A, Benoit, V, Grimaldi, R, Guyonnet, D, Holscher, HD, Hunter, K, Manurung, S, Obis, D, Petrova, MI, Steinert, RE, Swanson, KS, Sinderen, Dv, Vulevic, J & Gibson, GR 2021, 'Shaping the future of probiotic and prebiotics', Trends Microbiol, vol. 29, no. 8, pp. 667–685, https://doi.org/10.1016/j.tim.2021.01.003
de Araújo Etchepare, M, Nunes, GL, Nicoloso, BR, Barin, JS, Flores, EMM, de Oliveira Mello, R & de Menezes, CR 2020, 'Improvement of the viability of encapsulated probiotic using whey proteins', LWT-Food Science Technology, vol. 117, article 108601, https://doi.org/10.1016/j.lwt.2019.108601
Dinika, I, Nurhadi, B, Masruchin, N, Balia, RL & Utama, GL 2019, 'The roles of Candida tropicalis toward peptide and amino acid changes in cheese whey fermentation', International Journal of Technology, vol. 10, no. 8, pp. 1533-1540, https://doi.org/10.14716/ijtech.v10i8.3661
Ghosal, A, Pradhan, S, Patel, S & Dahikar, PG 2023, 'Effect of heat on protein: A review', International Journal of Food Science and Nutrition, vol. 8, no. 1, pp. 20-22
Gozan, M, Ramadhan, MYA, Harahap, AFP, Sari, CN, Muharam, Y, Purwanto, WW & Tristantini, D 2020, 'Techno-economic analysis of healthy herbal ice cream product', International Journal of Technology, vol. 11, no. 5, pp. 931-940, https://doi.org/10.14716/ijtech.v11i5.4327
Heidarzadeh-Esfahani, N, Soleimani, D, Hajiahmadi, S, Moradi, S, Heidarzadeh, N & Nachvak, SM 2021, 'Dietary intake in relation to the risk of reflux disease: A systematic review', Preventive Nutrition and Food Science, vol. 26, no. 4, pp. 367-379, https://doi.org/10.3746/pnf.2021.26.4.367
Jannah, SR, Rahayu, ES, Yanti, R, Suroto, DA & Wikandari, R 2022, 'Study of viability, storage stability, and shelf life of probiotic instant coffee Lactiplantibacillus plantarum subsp. plantarum Dad-13 in vacuum and nonvacuum packaging at different storage temperatures', International Journal of Food Science, vol. 2022, article 1-7, https://doi.org/10.1155/2022/1663772
Jin, Y, Tang, J & Sablani, SS 2019, 'Food component influence on water activity of low-moisture powders at elevated temperatures in connection with pathogen control', LWT-Food Science Technology, vol. 112, article 108257, https://doi.org/10.1016/j.lwt.2019.108257
Kang, SP 2011, 'Color in food evaluation’, In: Encyclopedia of Agrophysics, pp. 138-141, https://doi.org/10.1007/978-90-481-3585-1_236
Langrish, TAG & Premarajah, R 2013, 'Antioxidant capacity of spray-dried plant extracts: Experiments and simulations', Advanced Powder Technology, vol. 24, no. 4, pp. 771-779, https://doi.org/10.1016/j.apt.2013.03.020
Maftei, NM, Raileanu, CR, Balta, AA, Ambrose, L, Boev, M, Marin, DB & Lisa, EL 2024, 'The potential impact of probiotic on human health: An update on their health-promoting properties', Microorganisms, vol. 12, no. 2, article 234, https://doi.org/10.3390/microorganisms12020234
Meena, S, Prasad, W, Khamrui, K, Mandal, S & Bhat, S 2021, 'Preparation of spray-dried curcumin microcapsules using a blend of whey protein with maltodextrin and gum arabica and its in-vitro digestibility evaluation', Food Bioscience, vol. 41, article 100990, https://doi.org/10.1016/j.fbio.2021.100990
Mishra, J, Bohr, A, Rades, T, Grohganz, H & Löbmann, K 2019, 'Whey proteins as stabilizers in amorphous solid dispersions', European Journal of Pharmaceutical Sciences, vol. 128, pp. 144-151, https://doi.org/10.1016/j.ejps.2018.12.002
Olivares, A, Soto, C, Caballero, E & Altamirano, C 2019, 'Survival of microencapsulated Lactobacillus casei (prepared by vibration technology) in fruit juice during cold storage', Electronic Journal of Biotechnology, vol. 42, pp. 42-48, https://doi.org/10.1016/j.ejbt.2019.10.002
Paula, DdA, Martins, EMF, Costa, NdA, de Oliveira, PM, de Oliveira, EB & Ramos, AM 2019, 'Use of gelatin and gum arabic for microencapsulation of probiotic cells from Lactobacillus plantarum by a dual process combining double emulsification followed by complex coacervation', International Journal of Biological Macromolecules, vol. 133, pp. 722-731, https://doi.org/10.1016/j.ijbiomac.2019.04.110
Pisani, S, Dorati, R, Genta, I, Chiesa, E, Modena, T & Conti, B 2020, 'High efficiency vibrational technology (HEVT) for cell encapsulation in polymeric microcapsules', Pharmaceutics, vol. 12, no. 5, article 469, https://doi.org/10.3390/pharmaceutics12050469
Pourjafar, H, Noori, N, Gandomi, H, Basti, AA & Ansari, F 2020, 'Viability of microencapsulated and non-microencapsulated Lactobacilli in a commercial beverage', Biotechnology Reports (Amst), vol. 25, article e00432, https://doi.org/10.1016/j.btre.2020.e00432
Rajam, R & Subramanian, P 2022, 'Encapsulation of probiotic: past, present and future', Beni-Suef University Journal of Basic and Applied Sciences, vol. 11, article 46, https://doi.org/10.1186/s43088-022-00228-w
Rodrigues, FJ, Cedran, MF, Bicas, JL & Sato, HH 2020, 'Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications – A narrative review', Food Research International, vol. 137, article 109682, https://doi.org/10.1016/j.foodres.2020.109682
Rusch, JA, Layden, BT & Dugas, LR 2023, 'Signalling cognition: The gut microbiota and hypothalamic-pituitary-adrenal axis', Frontiers in Endocrinology, vol. 19, article 1130689, https://doi.org/10.3389/fendo.2023.1130689
Sahlan, M, Fadhan, AM, Pratami, DK, Wijanarko, A, Lischer, K, Hermansyah, H & Mahira, KF 2019, 'Encapsulation of agarwood essential oil with maltodextrin and gum arabic', International Journal of Technology, vol. 10, no. 8, pp. 1541-1547, https://doi.org/10.14716/ijtech.v10i8.3485
Shofinita, D & Langrish, TAG 2016, 'Redox (pro-oxidant/antioxidant) balance in the spray drying of orange peel extracts', Drying Technology, vol. 34, no. 14, pp. 1719-1725, https://doi.org/10.1080/07373937.2016.1175471
Shofinita, D, Bindar, Y, Samadhi, TW, Choliq, NS & Jaelawijaya, AA 2021, 'Increasing the yield of powder and bioactive materials during extraction and spray drying of dragon fruit skin extracts', Journal of Engineering & Technological Sciences, vol. 53, no. 6, article 210612, https://doi.org/10.5614/j.eng.technol.sci.2021.53.6.12
Shofinita, D, Bindar, Y, Samadhi, TW, Jaelawijaya, AA & Fawwaz, M 2020, ‘Reducing the stickiness of dragon fruit skin extract powder as food colorant by addition of maltodextrin during freeze drying’, in: AIP Conference Proceedings, vol. 2219, no. 1, https://doi.org/10.1063/5.0003030
Shofinita, D, Fawwaz, M & Achmadi, AB 2023b, 'Betalain extracts: Drying techniques, encapsulation, and application in food industry', Food Frontiers, vol. 4, no. 2, pp. 576-623, https://doi.org/10.1002/fft2.227
Shofinita, D, Lestari, D, Aliwarga, L, Sumampouw, GA, Ambarwati, SA, Gunawan, KC & Achmadi, AB 2023a, 'Drying methods of coffee extracts and their effects on physicochemical properties: A review', Food and Bioprocess Technology, vol. 17, pp. 47-72, https://doi.org/10.1007/s11947-023-03067-4
Siccama, JW, Pegiou, E, Zhang, L, Mumm, R, Hall, RD, Boom, RM & Schutyser, MAI 2021, 'Maltodextrin improves physical properties and volatile compound retention of spray-dried asparagus concentrate', LWT - Food Science and Technology, vol. 142, article 111058, https://doi.org/10.1016/j.lwt.2021.111058
Sun, H, Hua, X, Zhang, M, Wang, Y, Chen, Y, Zhang, J, Wang, C & Wang, Y 2020, 'Whey protein concentrate, pullulan, and trehalose as thermal protective agents for increasing viability of Lactobacillus plantarum starter by spray drying', Food Science of Animal Resources, vol. 40, no. 1, pp. 118-131, https://doi.org/10.5851/kosfa.2019.e94
Sundararajan, P, Moser, J, Williams, L, Chiang, T, Riordan, C, Metzger, M, Zhang-Plasket, F, Wang, F, Collins, J & Williams, J 2023, 'Driving spray drying towards better yield: Tackling a problem that sticks around', Pharmaceutics, vol. 15, no. 8, article 2137, https://doi.org/10.3390/pharmaceutics15082137
Teixeira, MF, de Oliveira, EB, de Oliveira, AL & Zabot, GL 2021, 'Influence of drying conditions on the morphology and agglomeration of lactose powder particles', Journal of Food Process Engineering, vol. 44, no. 5, article 13751
Wang, X, Ma, N, Lei, T, Größ, J, Li, G, Liu, F, Meusel, H, Mikhailov, E, Wiedensohler, A & Su, H 2019, 'Effective density and hygroscopicity of protein particles generated with spray-drying process', Journal of Aerosol Science, vol. 137, article 105441, https://doi.org/10.1016/j.jaerosci.2019.105441
Wardani, IE 2007, Uji kualitas VCO berdasarkan cara pembuatan dari proses pengadukan tanpa pemancingan dan proses pengadukan dengan pemancingan (VCO quality test based on the manufacturing method from the stirring process without priming and the stirring process with priming), Lib Unnes, pp. 1-100
Whelehan, M & Marison, IW 2011, 'Microencapsulation using vibrating technology', Journal of Microencapsulation, vol. 28, no. 8, pp. 669-688, https://doi.org/10.3109/02652048.2011.586068
Yadav, MK, Kumari, I & Singh, B 2022, 'Probiotic, prebiotics and synbiotics: Safe options for next-generation therapeutics', Applied Microbiology and Biotechnology, vol. 106, pp. 505-521, https://doi.org/10.1007/s00253-021-11646-8
Yonekura, L, Sun, H, Soukoulis, C & Fisk, I 2014, 'Microencapsulation of Lactobacillus acidophilus NCIMB 701748 in matrices containing soluble fibre by spray drying: Technological characterization, storage stability and survival after in vitro digestion', Journal of Functional Foods, vol. 6, pp. 205-214, https://doi.org/10.1016/j.jff.2013.10.008
