Sustainable Porous Silica Material Extracted from Volcanic Ash of Mount Sinabung Indonesia as Corrosion Inhibitor
Corresponding email: lisnawaty@unimed.ac.id
Published at : 10 Jul 2024
Volume : IJtech
Vol 15, No 4 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i4.6740
Simatupang, L., Siburian, R., Ginting, E., Pakpahan, B.M.T., Simatupang, K.A.P., Siagian, D.G., Laoli, E.R., Goei, R., Tok, A.I.Y., 2024. Sustainable Porous Silica Material Extracted from Volcanic Ash of Mount Sinabung Indonesia as Corrosion Inhibitor. International Journal of Technology. Volume 15(4), pp. 880-889
| Lisnawaty Simatupang | Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Negeri Medan, Jl. Willem Iskandar Psr. V, Medan Sumatera Utara, 20221, Indonesia |
| Rikson Siburian | Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, 20155, Medan, Indonesia |
| Elfrida Ginting | Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Negeri Medan, Jl. Willem Iskandar Psr. V, Medan Sumatera Utara, 20221, Indonesia |
| Binsar Maruli Tua Pakpahan | Department of Mechanical Engineering, Faculty of Engineering, Universitas Negeri Medan, Jl. Willem Iskandar Psr. V, Medan Sumatera Utara, 2022, Indonesia |
| Kristian Adinata Pratama Simatupang | Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Negeri Medan, Jl. Willem Iskandar Psr. V, Medan Sumatera Utara, 20221, Indonesia |
| Dea Gracella Siagian | Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Negeri Medan, Jl. Willem Iskandar Psr. V, Medan Sumatera Utara, 20221, Indonesia |
| Edward Relius Laoli | Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Negeri Medan, Jl. Willem Iskandar Psr. V, Medan Sumatera Utara, 20221, Indonesia |
| Ronn Goei | School of Materials Science and Engineering, Nanyang Technological University, 50 Nanyang Avenue, 639798, Singapore |
| Alfred Iing Yoong Tok | School of Materials Science and Engineering, Nanyang Technological University, 50 Nanyang Avenue, 639798, Singapore |
This study investigated the
potential of porous silica material extracted from volcanic ash of Mount
Sinabung, Indonesia, as a corrosion inhibitor. The new material was subjected
to comprehensive analysis using the X-Ray Diffraction (XRD), Fourier Transform
Infrared Spectroscopy (FTIR), Search Engine Marketing (SEM), and Atomic
Absorption Spectrophotometry (AAS). Corrosion test was conducted by coating the
metal surface with synthesized silica. XRD data showed the presence of
amorphous silica, while SEM indicated a rough and irregular pore cavity. Based
on AAS characterization, the concentration of silica in the Mount Sinabung
volcanic ash was 79.23 % (v/v) with a yield of 29.73 %(w/w). Furthermore,
coated and uncoated iron plates, with grit variations of 800, 1200, 1500, and
2000, were tested against HCl 15 % (v/v) and NaCl 3.5 % (w/v)
as model corrosive solutions. The SEM results showed that coated plates had
fewer holes and cracks formation while the XRD analysis of the same samples
presented a slight decrease in the intensity of iron phase. Among silica-coated
iron plates, the 1500 grit variation had the lowest corrosion rate and the
highest corrosion inhibitor efficiency in both HCl 15 % (v/v)
and NaCl 3.5 % (w/v) corrosive solutions, recording efficiencies
of 26.3 and 91.8 %, respectively.
Corrosion inhibitor; Grit; Natural silica; Silica coated iron; Volcanic ash
Mount Sinabung is one of
the active volcanoes in Indonesia, located in the North Sumatera Province.
According to The Indonesia Disaster Control Bureau (BNPB) data, Mount Sinabung
has emitted approximately 250 million tons of ash since the eruption in 2010. A
previous study discovered that the main component of volcanic ash was SiO2
(74.3%) (Karolina et al., 2020; Lubis et
al., 2019). Silica content is higher compared to other volcanoes in
the country, such as Mount Merapi (63.3 %) or Mount Kelud (70.6 %) (Nakada et al., 2019).
The abundance of volcanic ash
and high silica content presents significant potential for the production of silica-based material.
Silica has various applications in the pharmaceutical, ceramics, paints,
coatings, and chemical industries. This is due to the numerous advantageous
properties, including high porosity, mechanical strength, thermal stability, pore
surface area, stability in acidic environments, non-swelling characteristics,
and resistance to microbial attack (Salleh et
al., 2021; Boonmee and Jarukumjorn, 2020; Pan, Li, and Mao., 2020; Mainier et
al., 2018a; El-Fargani et al., 2017; Verma and Khan, 2016; Anderson
and Segall, 2011). These attributes support the potential for the
cost-effective production of silica-based composite material applied by various
niche (Beleuk-a Moungam et al., 2022;
Prabha et al., 2021; Silvana and Sunardi,
2020; Iguchi et al., 2012).
Several studies have reported the use of
volcanic ash, including its application as a base material for geopolymers and
in the synthesis of nano-silica (Hasanah et al.,
2021; Sinuhaji et al., 2018; Karolina et al., 2015).
Investigation has been conducted on the preparation of volcanic ash from Mount
Sinabung, a basic material for creating silica-based adsorbents. This study
also comprises the characterization of volcanic ash, modification of silica
surfaces for composite material, and its application in heavy metal adsorption (Simatupang and Devi, 2016). Based on previous
work, the characterization showed that the resulting silica gel was amorphous,
with a surface area of 375 m2/g and a pore diameter of 1.5 nm (Simatupang et al., 2020). The substantial
pore surface area renders silica gel suitable for adsorption purposes.
The common problem faced by
industrialized nations is metal corrosion, a process driven by oxidation
reactions, thereby leading to degradation in the quality of metal. Corrosion
could be caused by moisture, acids, salt, and high ambient temperatures (Pan et al., 2020; Yeganeh, Omidi, and Eskandari, 2018;
Javaherdashti, 2000). However, the process can be controlled by slowing
down oxidation (Assassi and Benharrats, 2021; Chasse,
Scardino, and Swain, 2020; Wang et al., 2020; Onyeachu et al., 2019;
Tansug et al., 2014). The adhesion
strength between the coating material and the ferrous metal surface is
influenced by the level of surface roughness. The rough iron plate specimens
produced areas with an unstable surface structure that experienced greater
corrosion due to the uneven distribution of the passive layer.
Several materials previously used as
corrosion inhibitor, include polyaniline, metal alloy, and imidiazole.
Furthermore, inhibitor material characteristics are surface area, small pore
size and heteroatom with N and O, lone pair electrons, as well as metal with
lower potential reduction standard (Mulyani et
al., 2023; Ningrum et al., 2023; Riyanto
et al., 2023; Assassi and Benharrats,
2021).
Sodium silicate is a chemical compound
that is often used as corrosion inhibitor due to its environmentally
friendliness and low cost (Mulyani et al.,
2023; Da-Silva, Saji, and Aoki, 2022; Saji, 2019). In coating
application, a mixture of silica from natural sand and rice husk ash serves as
a natural inhibitor for reinforcing concrete structures (Marzorati, Verotta, and Trasatti, 2019; Awizar et al., 2013). This study was
conducted specially to optimize the use of Sinabung volcanic ash as silica
precursor and coating material for corrosion inhibitor to protect the ferrous
metal from corrosion.
2.1. Preparation of Silicate from
Volcanic ash
The preparation
of silicate comprised
soaking 20 g of volcanic ash in 37 % (v/v) HCl (E-Merck)
for 2 hours at a temperature of 95°C with continuous stirring. After
filtration, the residue was rinsed in distilled water until reaching pH 7, then dried in an oven at 120 °C
for 6 hours. The dried volcanic ash was extracted with a 4, 6, or 8 M NaOH
solution (E-Merck) and boiled while stirring until the mixture thickened. The mixture was
then placed in a
furnace at 750 °C for 3 hours. After cooling, 200 mL of distilled water was
added, and the mixture was left overnight before being filtered. A total of 20 mL of Na2SiO3
solution was placed into a plastic container, and a few drops of 3M HCl
solution were added while stirring to form a white gel and neutral pH. Silica gel was filtered and rinsed
with distilled water, followed by drying in an oven at 120 °C. Silica yield from volcanic ash was calculated using Equation 1.
%
silica =
The schematic representation of the preparation of silica from volcanic ash is shown in Figure 1.
Figure 1 A Schematic Representation of the
Preparation of Silica from Volcanic Ash
Atomic Absorption Spectroscopy
(AAS)Z-2000 series was performed to determine silica content in the Na2SiO3
solution. FTIR SHIMADZU, Rigaku ZSX, XRD Perkin Elmer 3110 Shimadzu XRD 6000,
and SEM Zeiss type EPOMH 10 Zss were used to characterize the physicochemical
properties of material.
2.2. Corrosion
Testing of Iron Samples
Iron plate 3 × 3 cm2 with a thickness of 3
mm was used for corrosion testing. The samples were pre-treated with sandpaper
of varying grit numbers 800, 1200, 1500, and 2000 to smoothen and remove
scratches on the surface. Each iron plate grit was soaked in Inhibitor for 5 days.
Subsequently, the uncoated and coated iron plates were dipped in a corrosive
solution containing 15 % (v/v) HCl and 3.5 % (w/v)
NaCl for 96 hours. The HCl solution represents an acidic environment while NaCl
represents a salty atmosphere conducive to corrosion. Sets of silica-coated and
uncoated iron plates were analyzed using SEM and XRD before and after corrosion
tests.
Peaks at 3356.89 cm-1,
3454.12 cm-1, and 3446.02 cm-1, showed the presence of OH
strain vibrations from Si-OH, as presented in Figure 2. Furthermore, Si-O
asymmetric stretching vibrations in Si-O-Si were characterized by band
absorptions at 1184.45 cm-1 and 1095.57 cm-1, represented
by a wide and sharp peak in the 1000-1100 cm-1 wavenumber range. A
peak was observed at wave numbers 796.42 cm-1 and 789.21 cm-1,
which showed Si-O-Si stretching vibrations. The presence of the Si-O-Si
functional group was confirmed by the peaks observed at 326.46 cm-1,
attributed to the bending vibration, in both 6M and 8M NaOH solutions.
The XRD pattern, as presented in Figure 2, showed that silica
gel produced from the 3 variations of NaOH was amorphous, characterized by a
broad peak at = 23.36°;
= 22.68°;
= 23.40°, with the highest intensity
being
= 23.40°. The diffraction pattern, with a peak, widened around
=
20-24°, indicated a low crystallinity amorphous structure (Simatupang et al., 2018).
SEM image showed the existence of rough and irregular pore
cavities, as presented in Figure 3. The presence of amorphous silica was also
confirmed by the XRD results. Non-crystalline or amorphous silica possesses
pores with atoms or molecules arranged in random and irregular patterns, as
well as complex spherical structures.
Figure 2 (1) FTIR spectra of silica gel prepared using (A) 4M NaOH, (B) 6M NaOH, and (C) 8M NaOH, and (2) XRD pattern spectra of silica gel prepared using (A) 4M NaOH, (B) 6M NaOH, and (C) 8M NaOH
Figure 3 SEM Image of Silica Gel
Prepared Using (a) 4M NaOH, (b) 6M NaOH, (c) 8M NaOH
The concentration of NaOH and the length
of the extraction time affect silica formation process. The AAS data showed
that the highest silica content was discovered when volcanic ash was extracted
with 8M NaOH solution. This concentration resulted in a 79.23% (v/v) Na2SiO3
and a gel yield of 29.73% (w/w). The purpose of using 8M NaOH is due to the
higher concentration of NaOH, leading to greater extraction power.
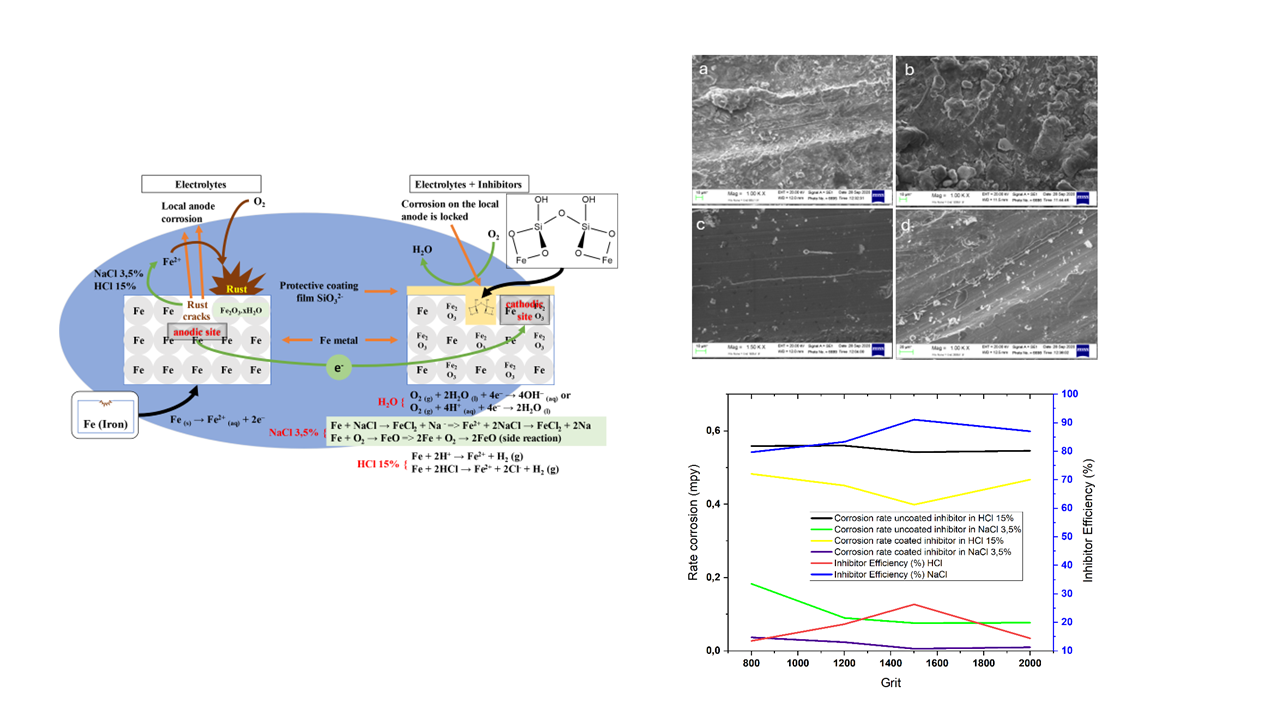
The sets of silicate-coated and uncoated iron plates were subjected to corrosion test using a corrosive solution containing 15 % HCl (v/v) and 3.5 % NaCl (w/v). The results were analyzed using SEM (Figures 4 and 5).
Figure 4 (1) SEM images grit of iron plate uncoated before treatment (a) 800 (b)
1200 (c) 1500 (d) 2000; (2) SEM images uncoated, in HCl 15 % (v/v;
(3) SEM images uncoated, in NaCl 3.5 % (w/v)
Figure 5 (1) SEM images of grit of iron plate coated before treatment, showing
(a) 800, (b) 1200, (c) 1500, and (d) 2000 grit levels; (2) SEM images of coated
iron plate after exposure to 15 % HCl (v/v); and (3) SEM
images of coated iron plate after exposure to 3.5 % NaCl (w/v)"
Figure 5
shows the morphology of iron plate after coating with silicate. The 1500-grit
iron plate has the smoothest surface compared to the other grits, which results
in a more even thickness of silicate layer. The morphology appeared to be less
perforated and fewer lumps were formed compared to uncoated iron plates with
inhibitor. This is in accordance with the theory that samples with the addition
of inhibitor will crack less, showing a smaller corrosion rate (Devianto et al., 2023; Goyal et al., 2020).
Based on the analysis data, it was observed that silicate is effective as
corrosion inhibitor.
The
reaction mechanism scheme is shown in Figure 6. Sodium silicate has an anodic
inhibitory capacity in a neutral medium. This implied that the SiO2
species migrated to the anode region of the metal surface, reacting with Fe2+
ions and forming a protective layer of iron silicate (FeSiO3).
Silicate was effective as inhibitor by reacting with OH-, thereby reducing
corrosion reaction in neutral solutions and decreasing corrosion rate. The new
peak that appeared after immersion at 2 = 30-40° was FeOOH- (Ningrum et al., 2023).
Figure 6 Mechanism of
silicate inhibition reaction
XRD patterns for all grit both uncoated and coated iron plates
in NaCl 3.5% (w/v) can be seen in Figure 7 showing that the peak on iron plate
was lower in intensity than the peak on iron plate before treatment.
The existence of a lower
intensity peak after immersion in corrosion solution showed sediment. The Fe
peak of the 2000 grit iron plate at 2 = 40-50° was sharper compared to the
1200 and 1500 grit. Furthermore, the
level of surface smoothness affected corrosion rate, with a new peak appearing at
= 20-30° for the
series of samples after immersion in NaCl 3.5% (w/v). The
intensity of each iron plate showed a
reduction in iron corrosion rate and a slight decrease in grit size variation, showing effective inhibition by
silicate inhibitor.
Corrosion test of iron plate was analyzed
for corrosion rate using the weight-loss method (Malaret, 2022). Where the CR is corrosion rate (mpy), W is mass loss (g), A is surface area (cm2), t is the exposure time (hour), D is density (g/cm3), C
is constant
3.45 x 106.
Based on Table 1, the 1500 grit iron plate, whether
silica-coated or uncoated, had lowest corrosion rate of 3.399 mpy and 0.006
mpy, alongside highest inhibitor efficiency of 26.3% and 91.1% respectively.
These results surpass those of a previous study utilizing tobacco extract and
sodium silicate as inhibitor in a3.5% NaCl (w/v) corrosive solution, which
achieved efficiencies of 24 - 69% and 79.55%, respectively.
Table 1 Data of Corrosion Rate and Inhibitor Efficiency for each Grit
of Iron Plate
|
No |
Grit |
Corrosion
Solution |
Corrosion
rate uncoated
iron (mpy) |
Corrosion
rate silicate-coated iron (mpy) |
Inhibitor
Efficiency (%) |
|
1 |
800 |
HCl
15% |
0.559 |
0.483 |
13.5 |
|
2 |
1200 |
0.560 |
0.451 |
19.4 | |
|
3 |
1500 |
0.542 |
0.399 |
26.3 | |
|
4 |
2000 |
0.546 |
0.467 |
14.4 | |
|
5 |
800 |
NaCl
3.5% |
0.183 |
0.037 |
79.7 |
|
6 |
1200 |
0.090 |
0.024 |
83.3 | |
|
7 |
1500 |
0.076 |
0.006 |
91.1 | |
|
8 |
2000 |
0.077 |
0.010 |
87.0 |
Figure 8 Corrosion Rate and Inhibitor Efficiency for each Grit
of Iron Plate uncoated silica inhibitor and coated silica inhibitor in
corrosive solutions
In conclusion, the highest silica
content from volcanic ash of Mount Sinabung was observed when 8M NaOH was used,
with a Na2SiO3 concentration of 79.23 v% (v/v)
and a yield of 29.73v% (w/w). According to the
characterization results by FTIR, the synthesized silica gel has -OH and Si-O
functional groups from Si-OH and Si-O-Si, respectively. XRD analysis suggested
that the as-synthesized silica gel had an amorphous structure. Micrograph SEM
showed rough and irregular pore cavities, while the effect of surface grit
variations on the performance of sodium silicate inhibitor synthesized in 15 %
HCl (v/v) and 3.5 % NaCl (w/v)
solutions, was lower corrosion rates. The lowest corrosion rate was observed on
the 1500 grit iron plate, and the addition of silica as inhibitor reduced the
rate in HCl 15 % (v/v) and NaCl 3.5 % (w/v)
corrosive solutions, leading to inhibitor efficiencies of 26.3 % and 91.8 %,
respectively.
The
authors acknowledge financial support from Universitas Negeri Medan for the
Financial Year 2021-2022 under Applied Product Research, Contract Numbers:
008/UN33.8/PL.PNBP/2021 and 104/UN33.8/KEP/PPKM/PT/2022.
Anderson,
K., Segall, P., 2011. Physics-based Models of Ground Deformation and Extrusion
Rate at Effusively Erupting Volcanoes. Journal of Geophysical Research:
Solid Earth, Volume 16(B7), pp. 1–20
Assassi,
F., Benharrats, N., 2021. Synthesis, Characterizations and Application of
Polyaniline-paint as Anticorrosion Agent. Inorganic and Nano-Metal Chemistry,
Volume 51(6), pp. 805–813
Awizar,
D.A., Othman, N.K., Jalar, A., Daud, A.R., Rahman, I.A., Al-Hardan, N.H., 2013.
Nanosilicate Extraction from Rice Husk ash as Green Corrosion Inhibitor. International
Journal of Electrochemical Science, 8(2), 1759–1769
Beleuk-a
Moungam, L.M., Lemougna, P.N., Kaze, R.C., Mohamed, H., Deutou-Nemaleu, J.G.,
Billong, N., Kamseu, E., Mvondo-Ze, A.D., Kenfack, I.T., 2022. Synthesis of
Volcanic Ash-based Porous Inorganic Polymers Using Biomass as Pore Inducing
Agent: Phase Evolution and Descriptive Microstructure. Silicon, Volume
14(6), pp. 2595–2608
Boonmee,
A., Jarukumjorn, K., 2020. Preparation and Characterization of Silica
Nanoparticles from Sugarcane Bagasse Ash for using as a Filler in Natural
Rubber Composites. Polymer Bulletin, Volume 77(7), pp. 3457–3472
Chasse,
K.R., Scardino, A.J., Swain, G.W., 2020. Corrosion and Fouling Study of
Copper-based Antifouling Coatings on 5083 Aluminum Alloy. Progress in
Organic Coatings, Volume 141, p. 105555
Da-Silva,
P.B., Saji, V.S., Aoki, I.V., 2022. Rapid and Eco-friendly One-step Synthesis
of Dodecylamine-encapsulated Mesoporous Silica Nanocontainers. Microporous
and Mesoporous Materials, Volume 341, pp. 112109
Devianto,
H., Nurdin, I., Widiatmoko, P., Silvia, D., Prakarsa, C., 2023. Tobacco Extract
for Inhibition of Carbon Steel Corrosion in H2S-contained NaCl Solution. International
Journal of Technology, Volume 14(5), pp. 1167–1176
El-Fargani,
H., Lakhmiri, R., El-Farissi, H., Albourine, A., Safi, M., Cherkaoui, O., 2017.
Removal of Anionic Dyes by Silica-chitosan Composite in Single and Binary Systems:
Valorization of Shrimp co-product “Crangon-Crangon” and “Pandalus Borealis.” Journal
of Materials and Environmental Science, Volume 8(2), pp. 724–739
Goyal,
M., Vashist, H., Kumar, S., Bahadur, I., Benhiba, F., Zarrouk, A., 2020. Acid
Corrosion Inhibition of Ferrous and Non-ferrous Metal by Nature Friendly
Ethoxycarbonylmethyltriphenylphosphonium Bromide (ECMTPB): Experimental and MD
Simulation Evaluation. Journal of Molecular Liquids, Volume 315, p.
113705
Hasanah,
M., Sembiring, T., Sebayang, K., Humaidi, S., Rahmadsyah, Saktisahdan, T.J.,
Handoko, F., Ritonga, S.I., 2021. Extraction Of Silica Dioxide (SiO2)
From Mount Sinabung Volcanic Ash with Coprecipitation Method. In: IOP
Conference Series: Materials Science and Engineering, Volume 1156(1), p. 012015
Iguchi,
M., Surono, Nishimura, T., Hendrasto, M., Rosadi, U., Ohkura, T., Triastuty,
H., Basuki, A., Loeqman, A., Maryanto, S., Ishihara, K., Yoshimoto, M., Nakada,
S., Hokanishi, N., 2012. Methods for Eruption Prediction and Hazard Evaluation
at Indonesian Volcanoes. Journal of Disaster Research, Volume 7(1), pp.
26–36
Javaherdashti,
R., 2000. How Corrosion Affects Industry and Life. Anti-Corrosion Methods
and Materials, Volume 47(1), pp. 30–34
Karolina,
R., Syahrizal, S., Putra, M.A., Prasetyo, T.A., 2015. Optimization of the use
of Volcanic Ash of Mount Sinabung Eruption as the Substitution for Fine
Aggregate. Procedia Engineering, Volume 125, pp. 669–674
Karolina,
R., Syahrizal., M.A.P., Handana., Wijaya, B., 2020. Utilization of Volcanic Ash
of Mount Sinabung as a Substitute for Cement to Flexure Strength of Geopolymer
Concrete. Icosteerr 2018, pp. 332–337
Lubis,
M., Sukeksi, L., Harahap, M.B., Ginting, M., Wici, H., Ayu, G.E., 2019. Use of
Silica Gel from Volcanic Ash as Chitosan Composite Membrane’s Filler. Asian
Journal of Chemistry, Volume 31(10), pp. 2303–2305
Mainier, F.B., Figueiredo, A.A.M., de-Almeida Junior, A.A.M.,
Almeida-Junior, B.B., 2018a. Proposal of the use Sodium Silicate as a Corrosion
Inhibitor in Hydrostatic Testing of Petroleum Tanks using Seawater. International
Journal of Advanced Engineering Research and Science, Volume 5(6), pp.
33–38
Malaret,
F., Yang, X.S., 2022. Exact Calculation of Corrosion Rates by the Weight-loss Method.
Experimental Results, Volume 3, p. E13
Marzorati,
S., Verotta, L., Trasatti, S.P., 2019. Green Corrosion Inhibitors from Natural
Sources and Biomass Wastes. Molecules, Volume 24(1), p. 48
Mulyani,
R.W.E., Nuruddin, A., Suprijanto, Sunendar-Purwasasmita, B., 2023.
Silica-Chitosan Nanocomposite Coatings for Enhancing Hydrophilicity of
Polyester Fabric. International Journal of Technology, Volume 14(4), pp.
761–769
Nakada,
S., Zaennudin, A., Yoshimoto, M., Maeno, F., Suzuki, Y., Hokanishi, N., Sasaki,
H., Iguchi, M., Ohkura, T., Gunawan, H., Triastuty, H., 2019. Growth process of
the lava dome/flow complex at Sinabung Volcano during 2013–2016. Journal of
Volcanology and Geothermal Research, Volume 382, 120–136
Ningrum,
E.O., Khoiroh, I., Nastiti, H.I., Affan, R.A., Karisma, A.D., Agustiani, E.,
Surono, A., Suroto, H., Suprapto, S., Taji, L.S., Widiyanto, S., 2023. Surface
Coating Effect on Corrosion Resistance of Titanium Alloy Bone Implants by
Anodizing Method. International Journal of Technology, Volume 14(4), pp.
749–760
Onyeachu,
I.B., Obot, I.B., Sorour, A.A., Abdul-Rashid, M.I., 2019. Green Corrosion
Inhibitor for Oilfield Application I: Electrochemical Assessment of
2-(2-pyridyl) Benzimidazole for API X60 Steel Under Sweet Environment in NACE
Brine ID196. Corrosion Science, Volume 150, pp. 183–193
Pan,
C., Chen, N., He, J., Liu, S., Chen, K., Wang, P., Xu, P., 2020. Effects of
Corrosion Inhibitor and Functional Components on the Electrochemical and
Mechanical Properties of Concrete Subject to Chloride Environment. Construction
and Building Materials, Volume 260, p. 119724
Pan,
C., Li, X., Mao, J., 2020. The Effect of a Corrosion Inhibitor on the
Rehabilitation of Reinforced Concrete Containing Sea Sand and Seawater. Materials,
Volume 13(6), p. 1480
Prabha,
S., Durgalakshmi, D., Rajendran, S., Lichtfouse, E., 2021. Plant-derived Silica
Nanoparticles and Composites for Biosensors, Bioimaging, Drug Delivery and
Supercapacitors: a Review. Environmental Chemistry Letters, Volume
19(2), pp. 1667–1691
Riyanto,
Jazuli, M.M., Sahroni, I., Musawwa, M.M., Cahyandaru, N., Wahyuni, E.T., 2023.
A Simple Technique for the Corrosion Inhibition of Underwater Cannonball from a
Shipwreck. International Journal of Technology, Volume14(4), pp. 843–853
Saji,
V.S., 2019. Supramolecular Concepts and Approaches in Corrosion and Biofouling
Prevention. Corrosion Reviews, Volume 37(3), pp. 187–230
Salleh,
S.Z., Yusoff, A.H., Zakaria, S.K., Taib, M.A.A., Seman, A.A., Masri, M.N.,
Mohamad, M., Mamat, S., Sobri, S.A., Ali, A., Ter-Teo, P., 2021. Plant Extracts
as Green Corrosion Inhibitor for Ferrous Metal Alloys: A Review. Journal of
Cleaner Production, Volume 304, p. 127030
Silvana,
S., Sunardi, S., 2020. Synthesis and Characterization of Si02/Zn0
Nanocomposites from Zinc Waste and Mount Merapi Volcanic Ash. Journal of
Scientific and Applied Chemistry, Volume 23(10), pp. 365–369
Simatupang,
L., Devi., 2016. The preparation and characterization of Sinabung volcanic ash
as silica based adsorbent. Jurnal Pendidikan Kimia (Journal of Chemistry
Education), Volume 8(3), pp. 159–163
Simatupang,
L., Siburian, R., Sitanggang, P., Doloksaribu, M., Situmorang, M., Marpaung,
H., 2018. Synthesis and Application of Silica Gel Base on Mount Sinabung’s Fly
Ash for Cd(II) Removal with Fixed Bed Column. Rasayan Journal of Chemistry,
Volume 11(2), p. 819–827
Simatupang,
L., Situmorang, M., Marpaung, H., Siburian, R., 2020. Fabrication of
Silica-based Chitosan Biocomposite Material from Volcanic Ash and Shrimp Husk
by Sol Gel Method for Adsorbent of Cadmium (Ii) Ions. Indian Journal of
Chemical Technology, Volume 27(5), pp. 387–394
Sinuhaji,
P., Sembiring, T., Magfirah, A., Piliang, AF., Nababan, S.M., 2018. Analysis of
Composition; Topography of Volcanic Materials Erupted from Mount Sinabung, Karo
Regency, Indonesia. In: Journal of Physics: Conference Series, Volume
1116(3), p. 032035
Tansug,
G., Tüken, T., Giray, E.S., Findikkiran, G., Sigircik, G., Demirkol, O., Erbil,
M., 2014. A New Corrosion Inhibitor for Copper Protection. Corrosion Science,
Volume 84, pp. 21–29
Verma,
D.K., Khan, F., 2016. Corrosion Inhibition of Mild Steel in Hydrochloric Acid
using Extract of Glycine Max Leaves. Research on Chemical Intermediates,
Volume 42(4), pp. 3489–3506
Wang, X., Jing, C., Chen, Y., Wang, X., Zhao, G., Zhang, X., Wu, L., Liu, X., Dong, B., Zhang, Y., 2020. Active Corrosion Protection of Super-hydrophobic Corrosion Inhibitor Intercalated Mg–Al Layered Double Hydroxide Coating on AZ31 Magnesium Alloy. Journal of Magnesium and Alloys, Volume 8(1), pp. 291–300
Yeganeh, M., Omidi, M., Eskandari, M., 2018. Superhydrophobic Surface of AZ31 Alloy Fabricated by Chemical Treatment in the NiSO4 Solution. Journal of Materials Engineering and Performance, Volume 27(8), pp. 3951–3960
