The Effect of Umbilical Cord Blood Serum and Platelet-Rich Plasma Coatings on the Characteristics of Poly(-caprolactone) Scaffolds for Skin Tissue Engineering Applications
Corresponding email: retno.wahyu01@ui.ac.id
Published at : 07 Dec 2023
Volume : IJtech
Vol 14, No 7 (2023)
DOI : https://doi.org/10.14716/ijtech.v14i7.6709
Nurhayati, R.W., Laksono, A.L., Salwa, A., Pangesty, A.I., Whulanza, Y., Mubarok, W., 2023. The Effect of Umbilical Cord Blood Serum and Platelet-Rich Plasma Coatings on the Characteristics of Poly (-caprolactone) Scaffolds for Skin Tissue Engineering Applications. International Journal of Technology. Volume 14(7), pp. 1596-1604
| Retno Wahyu Nurhayati | - Universitas Indonesia - |
| Auzan Luthfi Laksono | Universitas Indonesia |
| Assyafiya Salwa | Universitas Indonesia |
| Azizah Intan Pangesty | Universitas Indonesia |
| Yudan Whulanza | Universitas Indonesia |
| Wildan Mubarok | Osaka University |
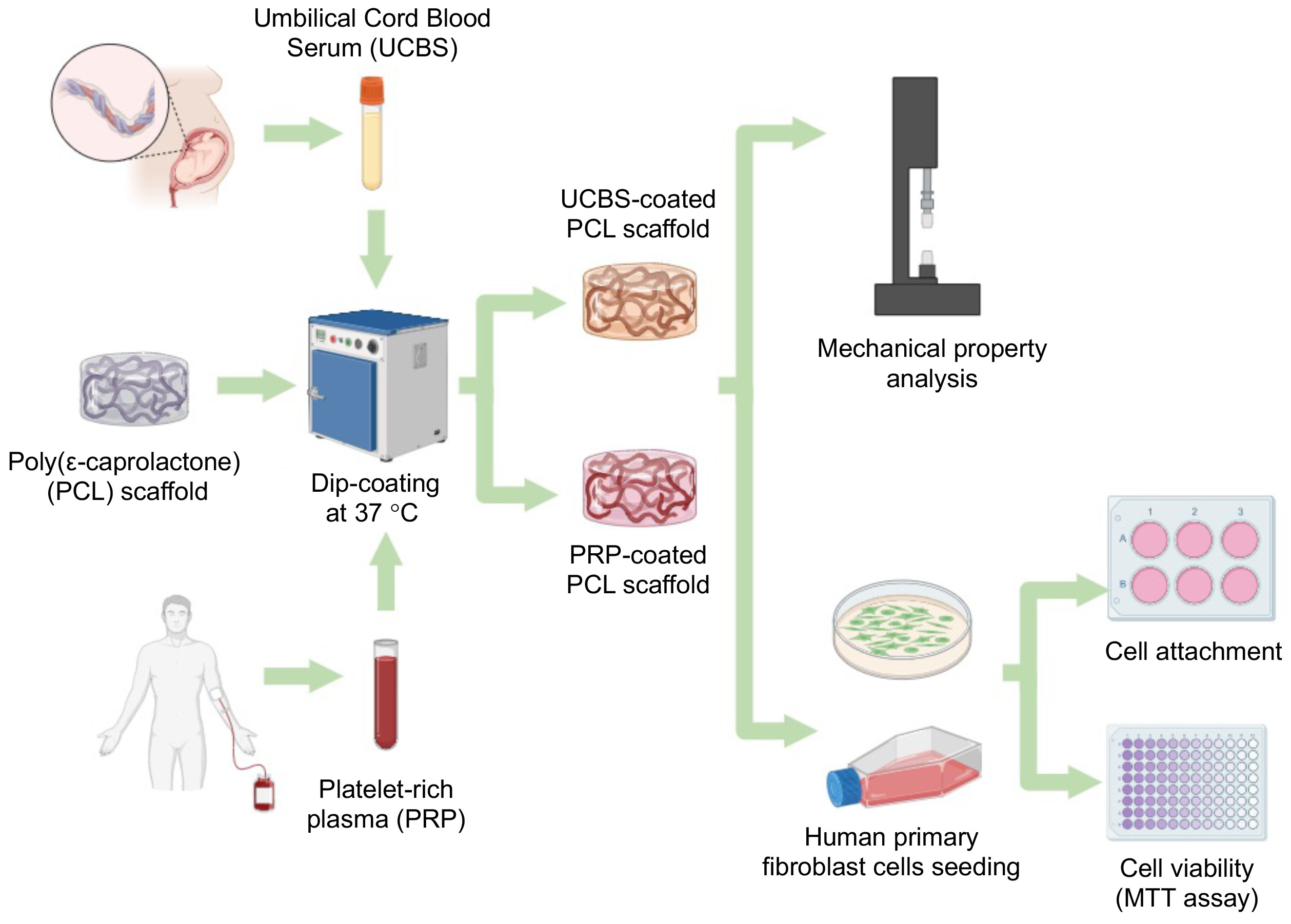
The need for effective artificial skin as a substitute
for damaged skin in chronic wound therapy is recently growing.
Poly(caprolactone) (PCL) has been identified as a potential material for
artificial skin scaffolds due to its exceptional mechanical properties and
biocompatibility. However, PCL lacks sufficient bioactivity, necessitating the
introduction of bioactive molecules to scaffolds. Human umbilical cord blood
serum (UCBS) and platelet-rich plasma (PRP), rich in bioactive molecules, are
promising coating materials for PCL-based scaffolds. Therefore, this research
aimed to investigate the effect of UCBS and PRP coatings on the mechanical
properties, cytotoxicity, and cell attachment ability of PCL scaffolds.
Scaffolds prepared through glutaraldehyde-mediated cross-linking of 20% (w/v)
PCL followed by freeze-drying were immersed with UCBS or PRP overnight. Coating
scaffolds with UCBS generated a significantly lower Young’s modulus (0.20 MPa)
compared to non-coated counterparts (0.27 MPa), while PRP-coated scaffolds
showed no substantial change (0.24 MPa). Both UCBS and PRP coatings
significantly increased (p < 0.05) the viability and attachment of primary
human fibroblast cells on scaffolds, showing the potential to enhance PCL
cytocompatibility for artificial skin.
Artificial skin; Fibroblasts; Human umbilical cord blood serum; Platelet-rich plasma; Poly(-caprolactone)
Natural A chronic wound is a significant healthcare challenge, affecting a large population globally, with an estimated occurrence ranging from 1.51 to 2.21 cases per 1,000 individuals (Martinengo et al., 2019). This is commonly managed with skin graft therapy, but the process presents various limitations, such as donor shortages, risk of disease transmission, immunogenicity, and high medical costs (Chandika et al., 2021). In response, the development of artificial skin has become a promising alternative treatment.
Artificial skin,
comprising cells, scaffolds, and bioactive molecules, holds great potential for
tissue regeneration and wound healing (Chung et
al., 2020). While cells and bioactive molecules are widely
available, current research focuses on identifying biologically and
physicochemically compatible scaffolds to create effective artificial skin
constructs.
Poly(caprolactone)
(PCL), a hydrophobic semi-crystalline polyester synthesized by ring-opening
polymerization of
caprolactone (Homaeigohar dan Boccaccini, 2022), possesses desirable properties for
scaffolds applications in tissue engineering (Vach-Agocsova
et al., 2023; Gao et al.,
2018; Siddiqui et al., 2018), including artificial skin. Despite
its biocompatibility, mechanical strength, low melting point (60°C), and
versatility in producing various shapes and porous structures, PCL has
relatively low inherent bioactivity (Petretta et
al., 2021). This limitation necessitates the incorporation of
additional bioactive molecules on the scaffold's surface.
Surface properties,
including surface topography, hydrophilicity, and chemical composition, often
influence cell-substrate interactions (Dewi et
al., 2020). Previous research explored the application of collagen
in coatings for promoting cell adhesion (Sharif et
al., 2017) and the modification of growth factors such as vascular
endothelial growth factors (VEGF) and bone morphogenic protein-2 (BMP-2) on
PCL-based scaffolds (Qin et al., 2022; Suárez-González et
al., 2012). However, the
limited availability, high cost, and immune-stimulating potential of these
factors, specifically those from non-human sources, pose significant challenges
(Mariani et al., 2019). Umbilical
cord blood serum (UCBS) and platelet-rich plasma (PRP) are promising
alternative sources rich in bioactive molecules that can enhance PCL scaffold
bioactivity. UCBS contains cytokines and extracellular matrix proteins (Maharajan et al., 2021), along with growth
factors produced by Umbilical Cord Mesenchymal Stem Cells (Nurhayati et al., 2021a). Meanwhile, PRP
comprises platelets, growth factors, and fibrinogen crucial for cell attachment
and migration (Maharajan et al., 2021;
Nurhayati et al., 2021a; Devereaux et al., 2020; Pavlovic et al., 2016).
Coating PCL
scaffolds with UCBS/PRP allows bioactive molecules to interact with seeded
cells, promoting attachment, proliferation, and functional behavior (Francavilla and O’Brien, 2022; Wheeler and Yarden, 2015).
Moreover, the immunomodulatory properties of UCBS and PRP can modulate
the immune response and mitigate inflammation, reducing rejection of the
artificial skin constructs (Sriram et al., 2023; Lotfinejad et al.,
2021). Using UCBS/PRP
as coating materials presents a cost-effective and easily accessible solution
compared to commercial growth factor supplements. properties.
Despite the
advantages of UCBS and PRP, their applications as coating materials have not
been explored. Therefore, this research aimed to investigate the effect of UCBS
and PRP coatings on the characteristics of PCL scaffolds for skin tissue
engineering, as well as evaluate the impact exerted on the viability and
adhesion of primary human fibroblast cells (Figure 1).
2.1. Ethical Clearance
Ethical clearance with Approval No.
KET-1003/UN2.F1/ETIK/PPM.00.02/2021 was obtained from the Ethical Committee for
Medical Research of the Faculty of Medicine Universitas Indonesia-Dr. Cipto
Mangunkusumo General Hospital.
2.2. Materials
The materials
used in this research included PCL (Mn=80,000), acetic acid, glutaraldehyde,
and calcium chloride (CaCl2), purchased from Sigma-Aldrich (St.
Louis, MO, USA). Additionally, outdated human PRP was obtained from the
Indonesian Red Cross (Jakarta, Indonesia). Dimethylsulfoxide (DMSO) was
procured from Molecular Probes (Eugene, OR, USA). Dulbecco's Modified Eagle
Medium low glucose (DMEM; glucose concentration 1 g/L), fetal bovine serum
(FBS), penicillin/streptomycin, phosphate-buffered saline (PBS; pH 7.4), and trypan
blue were acquired from Gibco (New York, NY, USA). The
2,5-diphenyl-2H-tetrazolium bromide (MTT) solution was purchased from
Invitrogen (Carlsbad, CA, USA).
2.3. Scaffolds Fabrication and Coating
A 20% (w/v) PCL
solution was prepared by dissolving PCL in glacial acetic acid at 60°C for 1 h.
This was then transferred into a 24-well plate, frozen at -20°C overnight, and
treated with 5% (v/v) glutaraldehyde for 30 min. The resulting frozen scaffolds
were freeze-dried for 5 h at 0.5 atm and -120°C. For coating, UCBS and PRP
solution were mixed with 0.5 mM CaCl2 at a ratio of 9:1. The
freeze-dried PCL scaffolds were washed with PBS and immersed in UCBS and PRP
solution at 37°C overnight, then stored at for subsequent analysis.
2.4. Mechanical
Property Measurement
The mechanical properties of scaffolds
were evaluated using an Instron Universal Testing Machine (UTM, 6800, Instron,
Norwood, MA, USA). Scaffolds with a 1 cm diameter were horizontally placed
between UTM pressure plates and subjected to a compressive force at a
controlled rate of 50 mm/min, reaching a maximum force of 500 N until complete
crushing occurred. Young’s modulus was determined based on the stress-strain
curve in the range of 0% -1.2% strain (Figure 2a).
2.5. Isolation
and Culture of Human Primary Fibroblast Cells
Human primary fibroblast cells were isolated from
discarded skin following C-section surgery using an explant method (Nurhayati et al., 2019). The
skin tissue was sterilized with 0.5% (v/v) povidone-iodine in PBS, cut into
small pieces (~5 mm), and placed in a 24-well plate. Approximately 200 µL of
culture medium containing DMEM, 1% (v/v) penicillin/streptomycin, and 10% (v/v)
FBS was added to the well to prevent tissue floatation and ensure complete coverage.
Fibroblast cells originating from the explanted tissue were collected through
trypsinization and subsequently cultured in a humidified incubator at 37 with 5%
CO2.
2.6. Isolation
of Umbilical Cord Blood Serum
Human UCBS was isolated
from umbilical cord blood obtained post-C-section surgery using a gradient
density centrifugation method (Nurhayati et al., 2021b). The blood was pipetted onto the Ficoll solution, centrifuged at 400 ×
g for 10 min, and the top layer containing UCBS was carefully transferred to a
new tube and filtered with a 0.2 µm membrane filter.
2.7. Cell
Viability Assay
Cell viability was
assessed using an MTT assay, where the prepared scaffolds were initially
immersed in a 1.5 mL culture medium. At 24, 48, and 72 h, 500 µL medium was
collected and stored at 4. Fibroblast cells were seeded in a 96-well plate at
a density of 7.0 × 103 cells/well. After reaching 80% confluence,
the culture medium was replaced with a 100
size for immersion at the
respective time points. After 24 h, cells were incubated with the culture
medium mixed with MTT solution at a ratio of 9:1. Following a 4 h period, DMSO
was added to stop the reaction, and the absorbance at 570 nm was measured using
a microplate reader (Varioskan Lux, Thermofisher Scientific, Waltham, MA, USA),
then cell viability was calculated with Formula (1):
Where ODs shows absorbance
of the sample (cells exposed to medium incubated with scaffolds), ODc signifies
absorbance of cultured cells, and ODm represents absorbance of culture medium
as blank.
2.8. Cell Attachment Test
Scaffolds were
individually placed on an ultra-low attachment 6-well plate supplied with 3 mL
culture medium to ensure complete submersion and fibroblast cells were seeded
at a density of 1.0 × 105 cells/well. After 2, 4, and 6 h of
culture, non-attached cells were counted using a hemocytometer based on a dye
exclusion method, and cell attachment was calculated using Formula (2):
Where n0 indicates
the initial cell number during seeding and nf denotes the number of
non-attached cells in the culture medium.
2.9. Statistical Analysis
All experiments were
performed three times (n = 3), and data were expressed as mean ± standard
deviation (S.D.). Statistical analyses were conducted with GraphPad Prism 9
(GraphPad Software Inc., Boston, MA, USA), using one-way ANOVA for Young’s
modulus data and two-way ANOVA for cell viability and attachment data. A
post-hoc t-test was carried out using Tukey HSD, considering
results with p < 0.05 as statistically significant.
3.1. Mechanical Properties
The influence of UCBS and PRP coatings was examined
on the PCL scaffold's mechanical properties. The mechanical properties of the
scaffold are a critical factor in tissue engineering as they govern the cell's
behavior (Mubarok, Elvitigala, and Sakai 2022; Mubarok, Qu, and Sakai, 2021; Nadhif et al., 2020). Herein, the mechanical properties were evaluated by measuring Young’s
modulus based on the stress-strain curve (Figure 2a), and the result was
depicted in Figure 2b. Coating PCL scaffolds with PRP caused no substantial
changes (p > 0.05, Tukey HSD), while UCBS-coated PCL showed a significantly
lower Young’s modulus (p < 0.05, Tukey HSD) compared to the non-coated
counterparts.
Figure 2
Influence of UCBS and PRP coatings on the mechanical
properties of PCL scaffolds. (a) Stress-strain curve and (b) Young’s modulus of
scaffolds. Error bars represent S.D. (n = 3). * p < 0.05, ns:
no significant difference (p > 0.05), determined by Tukey HSD
analysis
The differences among scaffolds could be
attributed to the inherent components of UCBS and PRP (Table 1). UCBS, known
for high esterase content (Welzing et al.,
2011), potentially degraded the ester linkage in PCL affecting the
mechanical properties of scaffolds. Additionally, it might infiltrate PCL,
altering the polymer network and weakening scaffolds. A similar effect was
reported in recent research concerning PCL surface modification through
hydrolysis with NaOH and aminolysis using hexamethylenediamine/isopropanol,
leading to decreased mechanical properties due to interconnected network
disruption (Yaseri et al., 2023).
Meanwhile, PRP might contain smaller or no esterase, resulting in minimal
interference with the PCL network. Despite these alterations, all PCL-based
scaffolds maintained Young’s modulus values between 0.20-0.27 MPa, close to the
range observed in human skin (0.135 MPa - 0.169 MPa) (Nokoorani
et al., 2021), ensuring a suitable physical environment for skin
cells.
Table 1 Summary of UCBS and PRP
components and the functions
3.2. Cell Viability Assay
The viability of human
primary fibroblast cells exposed to the culture medium pre-incubated with
scaffolds for 24 to 72 h was evaluated using an MTT assay capable of assessing
metabolic activity. The results showed that coating PCL scaffolds with UCBS and
PRP significantly improved cell viability (p < 0.05, two-way ANOVA) (Figure 3).
Figure 3 Viability of human primary fibroblast cells exposed to culture medium
incubated with PCL, PCL+UCBS, and PCL+PRP scaffolds. Data are represented as
mean ± S.D. (n = 3). *p < 0.05, ***p < 0.001, ns: no
significant difference (p > 0.05), determined by Tukey HSD analysis
The augmented cell viability
observed in UCBS- and PRP-coated scaffolds was due to the presence of growth
factors and cytokines stimulating the survival pathways (Chen et al., 2022). These coatings might prevent PCL
release or degradation, preserving cell viability. Antioxidants found in both
UCBS and PRP, such as superoxide dismutase, catalase, and glutathione (Shetty et al., 2007), neutralize reactive
oxidative stress (ROS) and free radicals, thereby protecting cells from
oxidative damage and enhancing viability (Sharif et
al., 2017).
3.3. Cell
Attachment Test
The attachment of human primary fibroblast
cells on scaffolds was evaluated at 2, 4, and 6 h post-seeding. According to Figure
4, the lowest percentage of cell attachment was observed at all time points.
UCBS and PRP coatings containing growth factors, such as VEGF, EGF, PDGF, FGF,
TGF, and IGF, significantly increased cell attachment (p < 0.05, two-way
ANOVA) by stimulating adhesion (Maharajan et
al., 2021; Pavlovic et al., 2016; Montero, Santos, and
Fernández, 2015).
The interaction between these growth factors and the fibroblast cell receptor
played a crucial role in enhancing attachment. Both UCBS and PRP contained
extracellular matrix components, supporting increased cell attachment. UCBS,
with fibronectin serving as a ligand for integrin expressed by fibroblasts,
facilitated attachment to the extracellular matrix (Morshed
et al., 2019). On the other hand, PRP comprised fibrinogen, which
was converted into a fibrin matrix during the fibrin polymerization process
aided by CaCl2 to function as a ligand for cell adhesion receptors (Devereaux et al., 2020). The interaction
between fibrin with
integrins on fibroblast cells further
promoted attachment. Additionally, the cytokine IL-6 found in UCBS upregulated
integrin expression on the cell surface, increasing the adhesion and stability
of attachment to scaffolds (Romanov et al.,
2019).
Figure
4 The attachment of human
primary fibroblast cells on PCL, PCL+UCBS, and PCL+PRP scaffolds. Data are
represented as mean ± S.D. (n = 3). *p < 0.05, ns: no
significant difference (p > 0.05), determined by Tukey HSD analysis
In
conclusion, the incorporation of UCBS and PRP as coating materials distinctly
impacted the mechanical and biological properties of PCL scaffolds. UCBS
moderately reduced (~25%) the stiffness of PCL scaffolds meanwhile PRP caused
no significant alteration in stiffness. Both UCBS and PRP coatings
significantly improved (p < 0.05) the viability and attachment of
human primary fibroblast cells. These coatings could be considered a promising
method for the development of artificial skin in tissue engineering
applications.
This research was
funded partially by the Q2 Scheme Research Grant (Hibah Publikasi Artikel di
Jurnal Internasional Kuartil Q2 (PUTI Q2)) (NKB-832/UN2.RST/HKP.05.00/2023)
provided by the Directorate of Research and Development at Universitas
Indonesia and the Bilateral Exchange Program DIKTI-JSPS Joint Research Projects
2023 (No. 058/E4.4/KO/2023) from the Indonesian Ministry of Education, Culture,
Research, and Technology (Kemendikbud RI) and Japan Society for the Promotion
of Science (JSPS). The author (WM) was funded by the JSPS Postdoctoral
Fellowship in Japan.
Chandika, P., Oh, G.-W., Heo, S.-Y., Kim, S.-C.,
Kim, T.-H., Kim, M.-S., Jung, W.-K., 2021.
Electrospun Porous Bilayer Nano-fibrous Fish Collagen/ Poly(-caprolactone) (PCL) Bio-composite Scaffolds with Covalently Cross-linked Chitooligosaccharides
for Full-thickness Wound-healing Applications. Materials
Science and Engineering: C, Volume 121, p. 111871
Chen, K., Rao, Z., Dong, S., Chen, Y., Wang, X.,
Luo, Y., Gong, F., Li, X., 2022. Roles of the Fibroblast Growth Factor Signal Transduction System in Tissue Injury Repair. Burns and Trauma, Volume 10, p. tkac005
Chung, J.J., Im, H., Kim, S.H., Park, J.W., Jung,
Y., 2020. Toward Biomimetic Scaffolds For Tissue Engineering: 3D Printing
Techniques In Regenerative Medicine. Frontiers in Bioengineering and Biotechnology,
Volume 8, p. 586406
Dewi, A.H., Yulianto, D.K., Ana, I.D., Rochmadi,
R., Siswomihardjo, W., 2020. Effect of Cinnamaldehyde, an Anti-Inflammatory
Agent, on the Surface Characteristics of a Plaster of Paris-CaCO 3 Hydrogel for
Bone Substitution in Biomedicine. International Journal of Technology,
11(5), pp. 963–973
Devereaux, J., Dargahi, N., Fraser, S., Nurgali,
K., Kiatos, D., Apostolopoulos, V., 2020. Leucocyte-rich Platelet-rich Plasma Enhances Fibroblast and Extracellular Matrix Activity: Implications in Wound Healing. International
Journal of Molecular Sciences, Volume 21(18), p. 6519
Francavilla, C., O’Brien, C.S., 2022.
Fibroblast Growth Factor Receptor Signalling Dysregulation and Targeting in Breast Cancer. Open
Biology, Volume 12(2), p. 210373
Gao, J., Chen, S., Tang, D., Jiang, L., Shi, J., Wang, S., 2018.
Mechanical Properties and Degradability of Electrospun Poly(-caprolactone) (PCL)/Plga Blended Scaffolds as Vascular Grafts. Transactions
of Tianjin University, Volume 25(2), pp. 152–160
Homaeigohar, S., Boccaccini, A.R., 2022.
Nature-derived and Synthetic Additives to Poly (-caprolactone) Nanofibrous
systems for Biomedicine; an Updated Overview. Frontiers
in Chemistry, Volume 9, p. 809676
Lotfinejad, P., Shamsasenjan, K., Baradaran, B.,
Safarzadeh, E., Kazemi, T., Movassaghpour, A.A., 2021. Immunomodulatory Effect of Human Umbilical Cord Blood-derived Mesenchymal Stem Cells on Activated
T-lymphocyte. Iranian Journal of Allergy, Asthma, and Immunology, Volume 20(6), PP. 711–720
Maharajan, N., Cho, G. W., Choi, J. H., Jang, C. H., 2021. Regenerative Therapy using Umbilical Cord Serum. In vivo
(Athens, Greece), Volume 35(2), pp. 699–705
Mariani, E., Lisignoli, G., Borzì, R.M., Pulsatelli,
L., 2019. Biomaterials: Foreign Bodies or Tuners for the Immune Response? International Journal of Molecular Sciences, Volume 20(3), p. 636
Martinengo, L., Olsson, M., Bajpai, R., Soljak,
M., Upton, Z., Schmidtchen, A., Car, J., Järbrink, K., 2019.
Prevalence of Chronic Wounds in the General Population: Systematic Review and Meta-analysis of Observational Studies. Annals
of Epidemiology, Volume 29, pp. 8–15
Montero, E.C., Santos, M.E.F, Fernández, R.S.,
2015. Platelet-rich Plasma: Applications in Dermatology. Actas
Dermo-Sifiliográficas (English Edition), Volume 106(2), pp. 104–111
Morshed, A., Abbas, A.B., Hu, J., Xu, H., 2019.
Shedding New Light on the Role of 3 and 5 1 Integrins in Rheumatoid Arthritis. Molecules,
Volume 24(8), pp. 1537–1537
Mubarok, W., Qu, Y., Sakai, S., 2021. Influence of Hydrogen Peroxide-mediated Cross-linking and
Degradation on Cell-adhesive Gelatin Hydrogels. American
Chemical Society (ACS) Applied Bio
Materials, Volume 4(5), pp. 4184–4190
Mubarok, W., Elvitigala, K.C.M.L., Sakai, S., 2022.
Tuning Myogenesis by Controlling Gelatin Hydrogel Properties Through Hydrogen Peroxide-mediated
Cross-linking and Degradation. Gels, Volume 8(6), p. 387
Nokoorani, Y.D., Shamloo, A., Bahadoran, M., Moravvej, H.,
2021. Fabrication and Characterization of Scaffolds Containing Different Amounts of Allantoin for Skin Tissue Engineering. Scientific
Reports, Volume 11(1), p. 16164
Nadhif, M.H., Assyarify, H., Waafi, A.K., Whulanza, Y.,
2020. Reflecting on Mechanical Functionalities in Bioreactors for Tissue
Engineering Purposes. International Journal of Technology, Volume 11(5), pp. 1066-1075
Nurhayati, R.W., Sandora, N., Suwarti, Nauli, R.,
Khoiriyah, Z., Wardhana, A., 2019. Brief Comparative Study on the Isolation and Culture Methods of Human Keratinocyte from
Skin Tissue. In: Aeronautical Information
Publication (AIP) Conference Proceeding Volume 2193(1)
Nurhayati, R.W., Lubis, D.S.H., Pratama, G.,
Agustina, E., Khoiriyah, Z., Alawiyah, K., Pawitan, J.A., 2021a. The Effects of Static and Dynamic Culture Systems on Cell Proliferation and
Conditioned Media of Umbilical Cord-derived Mesenchymal Stem Cells. International
Journal of Technology. Volume 12(6), pp. 1187–1197
Nurhayati, R.W., Cahyo, R.D., Pratama, G., Anggraini, D., Mubarok, W., Kobayashi, M., Antarianto,
R.D., 2021b. Alginate-chitosan Microencapsulated Cells for Improving cd34+ Progenitor Maintenance and Expansion. Applied
Science, 11, 7887.
Pavlovic, V., Ciric, M., Jovanovic, V., Stojanovic, P.,
2016. Platelet Rich Plasma: a Short Overview of Certain Bioactive Components. Open
Medicine, Volume 11(1), pp. 242–247
Petretta, M., Gambardella, A., Boi, M., Berni,
M., Cavallo, C., Marchiori, G., Maltarello, M.C., Bellucci, D., Fini, M.,
Baldini, N., Grigolo, B., Cannillo, V., 2021. Composite Scaffolds for Bone Tissue Regeneration
based on Poly(-caprolactone) (PCL) and Mg-containing Bioactive Glasses. Biology
(Basel), Volume 10(5), pp. 398
Qin, X., Wu, Y., Liu, S., Yang, L., Yuan, H.,
Cai, S., Flesch, J., Li, Z., Tang, Y., Li, X., Zhuang, Y., 2022. Surface Modification of Polycaprolactone Scaffold with Improved Biocompatibility
and Controlled Growth Factor Release for Enhanced Stem Cell Differentiation. Frontiers
in Bioengineering and Biotechnology, Volume 9, p. 802311
Romanov, Y.A., Vtorushina, V.V., Dugina, T.N.,
Romanov, A., Petrova, N.V., 2019. Human Umbilical Cord Blood Serum/plasma: Cytokine Profile and Prospective Application in Regenerative Medicine. Bulletin
of Experimental Biology and Medicine, Volume 168(1), pp. 173–177
Sharif, S., Ai, J., Azami, M., Verdi, J., Atlasi,
M.A., Shirian, S., Samadikuchaksaraei, A., 2017. Collagen-coated Nano-electrospun Poly(-caprolactone)
(PCL) Seeded with Human Endometrial Stem Cells for Skin Tissue Engineering Applications. Journal
of Biomedical Materials Research Part B: Applied Biomaterials, Volume 106(4), pp. 1578–1586
Shetty, P., Bharucha, K., Tanavde, V.,
2007. Human Umbilical Cord Blood Serum Can Replace Fetal Bovine Serum in the Culture of Mesenchymal Stem Cells. Cell
Biology International, Volume 31(3), pp. 293–298
Siddiqui, N., Asawa, S., Birru, B., Baadhe, R.,
Rao, S., 2018. Poly(-caprolactone) (PCL)-Based Composite Scaffold Matrices for Tissue Engineering Applications. Molecular
Biotechnology, Volume 60(7), pp. 506–532
Sriram, S., Hasan, S., Alqarni, A., Alam, T.,
Kaleem, S.M., Aziz, S., Dawasaz, A.A., Saeed, S., 2023. Efficacy of
Platelet-rich Plasma Therapy in Oral Lichen Planus: a Systematic Review. Medicina
(Kaunas), Volume 59(4), pp. 746–746
Suárez-González, D., Barnhart, K., Migneco, F.,
Flanagan, C., Hollister, S.J., Murphy, W.L., 2012. Controllable Mineral Coatings on Poly(-caprolactone)
(PCL) Scaffolds as Carriers for Growth Factor Release. Biomaterials,
Volume 33(2), pp. 713–721
Vach-Agocsova, S.V., Culenova,
M., Birova, I., Omanikova, L., Moncmanova, B., Danisovic, L., Ziaran, S.,
Bakos, D., Alexy, P., 2023. Resorbable
Biomaterials Used for 3D Scaffolds in Tissue Engineering: A Review. Materials,
Volume 16(12), p. 4267
Welzing, L., Ebenfeld, S., Dlugay, V., Wiesen,
M.H.J., Roth, B., Mueller, C., 2011. Remifentanil Degradation in Umbilical Cord Blood of Preterm Infants. Anesthesiology,
Volume 114(3), pp. 570–577
Wheeler, D.L., Yarden, Y. 2015. Receptor
Tyrosine Kinases: Family and Subfamilies. In: Springer International
Publishing, Switzerland
Yaseri, R., Fadaie, M., Mirzaei, E., Samadian, H., Ebrahiminezhad, A., 2023. Surface modification of
polycaprolactone nanofibers through hydrolysis and aminolysis: a comparative
study on structural characteristics, mechanical properties, and cellular
performance. Scientific Reports, Volume 13(1), p. 9434
