Synthesis of Mesoporous Silica from Sugarcane Bagasse as Adsorbent for Colorants Using Cationic and Non-Ionic Surfactants
Corresponding email: donanta.dhaneswara@ui.ac.id
Published at : 05 Feb 2024
Volume : IJtech
Vol 15, No 2 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i2.6721
Dhaneswara, D., Tsania, A., Fatriansyah, J.F., Federico, A., Ulfiati, R., Muslih, R., Mastuli, M.S., 2024. Synthesis of Mesoporous Silica from Sugarcane Bagasse as Adsorbent for Colorants Using Cationic and Non-Ionic Surfactants. International Journal of Technology. Volume 15(2), pp. 373-382
| Donanta Dhaneswara | 1. Department of Metallurgical and Materials Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, Jawa Barat, 16242, Indonesia, 2. Advanced Materials Research Center (AMRC), Fa |
| Audrey Tsania | Department of Metallurgical and Materials Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, Jawa Barat, 16242, Indonesia |
| Jaka Fajar Fatriansyah | 1. Department of Metallurgical and Materials Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, Jawa Barat, 16242, Indonesia, 2. Advanced Materials Research Center (AMRC), Fa |
| Andreas Federico | 1. Department of Metallurgical and Materials Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, Jawa Barat, 16242, Indonesia, 2. Advanced Materials Research Center (AMRC), Fa |
| Ratu Ulfiati | Research Center for Process and Manufacturing Industrial Technology, National Research and Innovation Agency (BRIN), Klaster Teknologi Energi, Kawasan Puspiptek, Banten 15314, Indonesia |
| Rifai Muslih | Nuclear Energy Research Organization, National Research and Innovation Agency (BRIN), Kawasan Puspiptek, Banten 15314, Indonesia |
| Mohd Sufri Mastuli | 1. School of Chemistry and Environment, Faculty of Applied Sciences, Unversiti Teknologi MARA, 40450 Shah Alam Selangor, Malaysia, 2. Centre for Functional Materials and Nanotechnology, Institute of S |
In Indonesia, the rising sugar production contributes to an increase in
sugarcane bagasse waste. This waste can be used for environmental purposes, such
as the treatment of colorants in the textile industry using mesoporous silica
materials. Previous studies showed the potential of synthesizing mesoporous
silica from agricultural waste including corn cob and rice husk. Therefore,
this study aimed to investigate the potential use of sugarcane bagasse as an
alternative waste source for synthesizing mesoporous silica. Sugarcane bagasse
ash, containing a high silica content ranging from 55.5% to 70%, served as the
main silica source for synthesis. A combination of surfactant templates,
Pluronic 123 (P123), and Cetyl Trimethyl Ammonium Bromide (CTAB) in a ratio of
1:1, was used to obtain optimal structure, surface area, pore radius, and pore
volume. These parameters were optimized to enhance the adsorption of colorants,
specifically methyl blue commonly found in textile waste. The characterization
techniques used included Small-Angle X-ray Scattering (SAXS), Scanning Electron
Microscopy (SEM), Fourier Transform Infrared Spectroscopy (FTIR),
Brunauer-Emmett-Teller (BET) analysis, and UV-Vis spectroscopy. The synthesized
mesoporous silica showed a surface area of 323.3 m2/g, a pore radius
of 2.437 Å, and a pore volume of 0.52 cc/g, with an adsorption capacity of
97.3%. This adsorbent product was expected to provide a cost-effective,
eco-friendly source of silica suitable for various dye adsorption applications
and the treatment of heavy metals in industrial waste.
Mesoporous silica; Organic colorants; Sugarcane bagasse ash; Surfactant
Indonesia is one of the largest producers of
agricultural commodities globally, including sugarcane, with a production of
2.4 million tons in 2022. The high quantity of
Bagasse waste can be burned to ash with high silica content ranging from
55.5 to 70% (Wilson and Mahmud, 2015). This
phenomenon presents a significant use of bagasse waste, specifically in the
creation of mesoporous ceramic material (Szewczyk
and Prokopowicz, 2020), characterized by a high surface area suitable
for adsorption purposes. Generally, adsorbent materials can be used in various
applications, such as in drug delivery systems, oil adsorption, heavy metals,
and dyes (Sohrabnezhad, Jafarzadeh, and Pourahmad, 2018;
Bulgariu and Bulgariu, 2018).
The application of adsorbent for dye removal is based on the urgent need
to overcome the environmental impact of azo dyes produced by textile industry
waste (Mota et al., 2019; Sudibandriyo and
Putri, 2020; Jawad et al. 2020). Moreover, the use of mesoporous
silica material as an adsorbent has shown good adsorption performance. Raya et al. (2021) conducted adsorption
testing of azo dyes methylene blue using tetraethyl orthosilicate (TEOS)-based
mesoporous silica material. The results showed a significant MB removal
percentage of 96.27% for 3 h adsorption of 100 ppm MB. However, the use of
silica material could be more efficient, considering that TEOS raw materials
are expensive, leading to high product costs. Thu et
al. (2019) fabricated rice husk biomass waste-based mesoporous
silica material to overcome the problem, but the adsorption performance was not
that high. To enhance the adsorption performance of mesoporous silica material,
one promising solution is the addition of other surfactants as cosurfactants. Dhaneswara et al. (2023) observed the
effect of Pluronic 123 (P123) and Cetyl Trimethyl Ammonium Bromide (CTAB)
surfactant ratio on the adsorption performance of methylene blue, methyl orange
on rice husk, and corn cob based mesoporous silica. The result yielded a
maximum adsorption and surface area at a P123 and CTAB ratio of 1:1.
This study aimed to synthesize mesoporous silica derived from sugarcane bagasse using the sol-gel method and testing the adsorption capacity of methyl blue. The analysis was conducted to investigate typesetting P123 and CTAB surfactants as templates and the interaction process between surfactant and precursor silica sources. The product of mesoporous silica was characterized by Small-Angle X-ray Scattering (SAXS), Scanning Electron Microscopy (SEM), Fourier Transform Infrared Spectroscopy (FTIR), Brunauer-Emmett-Teller (BET) analysis, and UV-Vis spectroscopy.
The material used in this study included silica
precursor raw material derived from sugarcane bagasse obtained at a
plantation in Blitar, East Java. The surfactants used as
templates were CTAB with a quantity of 7.5 g (Sigma-Aldrich) and P123 of 7.5 g
(Sigma-Aldrich). Hydrochloric acid (HCl) solution (Supelco) was used as the
acid solution, while sodium hydroxide (Supelco) was applied as the
base solution. Subsequently, all chemicals passed
through processes such as dilution and dissolution.
2.1. Synthesis Silica Mesoporous
Synthesis of mesoporous silica commenced
with the extraction of silica precursor from sugarcane bagasse ash (SCBA) using
a NaOH alkaline solvent (Shah et al., 2017;
Fatriansyah Situmorang, and Dhaneswara, 2018), which resulted in sodium
silicate (Na2SiO3) as the product (Equation 1). P123 and
CTAB surfactants were dissolved in an acid solution with continuous stirring
and added with Na2SO3 dropwisely. Subsequently, stirring
was continued at 28°C for 2 hours, followed by aging at 40°C for 24 hours,
resulting in hydrolysis and condensation of silica, as shown in Equations 2 and
3 (Sohrabnezhad, Jafarzadeh, and Pourahmad, 2018).
After the gel was formed, the gel was dried, followed by calcination at 550°C
for 5 h.
2.2. Characterization
Synthesized mesoporous silica was
characterized to determine its pore structure, morphology, and adsorption
capacity. The crystal structure and orientation of mesoporous silica were
characterized by SAXS Shimadzu XRD 6000 using CuK radiation (1.54 Å wavelength)
at a diffraction angle
ranging from 0.2 to 5°. Subsequently, the surface
morphology and particle shape of mesoporous silica were observed using field
emission scanning electron microscope (FE-SEM) FEI Inspect F50. The observation
was performed at magnifications of 5000x and 10,000x. The functional groups
present were determined using FTIR spectroscopy PerkinElmer in the infrared
adsorption wavelength (500-4000 cm-1). The pore structure and
characterization were evaluated using Quantachrome NOVA 1200e BET nitrogen
adsorption with the Barret-Joyner-Halenda (BJH) method. Finally, the adsorption
performance of mesoporous silica in MB was tested using Lambda 25 PerkinElmer
UV-Vis spectroscopy with wavenumbers ranging from 500 to 700 nm. The graph of the test
results was used as a reference to determine the adsorption capacity (qt) of
mesoporous silica and the percentage of methyl blue dye removed from the
solution (%removal) each hour using Equations (4) and (5):
Where
Ct and Co represent the concentrations of the Blanco
solution, and the dye solution adsorbed during a certain time, while Ao
and At shows the absorbance intensities of the Blanco solution and
the dye solution adsorbed during a certain time. qt represents the uptake
capacity at time t (mg.g-1), m is the mass of the adsorbent (g), and
V is the volume (Dhaneswara et al., 2022).
3.1. Identification of Crystal
Structure
Synthesized mesoporous silica sample was subjected to SAXS testing and the pattern obtained was shown in Figure 1. From the curve, an intense peak was observed at 1.2° and less intense peaks at 0.85° and 2°, representing (110), (200), and (211) planes, respectively. This confirmed the long-range order mesostructure of mesoporous silica (Dhaneswara et al., 2022).
3.2. Surface Morphology
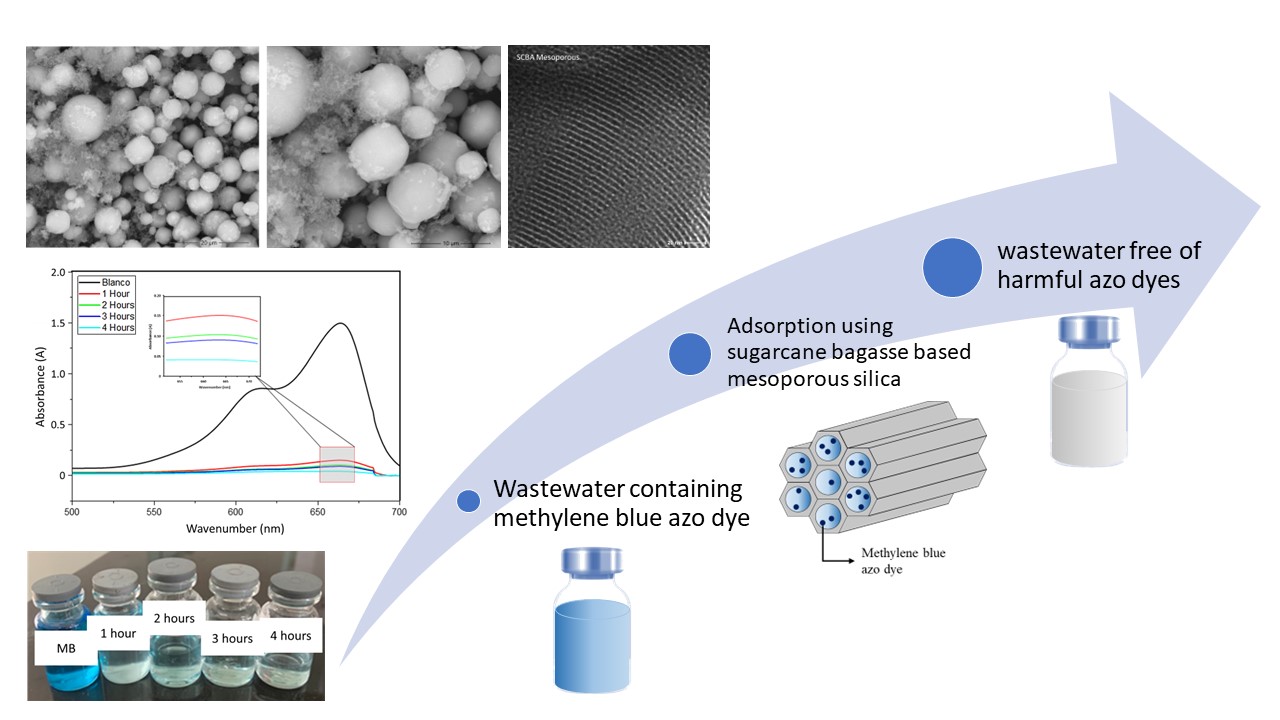
Based on the data from SEM analysis shown in Figure 2 at magnifications of 5000x and 10,000x, it was observed that mesoporous material synthesized using silica precursor from sugarcane bagasse adopted a spherical geometry. The properties of P123 lead to the formation of coarse aggregates among the perfectly agglomerated spherical particles. According to the literature, a surfactant ratio of 1:1 between CTAB and P123 resulted in the formation of perfectly agglomerated spherical particles. This ratio allows CTAB in P123 surfactant to influence the particle morphology through the formed micelles (Henao et al., 2020). The role of CTAB includes dehydrating the polypropylene oxide (PPO) groups on the carbon chain, and reducing the hydrophobic volume of the micelles (Henao et al., 2020). The reduction in PPO chains contributes to an increase in the ah/ac value in the packing parameters of the micelle, where ah represents the effective area of the hydrophilic chain, and as is the length of the hydrophobic chain (Nassar, Ahmed, and Raya, 2019). When CTAB concentration increases, resulting particles tend to adopt a spherical shape due to the interconnection of the hydrophobic alkyl chains, followed by the hydrophilic heads that have bonded with silica, merging and forming a bilayer (Nassar, Ahmed, and Raya, 2019). Consequently, SEM results suggest the presence of a few coarse aggregates due to an excessive concentration of P123. These coarse aggregates may be attributed to inaccuracies in weighing the P123 surfactant, which should be in a 1:1 ratio between P123 and CTAB. In addition to SEM, the morphology of mesoporous silica was also observed by using TEM, as shown in Figure 3. TEM images confirmed that mesoporous silica showed the ordered pore structure in the radial arrangement, showing the spherical-shaped particles (Abboud et al., 2020).
Figure 2 SEM images of SCBA Mesoporous: (a) 5000x magnification and (b) 10,000x magnification
Figure 3 TEM images of the obtained SCBA mesoporous at: (a) x 100 nm, (b) x 50 nm, and (c) x 20 nm magnification
3.3. Adsorption-Desorption
Curve
In BET analysis, the results showed crucial parameters such as surface area, pore radius, and pore volume of mesoporous silica material derived from sugarcane bagasse. This analysis also served to confirm the structural characteristics of a material through the formation of the adsorption-desorption isotherm curve, as shown in Figure 4. Synthesized mesoporous silica using sugarcane bagasse precursor tends to show Type IV in the classification of the adsorption isotherm curve according to IUPAC (Pingarrón et al., 2020). Type IV showed that the resulting mesoporous silica had mesopores. characterized by an almost horizontal line in the high relative pressure region. Meanwhile, when mesoporous adsorbate contained macropores, the horizontal line would not form (Wang et al., 2022). The adsorption in this type occurs due to interactions between the adsorbate-adsorbent and the adsorbate molecules in the condensed phase. Condensation is the phenomenon of a gas being condensed into a liquid-like phase in the pores (Thahir et al., 2019). Mesoporous silica derived from sugarcane bagasse precursor showed Type H1, showing a narrow pore size distribution (Pingarrón et al., 2020). Type H1 is commonly found in mesoporous silicas with uniform pores, such as MCM-41, MCM-48, and SBA-15 (Pingarrón et al., 2020). In this type, the pore network has minimal influence, resulting in steep, narrow curves, and loops, showing delayed condensation (Kingchok and Pornsuwan, 2020). This showed that mesoporous silica derived from sugarcane bagasse precursor contained interconnected pores forming a network with narrowed pore sizes, resembling an ink bottle shape.
Figure 4 Nitrogen
adsorption-desorption isotherm curve for SCBA mesoporous
3.4. Surface Area and Pore
Characteristics
BET analysis was conducted on mesoporous silica to determine the surface area and pore characteristics of the tested sample. The results showed that the tested mesoporous silica had a surface area of 323.297 m²/g, a pore radius of 24.37 Å, and a pore volume of 0.523 cc/g, as presented in Table 1. The surface area, pore radius, and pore volume characterized using BET were influenced by the mass content of the template used, namely P123 and CTAB (Dhaneswara et al., 2023; Pal, Lee, and Cho, 2020). A previous study showed the significant effect of using CTAB surfactant in decreasing the pore radius. This occurred because CTA+ ion in CTAB-Pluronic system affected hydrophilicity of Pluronic by incorporating the alkyl tail of the cationic surfactant, CTAB, into the polypropylene oxide (PPO) core (Tseng et al., 2017). This leads to a more hydrophilic PPO-PEO interface due to the reduction in PPO volume (Tseng et al., 2017). The presence of H1-type hysteresis loop showed a narrow pore size distribution and uniform particle sizes, observed in the distribution curve presented in Figure 5 (a). This narrow pore size distribution originated from the capillary condensation stage during silica condensation, a process that enhanced the regularity of mesoporous structure and improved its textural properties, such as the pore volume shown in Figure 5 (b) (Thahir et al., 2019).
Table 1 Physical properties of SCBA mesoporous sample obtained from N2 adsorption
measurements
|
Physical Properties |
Value |
|
Surface Area (m2/g) |
323.297 m2/g |
|
Pore Radius (Å) |
24.37 Å |
|
Pore Volume (cc/g) |
0.523 cc/g |
Figure 5 Pore size determination of SCBA mesoporous sample: (a) BJH desorption particle size distribution; (b) the relation between pore size and pore volume
3.5. Identification of
Functional Groups
FTIR
analysis results showed that peak wavelengths reflected the types of bonds
formed in mesoporous silica structure, including O-H, H-O-H, and Si-O-Si
groups, as shown in Figure 6. The transmission at peak wavelengths shows the
amount of groups formed in mesoporous silica. Moreover, lower transmission at
peak wavelengths suggests a higher content of formed groups in mesoporous
silica at specific wavenumbers. Table 2 shows the types of bonds formed in
mesoporous silica along with the respective transmissions. At a wavenumber of
3401 cm-1, a gradual curve shows the presence of O-H stretching
bonds in mesoporous silica (Baumgartner et al.,
2019). O-H bonds identified originate from remnants of the precursors
that are still present in the pore walls and have not fully decomposed. Another
identified group is H-OH functional group at a wavelength of 1633 cm-1 related to the water structure, showing the presence of absorbed H-O-H
vibration bonds (Baumgartner et al., 2019).
H-OH groups originate from water molecules or by-products of reactions trapped
in the Si-O-Si groups (Rashid et al., 2019).
Furthermore, the presence of hydroxyl groups, including O-H and H-OH, can
enhance the hydrophilic properties and modify the reactivity of the pore
channels (Rashid et al., 2019). In
mesoporous silica synthesized through the sol-gel process, Si-O-Si groups are
divided into three types, namely asymmetric Si-O-Si at a wavenumber of 1072 cm-1,
symmetric Si-O-Si at 789 cm-1, and Si-O-Si torque at a 443 cm-1.
The formation of Si-O-Si groups, which are the most abundant, shows the success
of the sol-gel process in producing mesoporous silica (Purwaningsih
et al. 2018).
Figure 6 FTIR spectra for SCBA Mesoporous
Table 2 The types of bonds in SCBA Mesoporous
|
Bonds |
SCBA Mesoporous | |
|
O-H |
Wavenumber (cm-1) |
3401 |
|
Transmittance (%) |
98.7 | |
|
H-OH |
Wavenumber (cm-1) |
1633 |
|
Transmittance (%) |
98.72 | |
|
Si-O-Si (Asymmetry) |
Wavenumber (cm-1) |
1072 |
|
Transmittance (%) |
78.7 | |
|
Si-O-Si (Symmetry) |
Wavenumber (cm-1) |
789 |
|
Transmittance (%) |
92 | |
|
Si-O-Si (Torque) |
Wavenumber (cm-1) |
443 |
|
Transmittance (%) |
63.4 | |
3.6. Adsorption of Methyl Blue
The absorption testing of mesoporous silica for methyl blue solution was conducted for 4 hours with an initial concentration of 10 ppm. Samples of the absorbed mesoporous silica that were synthesized from sugarcane bagasse precursor were taken every hour and compared with the blanco solution. The results of UV-Vis testing for the blanco solution of methyl blue, along with the variation in absorption over time, are presented in Figure 7 (a). In the Blanco solution, the peak with the highest absorbance at a wavenumber of 664 nm and an absorbance intensity of 1.49 nm showed red visible light (Dhaneswara et al., 2020). This red spectrum would reflect the blue color in the increasingly concentrated methyl blue solution (Pratiwi and Nandiyanto, 2022). Furthermore, it was observed that the methyl blue solution absorbed by mesoporous silica has a lower absorbance intensity compared to the blanco solution. The decrease in absorbance intensity at the maximum wavenumber reduces as the absorption time with mesoporous silica adsorbent increases. Based on the results, the sample absorbed for 4 hours has the lowest intensity, reaching 0.0409 AU. The calculated uptake capacity and %Removal results are presented in Table 3. From the data, synthesized mesoporous silica showed potential as an adsorbent for methyl blue solution. This was evidenced by a %removal exceeding 90% over 4 hours. The adsorption mechanism of methyl blue included the migration of the dye solution to the surface of mesoporous silica due to interactions between adsorbate and adsorbent. Methyl blue, being a cationic dye, would interact electrostatically with the negatively charged silica at normal pH (pH = 7). As presented in Figure 7 (b), the absorption of methylene blue by mesoporous silica for 4 hours resulted in a nearly colorless solution.
Table 3 The result of adsorption uptake capacity and %removal calculation of
SCBA mesoporous
|
Dyes |
|
1 Hour |
2 Hours |
3 Hours |
4 Hours |
|
Methylene Blue |
qt (mg/g) |
9 |
9.4 |
9.32 |
9.73 |
|
%Removal |
90% |
94% |
93.20% |
97.30% |
Figure 7 (a) Adsorption Spectra of aqueous methyl blue
dye solution in the presence of adsorbent; (b) visual image of aqueous methyl
blue dye solution in the presence of adsorbent
In conclusion, this study successfully synthesized mesoporous silica
material using a precursor derived from sugarcane bagasse, with cationic (CTAB)
and non-ionic (P123) surfactant template masses. This process resulted in a
two-dimensional body-centered cubic (BCC) crystal structure with Im3m symmetry.
The surface morphology of SCBA mesoporous material consisted mostly of
spherical particles and some coarse aggregates influenced by the surfactants
used. The synthesized material showed a surface area of 323.297 m²/g, a pore
radius of 24.37 Å, and a pore volume of 0.523 cc/g. H1-type isotherm curve
suggested a narrow and uniform pore size distribution, contributing to an
increased pore volume. FTIR analysis results showed spectra indicating the
presence of O-H, H-O-H, and Si-O-Si groups. The presence of three Si-O-Si
groups in synthesized mesoporous silica showed the successful sol-gel process.
After a 4-hour contact time, SCBA mesoporous material showed the capability to
adsorb methyl blue dye with a removal efficiency of 97.3% and an uptake
capacity of 9.73 mg/g.
This study was
supported by a research grant from the Indonesian Ministry of Education,
Culture, Research, and Technology No.NKB-1143/UN2.RST/HKP.05.00/2023. The
authors are grateful to the Advanced Materials Laboratory DTMM-FT, Universitas
Indonesia.
Abboud,
M., Sahlabji, T., Haija, M.A., El-Zahhar, A.A., Bondock, S., Ismail, I., Keshk,
S.M., 2020. Synthesis and Characterization of
Lignosulfonate/Amino-Functionalized SBA-15 Nanocomposites for the Adsorption of
Methylene Blue from Wastewater. New Journal of Chemistry, Volume 44(6),
pp. 2291–2302
Baumgartner,
B., Hayden, J., Loizillon, J., Steinbacher, S., Grosso, D., Lendl, B., 2019. Pore
Size-Dependent Structure of Confined Water in Mesoporous Silica Films from
Water Adsorption/Desorption Using Atr–Ftir Spectroscopy. ACS Publications,
Volume 35(37), pp. 11986–11994
Bulgariu,
L., Bulgariu, D., 2018. Functionalized Soy Waste Biomass - A Novel
Environmental-Friendly Biosorbent for the Removal of Heavy Metals from Aqueous
Solution. Journal of Cleaner Production, Volume 197, pp. 875–885
Dhaneswara,
D., Fatriansyah, J.F., Situmorang, F.W., Haqoh, A.N., 2020. Synthesis of Amorphous
Silica from Rice Husk Ash: Comparing Hcl and CH3COOH Acidification Methods and
Various Alkaline Concentrations. International Journal of Technology,
Volume 11(1), pp. 200–208
Dhaneswara,
D., Marito, H.S., Fatriansyah, J.F., Sofyan, N., Adhika, D.R., Suhariadi, I.,
2022. Spherical SBA-16 Particles Synthesized from Rice Husk Ash and Corn Cob
Ash for Efficient Organic Dye Adsorbent. Journal of Cleaner Production,
Volume 357, p. 131974
Dhaneswara,
D., Zulfikar, N., Fatriansyah, J.F., Mastuli, M.S., Suhariadi, I., 2023.
Adsorption Capacity of Mesoporous SBA-15 Particles Synthesized from Corncobs
and Rice Husk at Different CTAB/P123 Ratios and Their Application for Dyes
Adsorbent. Evergreen, Volume 10(2), pp. 924–930
Fatriansyah,
J.F., Situmorang, F.W., Dhaneswara, D., 2018. Ekstraksi silika dari sekam padi:
metode refluks dengan NaOH dan pengendapan menggunakan asam kuat (HCl) dan asam
lemah (CH3COOH) [Extraction of silica from rice husk: reflux method with NaOH and precipitation
using strong acid (HCl) and weak acid (CH3COOH)]. In: Prosiding Seminar
Nasional Fisika Universitas Riau, Volume 3, pp.123-127
Jawad, A.H., Hum, N.N.M.F., Farhan, A.M., Mastuli,
M.S., 2020. Biosorption of methylene blue dye by rice (Oryza sativa L.) straw:
adsorption and mechanism study. Desalin Water Treat, Volume 190,
pp.322-330
Kingchok, S., Pornsuwan, S., 2020. Comparison of Spherical and Rod-Like Morphologies of SBA-15
For Enzyme Immobilization. Journal of Porous Materials, Volume 27(5), pp.
1547–1557
Mota,
T.L.R., Gomes, A.L.M., Palhares, H.G., Nunes, E.H.M., Houmard, M., 2019.
Influence of the Synthesis Parameters on the Mesoporous Structure and
Adsorption Behavior of Silica Xerogels Fabricated by Sol–Gel Technique. Journal
of Sol-Gel Science and Technology, Volume 92(3), pp. 681–694
Nassar,
M.Y., Ahmed, I.S., Raya, M.A., 2019. A Facile and Tunable Approach for
Synthesis of Pure Silica Nanostructures from Rice Husk for the Removal of
Ciprofloxacin Drug from Polluted Aqueous Solutions. Journal of Molecular
Liquids, Volume 282, pp. 251–263
Pal, N.,
Lee, J.H., Cho, E.B., 2020. Recent Trends in Morphology-Controlled Synthesis
and Application of Mesoporous Silica Nanoparticles. Nanomaterials,
Volume 10(11), pp. 2122
Pingarrón,
J.M., Labuda, J., Barek, J., Brett, C.M.A., Camões, M.F., Fojta, M., Hibbert,
D.B., 2020. Terminology of Electrochemical Methods of Analysis (IUPAC
Recommendations 2019). Pure and Applied Chemistry, Volume 92(4), pp.
641–694
Pratiwi,
R.A., Nandiyanto, A.B.D., 2022. How to Read and Interpret UV-VIS Spectrophotometric
Results in Determining the Structure of Chemical Compounds. Indonesian
Journal of Educational Research and Technology, Volume 2(1), pp. 1–20
Purwaningsih,
H., Pratiwi, V.M., Purwana, S.A.B., Nurdiansyah, H., Rahmawati, Y., Susanti,
D., 2018. Fabrication of Mesoporous Silica Nanoparticles by Sol Gel Method
Followed Various Hydrothermal Temperature. AIP Conference Proceedings,
Volume 1945(1), pp. 1–8
Rashid,
R., Afroze, F., Ahmed, S., Miran, M.S., Bin, A., Susan, H., 2019. Control of
the Porosity and Morphology of Ordered Mesoporous Silica by Varying Calcination
Conditions. Materials Today: Proceedings, Volume 15, pp. 546–554
Raya,
I., La Nafie, N., Thahir, R., Yasser, M., Ismail, S., 2021. Prepare and utilize
mesoporous silica SBA-15 for efficient photocatalytic adsorption of methylene
blue and copper (II). Journal of Physics: Conference Series, Volume 2049(1),
pp. 012083
Shah,
B.A., Patel, A. V, Bagia, M.I., Shah, A. V, 2017. Green Approach towards the Synthesis
of MCM-41 from Siliceous Sugar Industry Waste. International Journal of
Applied Chemistry, Volume 13(3), pp. 497–514
Sohrabnezhad,
S., Jafarzadeh, A., Pourahmad, A., 2018. Synthesis and Characterization of
MCM-41 Ropes. Materials Letters, Volume 212, pp. 16–19
Sudibandriyo,
M., Putri, F.A., 2020. The Effect of Various Zeolites as an Adsorbent for
Bioethanol Purification Using a Fixed Bed Adsorption Column. International
Journal of Technology, Volume 11(7), pp. 1300–1308
Szewczyk,
A., Prokopowicz, M., 2020. Mesoporous Silica Pellets–A Promising Oral Drug
Delivery System? Journal of Drug Delivery Science and Technology, Volume
56, pp. 101491
Thahir,
R., Wahab, A.W., Nafie, N. La, Raya, I., 2019. Synthesis of High Surface Area
Mesoporous Silica SBA-15 By Adjusting Hydrothermal Treatment Time and the
Amount of Polyvinyl Alcohol. Open Chemistry, Volume 17(1), pp. 963–971
Thu,
H.T., Dat, L.T., Tuan, V.A., 2019. Synthesis of mesoporous SiO2 from rice husk
for removal of organic dyes in aqueous solution. Vietnam Journal of
Chemistry, Volume 57(2), pp.175-181
Tseng,
H.H., Chuang, H.W., Zhuang, G.L., Lai, W.H., Wey, M.Y., 2017.
Structure-controlled Mesoporous SBA-15-Derived Mixed Matrix Membranes for H2 Purification
and CO2 Capture. International Journal of Hydrogen Energy, Volume
42(16), pp. 11379–11391
Wang,
Y., Zhuang, Y., Wang, S., Liu, Y., Kong, L., Li, J., Chen, H., 2022.
Preparation and Characterization of Porous Palygorskite/Carbon Composites
through Zinc Chloride Activation for Wastewater Treatment. Clays and Clay
Minerals, Volume 70(3), pp. 450–459
Wilson,
L.D., Mahmud, S.T., 2015. The Adsorption Properties of Surface-Modified
Mesoporous Silica Materials with ß-cylodextrin. International Journal of
Technology, Volume 6(4), pp. 533–545
