Modified Polymetallic Zeolite-Based Catalysts for Hydroprocessing Diesel Oil Fraction and Tetradecane
Corresponding email: Erdos.Ongarbaev@kaznu.kz
Published at : 10 Jul 2024
Volume : IJtech
Vol 15, No 4 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i4.6746
Tuktin, B., Omarova, A., Saidilda, G., Nurzhanova, S., Tungatarova, S., Ongarbayev, Y., 2024. Modified Polymetallic Zeolite-Based Catalysts for Hydroprocessing Diesel Oil Fraction and Tetradecane. International Journal of Technology. Volume 15(4), pp. 812-823
| Balga Tuktin | D.V.Sokolsky Institute of Fuel, Catalysis and Electrochemistry, 142 Kunaev Str., Almaty, 050010, Kazakhstan |
| Aizhan Omarova | Faculty of Chemistry and Chemical Technology, Al-Farabi Kazakh National University, 71 Al-Farabi Pr., Almaty, 050040, Kazakhstan |
| Galymzhan Saidilda | D.V.Sokolsky Institute of Fuel, Catalysis and Electrochemistry, 142 Kunaev Str., Almaty, 050010, Kazakhstan |
| Saule Nurzhanova | D.V.Sokolsky Institute of Fuel, Catalysis and Electrochemistry, 142 Kunaev Str., Almaty, 050010, Kazakhstan |
| Svetlana Tungatarova | D.V.Sokolsky Institute of Fuel, Catalysis and Electrochemistry |
| Yerdos Ongarbayev | Faculty of Chemistry and Chemical Technology, Al-Farabi Kazakh National University, 71 Al-Farabi Pr., Almaty, 050040, Kazakhstan |
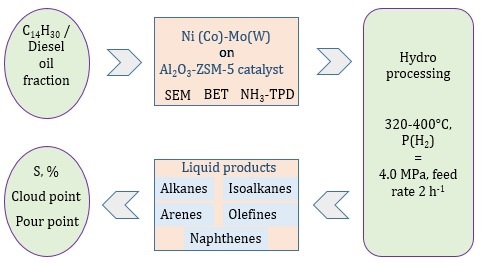
According to international standards, motor fuels require limits on the content of sulfur, benzene, aromatic and olefinic hydrocarbons. To achieve this goal, it is necessary to create new, active and selective catalysts and improve technologies for deep hydrotreating and hydroprocessing. Tetradecane and diesel oil fraction hydroprocessing on modified zeolite-containing Ni(Co)-Mo(W)/Al2O3-ZSM-5 catalysts were studied under varying process conditions. The novelty of the work lies in the synthesis of new catalysts based on ZSM-5 type zeolite, modified with metals Ni(Co)-Mo(W) and active additives of rare earth metals and phosphorus. After hydrotreating the diesel oil fraction on the catalyst KT-3, the pour point decreases from -27 to -57°C, the sulfur level decreases from 0.141 to 0.059%, and the yield of the liquid phase is 92.2%. The lowest content of residual sulfur (0.005%) is observed on the KT-4 catalyst at 400°C. Electron microscopic examinations have demonstrated that the modified zeolite-containing catalysts are distributed in a highly dispersed manner, and the metal components in the active phase are primarily in the oxidized state, generating associate clusters on the surface, the dispersion, structure and state of which is influenced by the catalyst components' characteristics. The developed modified zeolite-containing catalysts have multifunctional properties and simultaneously carry out the reactions of hydrocracking, hydroisomerization, hydrodesulfurization and dehydrogenation.
Catalyst; Diesel fraction; Hydroprocessing; Motor fuel; Tetradecane
The
efficient use of petroleum feedstock is potentially achieved by catalytic
processing of hydrocarbons into practical important products. In this context,
a key source of energy is diesel fuel, which is economical, environmentally
friendly, practical, and generally effective in many industrial processes
requiring large amounts of energy. Diesel engines are capable of providing stable
and reliable power for a variety of industrial applications.
The
growing trend of performance requirements for motor fuel underscores the need
to develop new technological and environmental solutions for oil refining. This fuel is the main component of plastics,
synthetic fibers, resins, rubbers, dyes, surfactants, and pharmaceutical
products.
Hydrotreating stage in petroleum raw material
processing ensures the production of clean motor fuel that meets environmental standards. Further intensification of
production relies on developing new, more active, and selective catalysts. In
this context, an important and topical issue is the formation of efficient
catalytic systems for hydroprocesses. Zeolite catalysts, particularly when based on highly silica
zeolite of the pentasil family, are a promising material in the petrochemical
and oil refining industry (Suhartono et al.,
2023). These catalysts are widely used due to the unique microporous structure
and acid-base properties, which enable the conversion of light alkanes into
valuable products during petrochemical synthesis (Zhang
et al., 2023).
Various studies
have synthesized hydrocracking catalysts with different zeolites to investigate
the role of modified zeolite-Y in crude oil hydroconversion (Ding et al.,
2021). The results showed that catalysts with high acidity, strong acid
sites, and mesopores could improve crude oil conversion.
A previous study (Huseynova et al.,
2022) provided an overview of zeolite-based catalysts used in the
alkylation of benzene and toluene with olefins, isobutane, and butenes with
butane-butene fractions, gasoline and oil fractions with olefins, as well as
propane-propylene and butane-butylene fractions of catalytic cracking. Developing novel technologies in
this field requires a new generation of catalysts for the processing of
hydrocarbon raw materials. International standards mandate that motor fuel
contains a significant amount of sulfur, benzene, aromatic hydrocarbons, and
olefin hydrocarbons.
Catalytic hydroprocessing of sulfurous and
paraffinic oils, comprising hydrotreatment, hydroisomerization, and
hydrogenation, achieves a high degree of quality for commercial products.
Hydrodesulfurization, a chemical change that removes sulfur from gas and
refined crude products such as gasoline, jet fuel, kerosene, alongside diesel
and fuel, upgrades the octane number of the resolvent streams (Sikkander et al., 2022).
A study by (Majodina et al.,
2023) compared traditional hydroprocessing and recently improved
catalysts, detailing the chemical considerations underlying the selection of
mineral materials used in both. Furthermore, investigations into the electronic
interactions of more economical and abundant metals including Nb, V, and Fe
with other elements and supporting materials have led to a better understanding
of the synergistic effects that help access noble metal-like properties.
To increase the production of petroleum
products, expand the range, and improve quality, it is necessary to change the
existing oil refining technology using highly efficient catalysts. A highly
promising CoMo catalyst for hydrotreating low-pressure diesel fuel on carriers
made of thermally activated aluminum hydroxide was investigated in comparison
with a commonly imported analog (Salnikov et al., 2023). Previous studies
also developed technologies
suitable for processing rehydrated pseudoboehmites from flash-calcined aluminum
hydroxide to prepare
catalysts for hydrotreating light and heavy oil fractions (Bayanov
et al., 2023). Modification with metals enhances
catalyst acidity, improving catalytic performance, conversion, and selectivity/yield
toward the product (Mavai, 2022). To advance the field of study, new multifunctional catalysts are
needed to effectively hydrotreat diesel fractions in a single stage. This
entails removing sulfur-containing compounds, hydrogenating unsaturated and
aromatic compounds, hydroisomerization, and selective hydrocracking of
n-paraffin hydrocarbons (Tuktin et al., 2021).
The development of new and highly efficient
catalysts for hydroimprovement of diesel fractions is crucial (Janardhan, Shanbhag and Halgeri, 2014; Zhang et al., 2010; Saih and Segawa, 2009; Rodriguez-Castellon,
Jimenez-Lopez, and Eliche-Quesada, 2008). A previous study (Fitri et al.,
2022) used citronella oil as a dietary supplement to diesel fuel.
Citronella fractions and oil have shown great potential as bio-additives to
diesel fuel, evidenced by acceptable density and viscosity in tested various
concentrations (0.1 - 0.5%). Co-Ni/HZSM-5 catalyst with a hierarchical
porous structure was tested for hydrocracking of vegetable oils at a
temperature of 400°C, and a
pressure of 20 bar for 2 hours. The liquid product had similar hydrocarbon
compounds to petroleum diesel fuel, with the most common being pentadecane and
heptadecane (Marlinda et al., 2022).
The influence of technological regimes on the
yield and hydrocarbon composition of products formed during the cracking of
commercial and M-100 fuel oil in the presence of air in the reactor was studied
by (Shakiyeva
et al., 2022). Natural Taizhuzgen zeolite
and Narynkol clay were used to prepare catalysts.
Sulfide catalysts were reportedly obtained
through mechanochemical combination of commercial powders including molybdenum,
cobalt, and nickel (Fedushchak et al., 2019). The study discussed the activity of catalysts
in model hydrodesulfurization reactions of dibenzothiophene and
4,6-dimethyldibenzothiophene as well as in hydrotreating S-components of diesel
fraction. Based on previous reports, the higher activity of Ni-based catalysts
is due to the superior hydrogenizing capacity. Studies by (Altynov et al.,
2023; Aleksandrov, Buhtiyarova and Reshetnikov, 2019) examined the
behavior of CoMo/Al2O3 catalyst in hydrotreating a
straight-run diesel fraction with a high sulfur content (more than 2 wt.%) mixed
with light gas oil in the temperature range of 335 - 365°C at a volumetric feed
rate of 0.8 - 2.5 h-1. Adding gas oil to the straight-run diesel
fraction during hydrotreating has diverse effects based on the temperature and
feed rate of the raw materials (Tanimu et al., 2022).
A study examined catalytic abilities of
trimetallic NiMoWS catalysts supported on aluminum oxide during hydrotreating
of straight-run diesel fraction (Nikul’shina et al., 2019). The results showed
that the oxide precursor’s nature had a significant effect on catalytic
activity. Mixing n-heptane, n-dodecane, tetralin, and decalin with diesel fuel
linearly changes density, viscosity and improves atomization (Wei et al.,
2022). The study by (Jaroszewska et al., 2021) showed that
catalysts containing titanium (HTiMCM-41 and NiMo/HTiMCM-41) improved textural
properties, as well as acidity and binding energy with the metal substrate than
Al-based analogs (HAlMCM -41 and NiMo/HAlMCM-41). Replacing aluminum or
titanium in modifying MCM-41 zeolite significantly affects the properties and
activity of Ni catalysts.
Typical transition metal
sulfides Ni/Co-promoted Mo, as well as W-based bi- and tri-metallic catalysts,
are used for selective removal of sulfur from refractory compounds. The review (Prihadiyono et
al., 2022; Leon et al., 2019) examined three very
specific topics of catalysts to produce ultra-low sulfur diesel. Furthermore, (Winarto et al., 2024) explored a biphasic hydrogenation
approach using solid NR dissolved in a solvent and a hydrogen source (hydrazine
hydrate and hydrogen peroxide) mixed in water. The choice of solvents,
catalysts, and the water-to-solvent volume ratio were examined for the impact on
hydrogenation.
Studies on developing effective catalysts and
technologies to process diesel fractions into valuable chemical products and
motor fuel are crucial (Laredo et al., 2020; Kar, Göksu and Yalman, 2018). The production of high-quality commercial petroleum products can
be achieved using catalysts prepared based on aluminosilicates with a zeolite
structure of the ZSM-5 type for hydrorefining diesel oil fractions (Tuktin et al.,
2022).
This study investigated hydroprocessing of tetradecane and diesel oil fraction oil on zeolite catalysts modified using
metals of variable valence. A series of new
catalysts with matrix structure Ni(Co)-Mo(W)/Al2O3-ZSM-5
were synthesized as shown in Table 1.
Table
1 Composition
of the developed catalysts
|
No. |
Catalyst sample |
Catalyst components |
|
1 |
KT-1 |
CoO-MoO3-P2O5-La2O3/Al2O3-ZSM-5 |
|
2 |
KT-2 |
CoO-WO3-P2O5-La2O3/Al2O3-ZSM-5 |
|
3 |
KT-3 |
NiO-MoO3-P2O5-La2O3/Al2O3-ZSM-5 |
|
4 |
KT-4 |
CoO-NiO-MoO3-P2O5-La2O3/Al2O3-ZSM-5 |
The synthesis of modified zeolite-based catalysts was performed by saturating a mixture of peptized aluminum hydroxide and zeolite ZSM-5 (China) with aqueous solutions of metal salts. These include (NH4)10W12O41×5H2O (Cherkasy chemical reagents plant, Russia), Ni(NO3)2×6H2O (Ural Chemical Plant, Russia), Co(NO3)3×6H2O (Novosibirsk Rare Metals Plant, Russia), (NH4)6MO7O24×4H2O (Cherkasy chemical reagents plant, Russia), La(NO3)3×6H2O (Novosibirsk Rare Metals Plant, Russia) and modifying additives. After molding, catalysts were dried at 150°C and calcined at 550°C for 5 hours. Before the experiments, catalysts were subjected to preliminarily sulphidation to increase activity in the reactor. The synthesized catalysts were tested through hydroprocessing tetradecane and diesel oil fraction using flow-through installation (Figure 1) with a stationary catalyst bed. The testing conditions included temperature ranging from 320-400°C, hydrogen pressure of 4.0 MPa, and a volumetric feed rate of 2 h-1.
Hydrocarbon composition of reaction products
was analyzed using Khromatek-Kristall and Khromatek-Kristall 5000
chromatographs (Russia). The chromatograph calculates the fractional
composition automatically. For the analysis of hydrocarbons, a glass column 3 m
long, 4 mm in diameter, filled with g-Al2O3 was used. The
integral selectivity (S) of aromatization, dehydrogenation, and cracking was
calculated using the formula S = Y/X, where Y is the yield of products; and X
is the feedstock degree of conversion.
The analysis of sulfur content, pour point, as well as cloud point of diesel oil fraction and products of hydroprocessing was carried out at Oilsert International LLP (Almaty, Kazakhstan). Various methods were used to analyze the physical and chemical characteristics of the developed catalysts. Surface area and porosity were measured by BET method on an AccuSorb unit manufactured by Micromeritics (USA), while electron microscopy was performed using an EM-125K transmission electron microscope (Williams and Carter, 2009). Microdiffraction images were interpreted using standard ASTM tables. To examine the number of acid sites and their strength distribution, the method of temperature-programmed desorption of ammonia was used (Mustafayeva, 2012).
Figure 1 Scheme of a laboratory installation
of
hydroprocessing, where 1 – burette, 2 – pump; 3, 7, 12, 14 – valves; 4, 8 –
pressure gauges; 5 – hydrogen cylinder; 6 – reducer; 9 – reactor; 10 –
refrigerator; 11 – rotameter; 13 – separator; 15 – flask
The development of
new catalysts effective for processing low-solidification diesel oil fractions
into high-octane products is currently a major concern. The original diesel oil fraction
has the following characteristics: pour point -27°C, cloud point -18°C, and a high content of
n-alkanes, which solidifies at higher temperatures compared to branched analogs. In
addition, the sulfur
content is approximately 0.141%.
Tetradecane, an n-alkane, and a component of
diesel oil fraction was used as a hydrocarbon model. The conversion of
tetradecane on the developed modified zeolite-based catalysts was studied by
varying the technological parameters of the process.
During hydroprocessing of n-tetradecane on KT-1 catalyst (Figure 2), the liquid catalysate formed
n-alkanes, isoalkanes,
olefins, aromatic
hydrocarbons, and naphthenes. With an increase in temperature from 320 to 400°C, the degree of
conversion increased from 41.2 to 74.5%. The yield of the liquid phase
decreased from 92.3 to 54.1% with an increase in temperature. In addition, the yield of
isoalkanes and aromatic hydrocarbons on KT-1 catalyst increased from 12.9
to 22.3% and 5.5 to 18.3%, respectively. Based on the results, the content of
olefins and naphthenic hydrocarbons varied insignificantly.
On KT-2 catalyst, hydroprocessing of tetradecane led to an increased content of isoalkanes, rising from 15.0 to 35.3% as the temperature rose from 320 to 400°C, while the amount of aromatic
hydrocarbons decreased from 5.1% to 6.3%. During hydroprocessing
of tetradecane on synthesized catalysts, the highest yield of isoalkanes was
observed on KT-3 catalyst at 400°C. Based on the results, a sharp rise was
observed in the yield of isoalkanes from 17.1 to 37.7% with an increase in
temperature.
Compared to
other catalysts, KT-4 produced a smaller quantity of olefinic hydrocarbons as
reaction products. A rise in the temperature from 320 to 400°C led to a change
in the conversion from 38.8 to 83.4%. Under these conditions, isoalkanes and
aromatic hydrocarbons increased from 13.4 to 30.5% and 6.2 to 19.2%,
respectively, while the naphthenes content decreased from 3.0 to 2.3%.
For all catalysts studied, the degree of tetradecane conversion increased with rising reaction temperature. Based on the results, the developed modified zeolite-based catalysts showed polyfunctional properties, simultaneously facilitating isomerization of n-alkanes, dehydrogenation, and dehydrocyclization.
Figure 2
Results and composition of tetradecane hydroprocessing products using catalysts
CoO-MoO3-P2O5-La2O3/Al2O3-ZSM-5
(KT-1), CoO-WO3-P2O5-La2O3/Al2O3-ZSM-5
(KT-2), NiO-MoO3-P2O5-La2O3/Al2O3-ZSM-5
(KT-3) and CoO-NiO-MoO3-P2O5-La2O3/Al2O3-ZSM-5
(KT-4)
Catalysts were evaluated during hydroprocessing of diesel oil fraction (Figure 3). At a temperature of 400°C, the lowest sulfur content (0.005%) was observed on KT-4 catalyst. The pour point of diesel oil fraction decreased by 15 - 30°C compared to the feedstock after hydroprocessing on the developed catalysts.
Figure 3 Results of diesel oil fraction
hydroprocessing on KT-3 and KT-4
catalysts
KT-3 catalyst produced the most
significant drop in pour point. After hydroprocessing of diesel fraction at
400°C, the pour point dropped to -57°C, and the yield of the liquid phase was
92.2%.
During hydroprocessing of diesel fraction on
the developed catalysts, simultaneous reactions of hydroisomerization,
hydrotreatment, and hydrocracking were observed. For example, after
hydroprocessing of diesel oil fraction on KT-3 catalyst at 400°C, the sulfur
content decreased from 0.141 to 0.059%, and the pour point reduced from -27 to
-57°C. On KT-4 catalyst at 400°C, the reduction reached 0.005% and -55°C respectively.
Catalyst activity is influenced by surface
structure, composition, and state of active sites. Different methods can be
used to investigate the physicochemical traits of catalysts including BET,
temperature-programmed desorption of ammonia, and electron microscopy.
BET analysis showed that the surface area of
the developed catalysts ranged from 221.0-285.0 m2/g and the pore
diameter was between 1.5-3.5 nm. Furthermore, the acid-base characteristics of
catalysts are essential for hydrocarbon processing process. In a previous study
(Wei et al., 2020), Ni-Mo catalysts
supported on Ni-modified ZSM-5 zeolites prepared by co-impregnation method
showed higher stability and isomerization selectivity in n-octane
hydroconversion. This enhanced property was attributed to the synergistic
effect between Brønsted acid sites and Lewis acid sites on catalysts. The
acid-base properties of catalysts were determined through
temperature-programmed desorption of ammonia (Table 2).
Table 2 Acid-base properties of catalysts
|
Catalyst |
Desorption
temperature, 0C |
Desorbed
NH3, 10-4 mole/g catalyst |
|
KT-1 |
195 |
23.4 |
|
KT-2 |
185 |
28.0 |
|
KT-3 |
220 |
32.5 |
|
KT-4 |
225 |
29.6 |
The data showed that acid centers with a desorption temperature of 195°C predominated on the surface of KT-1 The data showed that acid centers with a desorption temperature of 195°C predominated on the surface of KT-1 catalyst, with a concentration of 23.4×10-4 mole/g. The amount of ammonia desorbed from the surface of KT-2 catalyst was 28.0×10-4 mole/g and the concentration of acid sites on KT-3 catalyst was 32.5×10-4 mole/g. On the surface of KT-4 catalyst, ammonia was adsorbed in two forms with Tmax equal to 185 and 225°C. KT-3 catalyst, characterized by the highest concentration of acid sites (32.5×10-4 mole/g) and an average binding energy corresponding to desorption temperature of 220°C, demonstrated high hydroisomerization activity in hydroprocessing of tetradecane and diesel oil fraction. Metals with different degrees of oxidation can be found in the composition of acid sites, attached both inside and on the outer surface of the zeolite cavities, ensuring the multifunctionality of catalytic system.
Catalyst activity is influenced by the
surface structure, phase composition, and the state of modifying additives. In
the study by (Abdullaev et al., 2021), the surface of SSZ-13 zeolite was modified with varying amounts
(1-15%) of tungsten oxide, demonstrating significantly improved selectivity and
yield of propylene from ethylene. This enhancement was attributed not only to
softer and reduced strong acid sites but also to limited diffusion of bulky
products, as confirmed by scanning transmission electron microscopy and
energy-dispersive X-ray spectroscopy (STEM-EDS) data. An electron microscopy
study was carried out to examine the structure and state of active centers in
catalysts promoted by Co, Ni, Mo, and W, among others.
Electron microscopic studies showed that on
the surface of KT-1 catalyst (Figure 4a), extensive accumulations of small
particles were observed with diameter 3.0 - 5.0 nm, corresponding to a mixture
of MoOPO4, La2O3, MoO5, and La2MoO3
phases. Small accumulations of highly dispersed particles with 8.0 - 10.0
nm in size were also found, which could be attributed to La4(P2O7)3.
Furthermore, there were small transparent aggregates with d 20.0 nm related
to LaAlO3 in the
La° mixture. The emergence of
La° could be
connected to redox processes occurring between the active phase components.
The nature of the components in complex
polymetallic catalysts for hydroprocessing of hydrocarbons has a significant
effect on the dispersion and state of active centers. Formations with diameter
4.0 - 6.0 nm, consisting of AlLa3, Co2O3, Co2SiO3,
and La6O11, were prevalent on the surface of KT-1 catalyst (Figure 4b). Cobalt
formed single structures of metallic Co° with a diameter of 2.5 nm on the
surface. Additionally, there were accumulations (d 15.0 nm) of small
particles (d
2.5 nm) consisting of CoSi, CoSi2, and MoPCo2;
lamellar particles (d
15.0 nm) such as MoPCo2Si, CoO, and Mo3Si;
semitransparent structures (d
2.5 nm) namely MoO(OH)2, Co2O3,
MoP2, Co3Al3Si4, and SiP2O7;
alongside particles with d
6.0 - 10.0 nm, including LaP, MoO3, and
Mo3Si. Accumulations containing Co2Mo3O8,
LaP2O7, AlPO4, CoOOH, MoOPO4,
Co(P2O7), and La(MoO4)2 had
particles with different shapes in the range of 50-200 nm in diameter. In
addition, there were particles 4-5 nm in size including La4(P2O7)3,
CoSi, MoP, MoSi2, La6O11, and CoMoP2
Highly
dispersed structures of AlNi, Ni2O3, Mo3O5,
and Mo5Si3 with d 2.0 nm were predominant on the
surface of KT-3 catalyst
(Figure 5). There were well-spaced small accumulations of MoNiSi, Ni2O3,
MoSi2, and M6O11 particles with diameter 6.0 -
10.0 nm. In addition, the oxidized states of nickel Ni2O3
formed single islands, with a size ranging from 5.0 to 10.0 nm (Figure 5a).
KT-3 catalyst was defined by clusters measuring 3.0 - 4.0 nm, formed by fine
particles with d = 0.05 nm, containing NiSi2 and Ni2O3.
Particles with hexagonal faceting and diameters between 15.0 and 30.0 nm were
also found, composed of AlNi2Si, AlNi, La2O3,
MoO(OH)2)2, AlMo3, MoSi2, and Al3La
(Figure 5b). The discovered structures of AlNi2Si, AlMo3,
AlNi, MoSi2, and LaAlO3 NiSi2 indicated the
incorporation of metal components from the active phase into the zeolite
structure with the formation of new centers that could work as Lewis acid
centers.
During
hydroprocessing on polyfunctional catalysts with dehydrogenating,
hydrogenating, and acidic abilities, n-alkane dehydrogenation occurs first on
the metal centers, and the olefin produced on the acid center is converted into
a carbonium ion, which is easily isomerized (Tuktin et al., 2019). Mo5O7(OH)2, MoO3,
NiMO4, CoOOH, NiOOH, AlLa3, P and La, MoNiP, Ni2O3
particles with diameter ranging from 10.0 - 15.0 nm along the edge of dense and
large aggregates were discovered on the surface of KT-4 catalyst (Figure 6a). Additionally,
numerous loose formations of Mo3O5, Mo5Si3,
Mo6O11, Al3Ni,
Ni2O3, and PLa2 were observed with diameter of
3.0 - 5.0 nm. There were also clusters of AlNi2Si and Mo3O5
particles with diameters of 3.5 - 4.0 nm (Figure 6b).
Electron microscopy studies showed that particles
on the surface of the developed catalysts were highly dispersed, with sizes ranging
from 2.0 to 10.0 - 20.0 nm depending on the nature of the modifier metal and
the amount of zeolite introduced. All the studied catalysts were characterized
by the incorporation of modifier metals into the matrix structure with the
formation of CoSi, CoSi2, MoPCo2Si, Co3Al3Si4,
Mo3Si, Co2Si, Mo5Si3, AlNi, MoNiSi,
MoSi2, AlNi2Si, and W(Si, Al)2. These
structures can function as Lewis acid sites, while the phosphorus-containing
centers including SiP2, PLa2, MoNiP, LaP, and Co2P
are also quite strong acidic centers. The modification of catalysts leads to
the formation of highly dispersed heteronuclear clusters of complex
composition, which are active in hydrotreatment and hydroisomerization reactions
of diesel oil fraction (Gackowski et
al., 2020).
Studies have identified several types of surface
structures found on the surface of these catalysts, differing significantly in
size and chemical state of components. For instance, a small amount of
hierarchical Y zeolite (10 %) mixed with alumina, nickel nitrate, and
molybdenum oxide in the light diesel fuel hydrocracking catalyst, played a key
role in selectively hydrogenating naphthalene and further ring-opening activity.
The mesoporous structure of zeolite provided an effective interface and
improved the accessibility of acid sites for bulk reagents (Zhang et al., 2020).
According to electron microscopy data, the active
phase catalyst components are mostly in the oxidized state, creating associate
clusters on the surface. The dispersion, structure, and nature of the modifying
additives in catalyst determine the active state.
In conclusion, this study evaluated
hydroprocessing of tetradecane and diesel oil fraction using new modified zeolite-based
Ni(Co)-Mo(W)/Al2O3-ZSM-5 catalysts under varying process
conditions. The developed catalysts used in hydroprocessing diesel oil fraction showed polyfunctional properties, simultaneously facilitating hydrocracking, hydroisomerization,
hydrodesulfurization, and dehydrogenation reactions. These results suggest that modification using
metals with variable valence allows proper control of hydroisomerization,
hydrodesulfurization, and hydrocracking activity of catalysts in
hydroprocessing of diesel oil fraction, facilitating the production of fuel
with low sulfur and pour point.
The authors are grateful to the
Committee of Science, the Ministry of Science and Higher Education of the Republic
of Kazakhstan for funding this study through the project “ARO8857065 Creation of scientific
foundations for the development of new efficient catalysts and technology for
deep hydroprocessing of vacuum gas oil to produce high-quality motor fuel” (2020-2022).
Abdullaev, M.U., Lee, S., Kim, T.-W., Kim, C.-U., 2021. Tungsten
Oxide-Modified SSZ-13 Zeolite as an Efficient Catalyst for
Ethylene-To-Propylene Reaction. Catalysts, Volume 11,
p. 553
Aleksandrov,
P.V., Buhtiyarova, G.A., Reshetnikov, S.I., 2019. Study of The Influence of
Coker Gas Oil to The Straight-Run Gas Oil on The Process of Hydrotreating in
The Presence of CoMo/Al2O3 catalyst. Russian Journal of Applied
Chemistry, Volume 92,
pp. 1077–1083
Aljajan, Y., Stytsenko, V., Rubtsova, M., Glotov, A.,
2023. Hydroisomerization Catalysts for High-Quality Diesel Fuel Production. Catalysts, Volume 13, p. 1363
Altynov, A., Bogdanov, I., Lukyanov, D., Kirgina, M., 2023. Natural
Gas Liquids into Motor Gasolines: Methodology for Processing on a Zeolite
Catalyst and Development of Blending Recipes.
ChemEngineering, Volume 7, p. 93
Bayanov, V.A., Gizetdinova, A.F., Vishnevskaya, A.L.,
Tagandurdyeva, N.A., Kleymenov, A.V., Trafimov A.V., 2023. Processing Promising
Supports for Hydroprocessing Catalysts Based on Flash-Calcined Aluminium
Hydroxide. Oil & Gas Chemistry, Volume 2, pp. 59–62
Ding, L., Sitepu, H., Al-Bogami, S.A., Yami, D.,
Tamimi, M., Shaik, K., Sayed, E., 2021. Effect of Zeolite-Y Modification on Crude-Oil Direct
Hydrocracking. ACS Omega, Volume 6,
pp. 28654-28662
Fedushchak,
T.A., Uimin, M.A., Maikov, V.V., Mikubaeva, E.V., Akimov, A.S., Morozov, M.A.,
Zhuravkov, S.P., Petrenko, T.V., Vosmerikov, A.V., Zhirov, N.A., Kogan, V.M.,
2019. Two-Component
Ni(Co)-Promoted MoS2 Bulk Catalysts and Their
Hydrodesulphurising Ability in Model Reactions and Diesel Fraction
Hydrotreatment. Chemistry of Sustainable Development, Volume 27, pp. 71–77
Fitri,
N., Riza, R., Akbari, M.K., Khonitah, N., Fahmi, R.L., Fatimah, I., 2022.
Identification of Citronella Oil Fractions as Efficient Bio-Additive for Diesel
Engine Fuel. Designs, Volume
6, p. 15
Gackowski, M., Podobinski, J.,
Broclawik, E., Datka, J., 2020. IR and NMR Studies of the Status of Al and Acid
Sites in Desilicated Zeolite Y. Molecules,
Volume 25, pp. 31
Huseynova, G., Muxtarova, G., Aliyeva, N., Gastimova, G., Rashidova, S., 2022. Zeolite-Containing Catalysts in Alkylation
Processes. Catalysis Research, Volume
2(3, pp. 1–12
Janardhan,
H.L., Shanbhag, G.V., Halgeri, A.B., 2014. Shape-Selective Catalysis by
Phosphate Modified ZSM-5: Generation of New Acid Sites with Pore Narrowing. Applied Catalysis A: General, Volume
471, pp. 12–18
Jaroszewska,
K., Lewandowski, M., Góra-Marek, K., Grzechowiak, J., Djéga-Mariadassou, G.,
2021. Hydrodesulfurization of 4,6-Dimethyldibenzothiophene and the Diesel Oil
Fraction on NiMo Catalysts Supported over Proton-Exchanged AlMCM-41 and
TiMCM-41 Extrudates. Catalysts,
Volume 11, p. 1086
Kar, Y., Göksu, D.S., Yalman, Y., 2018.
Characterization of Light Diesel Fraction Obtained from Upgraded Heavy Oil. Egyptian Journal of Petroleum, Volume
27, pp. 1301–1304
Laredo, G.C., Pérez-Romo,
P., Águeda-Rangel, R., Escobar, J., Vega-Merino, P., 2020. Detailed Characterization of Light Cycle Oil
for BTX Production Purposes. International Journal of Petroleum and
Petrochemical Engineering, Volume 6, Issue 3, pp. 1–12
León, J.N.D., Kumar, C.R., Antúnez-García, J., Fuentes-Moyado, S., 2019. Recent Insights in Transition Metal Sulfide
Hydrodesulfurization Catalysts for the Production of Ultra Low Sulfur Diesel: A
Short Review. Catalysts,
Volume 9, p. 87
Majodina, S., Poswayo, O., Dembaremba, T.O., Tshentu,
Z.R., 2023. Towards Improvement of Hydroprocessing Catalysts - Understanding
The Role of Advanced Mineral Materials In Hydroprocessing Catalysts. Minerals
and Mineral Materials, Volume 2, p. 13
Marlinda, Al-Muttaqii, M.,
Roesyadi, A., Prajitno, D.H., 2022. Formation Of Hydrocarbon Compounds During
the Hydrocracking of Non-Edible Vegetable Oils With Cobalt-Nickel Supported On
Hierarchical HZSM-5 Catalyst. In IOP Conference Series: Earth and Environmental
Sciences, Volume 67, p. 012022
Mavai,
J., 2022. Methanol Synthesis and Converted into Hydrocarbons Over Zeolite
Catalyst a Review. Journal of Emerging
Technologies and Innovative Research, Volume 9, pp. 221–256
Mustafayeva,
R.M., 2012. Zeolite-Containing Catalysts in The Processes of
Obtaining Aromatic Hydrocarbons.
Azerbaijan: Baku
Nikul’shina,
M.S., Mozhaev, A.V., Lancelot, C., Blanchard, P., Lamonier, C., Nikul’shin,
P.A., 2019. Hydrotreating of Straight-Run Diesel Fraction Over Mixed Nimows/Al2O3 Sulfide Catalysts. Petroleum Chemistry, Volume 59, pp. 529–534
Prihadiyono,
F.I., Lestari, W.W., Putra, R., Aqna, A.N.L., Cahyani, I.S., Kadja, J.T.M.,
2022. Heterogeneous Catalyst Based on Nickel Modified into Indonesian Natural
Zeolite in Green Diesel Production from Crude Palm Oil. International Journal of Technology, Volume 13 (4), pp. 931–943
Rodriguez-Castellon,
E., Jimenez-Lopez, A., Eliche-Quesada, D., 2008. Nickel And Cobalt Promoted
Tungsten and Molybdenum Sulfide Mesoporous Catalysts For Hydrodesulfurization. Fuel, Volume 87, pp. 1195–1206
Saih,
Y., Segawa, K., 2009. Catalytic Activity of CoMo Catalysts Supported on
Boron-Modified Alumina for the Hydrodesulphurization of Dibenzothiophene and
4,6-Dimethyl Dibenzothiophene. Applied Catalysis A, Volume 353, pp. 258–265
Salnikov, V.A., Polyakov, N.A., Nevolina, S.A.,
Korotkova, N.V., Reznichenko, I.D., Ovchinnikov, K.A., 2023. A Promising
Catalyst for Hydrotreating Diesel Fuels on A Carrier Made of Thermally
Activated Aluminum Hydroxide. Oil &
Gas Chemistry, Volume 2, pp. 49–52
Shakiyeva, T.V., Sassykova, L.R., Khamlenko, A.A.,
Dzhatkambayeva, U.N., Sassykova, A.R., Batyrbayeva, A.A., Zhaxibayeva, Z.M.,
Ismailova, A.G., Sendilvelan, S., 2022. Catalytic Cracking of M-100 Fuel Oil:
Relationships Between Origin Process Parameters and Conversion Products. Chimica Techno Acta, Volume 9(3), p.
20229301
Sikkander, A. M., Kavitha, K., Ramanachiar, R.,
Anitha, V., Sasikala, S., Sivaraj, C., Balu, T.N., Mishra, S.R., Yasmeen, K.,
2022. Hydrodesulphurization of Petroleum. Petroleum
and Chemical Industry International, Volume 5 (3), pp. 150–152
Suhartono,
Romli, A., Hari Prabowo, B., Kusumo, P., Suharto, 2023. Converting Styrofoam
Waste into Fuel Using a Sequential Pyrolysis Reactor and Natural Zeolite
Catalytic Reformer. International Journal
of Technology, Volume 14 (1), pp. 185–194
., 2022. Metal-Free
Catalytic Oxidative Desulfurization of Fuels. A Review. Energy & Fuels, Volume 36 (7), pp. 3394–3419
Tuktin, B., Zhandarov, E., Nurgaliyev, N.,
Tenizbayeva, A., Shapovalov, A., 2019. Hydrotreating of Gasoline and Diesel Oil
Fractions Over Modified Alumina/Zeolite Catalysts. Petroleum Science and Technology, Volume 37, pp. 1770–1776
Tuktin, B.T., Omarova, A.A., Saidilda, G.T., Sassykova,
L.R., 2022. Modified Zeolite Catalysts for Efficient Processing Of N-Hexane and
Gasoline Fraction. Rasayan Journal of
Chemistry, Volume 15, pp. 2442–2449
Tuktin, B.T., Temirova, A.M., Omarova, A.A.,
Myltykbaeva, Z.K., Anisimov, A.V., 2021. Aromatization of Low-Molecular-Weight
Hydrocarbons on Modified Zeolite Catalysts. Theoretical
Foundations of Chemical Engineering, Volume 55, pp. 1016–1022
Wei, Q., Zhang, P., Liu,
X., Huang, W., Fan, X., Yan, Y., Zhang, R., Wang, L., Zhou, Y., 2020. Synthesis
of Ni-Modified ZSM-5 Zeolites and Their Catalytic Performance in n-Octane
Hydroconversion. Frontiers
in Chemistry, Volume 8, p. 586445
Wei,
Y.J., Zhang, Y.J., Zhu, X.D., Gu, H.M., Zhu, Z.Q., Liu, S.H., Sun, X.Y., Jiang,
X.L., 2022. Effects of Diesel Hydrocarbon Components on Cetane Number and
Engine Combustion and Emission Characteristics. Applied Sciences, Volume 12, p. 3549
Williams,
D.B., Carter, C.B., 2009. Transmission
Electron Microscopy. New York, USA:
Springer
Winarto,
D.A., Liza, C., Fathurrohman, M.I., Masa, A., Chalid M., 2024. Study of Solvent
and Catalyst in Diimide Biphasic Hydrogenation System of Natural Rubber. International Journal of Technology,
Volume 15 (2), pp. 414–424
Zhang,
D., Duan, A., Zhao, Z., Xu, C., 2010. Synthesis, Characterization and Catalytic
Performance of NiMo Catalysts Supported on Hierarchically Porous Beta-KIT-6
Material In The Hydrodesulfurization of Dibenzothiophen. Journal of
Catalysis, Volume 274, pp.
273–286
Zhang, M., Qin, B., Zhang, W., Zheng, J., Ma,
J., Du, Y., Li, R., 2020. Hydrocracking of Light Diesel Oil over Catalysts with
Industrially Modified Y Zeolites. Catalysts, Volume 10, p. 815
Zhang, W., Fang, D., Huang, G., Li, D., Zheng, Y.,
2023. Research and Application Development of Catalytic Redox Technology for
Zeolite-Based Catalysts. Catalysts, Volume 13, p. 1197
