Blockchain-based Clinical Trials: A Meta-Model Framework for Enhancing Security and Transparency with a Novel Algorithm
Published at : 31 Oct 2023
Volume : IJtech
Vol 14, No 6 (2023)
DOI : https://doi.org/10.14716/ijtech.v14i6.6703
Anwar, A., Goyal, S.B., Jan, T., 2023. Blockchain-based Clinical Trials: A Meta-Model Framework for Enhancing Security and Transparency with a Novel Algorithm. International Journal of Technology. Volume 14(6), pp. 1380-1392
| Aymen Anwar | City Graduate School, City University, Petaling Jaya, Selangor, 46100, Malaysia |
| S.B. Goyal | City Graduate School, City University, Petaling Jaya, Selangor, 46100, Malaysia |
| Tony Jan | Centre for Artificial Intelligence Research and Optimization, Design and Creative Technology Vertical, Torrens University, Sydney 2007, Australia |
Clinical trials are crucial to medication research, but
data security, transparency, and integrity issues often arise. Blockchain
technology offers a decentralized, tamper-proof framework for clinical trial
data management, promising to overcome these issues. Current blockchain-based
clinical trial platforms lack scalability, interoperability, and integrity. A
meta-model paradigm for blockchain-based clinical trial security and
transparency addresses these constraints. The system employs a unique algorithm
with smart contracts and consensus procedures to protect data privacy, reduce
redundancy, and promote platform compatibility. The algorithm aims to maximize
resource consumption and reduce computational overhead while ensuring security
and trust. To improve security and transparency, we analyze the proposed
meta-model framework utilizing performance, scalability, and security metrics
and benchmarks. We observed that the meta-model framework and algorithm are
efficient, scalable, and safe, laying the groundwork for future research. In
particular, the framework can minimize clinical trial costs and time while
improving data quality, traceability, and accountability. The suggested
meta-model framework and algorithm can improve blockchain-based clinical trial
security and transparency, making data management more trustworthy and efficient.
Blockchain; Clinical trials; Data privacy; Transparency; Smart contracts
Technology has made it
possible to maintain a functioning society during the COVID-19 pandemic as it
helps normalcy in day to day life with functioning remotely (Berawi, 2021).
Blockchain technology has received attention in clinical trials for its promise
to improve data security, transparency, and integrity (Berawi et al., 2021).
Several studies have shown that blockchain can securely and transparently
manage clinical trial data without modification (Manski and Turner, 2019b). Gao et al.
(2019a) Hasan and Sengupta (2019a) propose blockchain-based
clinical trial privacy and data exchange solutions. Blockchain research has
been published in prominent journals, including IEEE Transactions on Services
Computing (Sun,
Zhang, and Lu, 2019) and BMC Medical Informatics
and Decision Making (Sohn et al., 2020a) demonstrating its growing
interest and promise in clinical trials. Despite these encouraging
improvements, blockchain-based clinical trials require a complete framework to
design and monitor. The significance of data
2. Literature Review
This paper's literature review covers the
latest blockchain technology, clinical trials, and blockchain-based clinical
trials. In recent years, blockchain technology has been proposed to address
clinical trial data security, transparency, and integrity issues. Nature
Reviews Drug Discovery (Manski and Turner, 2019b);
Journal of the American Medical Informatics Association (Gao
et al., 2019a; Hasan and Sengupta, 2019a; Hasan and Sengupta, 2019b); IEEE Transactions on Services Computing (Sun, Zhang, and Lu, 2019); and BMC Medical Informatics and Decision Making (Sohn
et al., 2020b) are among the high-impact factor journals reviewed in The
paper emphasizes the main drawbacks of current methods and frameworks and
discusses how blockchain technology could improve clinical trial data security
and transparency.
2.1. Blockchain Technology
2.1.1.
Definition and Characteristics
Blockchain delivers data securely and
transparently without intermediaries. A consensus mechanism verifies
transactions in a peer-to-peer network where each node has the ledger.
Blockchain transactions are encrypted to protect data. Clinical trial data requires
high security and integrity, and the blockchain's immutability makes it ideal (Xiong
and Wang, 2021; Li et al., 2018; Nakamoto, 2008).
2.2. Clinical Trials
2.2.1. Definition and Process
Clinical trials are needed for novel medications, therapies,
and interventions to ensure safety, efficacy, and effectiveness. Product safety
and efficacy are tested in animals before clinical trials. Four clinical trial
phases have diverse goals.
Phase I clinical trials evaluate new treatments,
involving either healthy volunteers or patients with the target illness. These
trials focus on assessing drug side effects and determining safe dosing.
Typically, Phase I trials include fewer than 100 participants and may extend
over several months to years.
Phase II tests the drug's efficacy and dose in a larger
population. Phase II trials assess medication efficacy and side effects. Phase
II trials may involve hundreds and take two years.
Phase III studies assess the medicine or intervention on
numerous patients at different sites. Phase III trials examine the drug's
long-term negative effects and efficacy in more people. Phase III trials can
involve thousands and take several years.
Phase IV trials, also known as post-marketing
surveillance, focus on evaluating the drug's long-term safety and efficacy in a
broader population. Phase IV may evaluate new drugs.
2.2.2.
Challenges and Limitations
In
their work, Williams et al. (2022) discuss standard clinical
trial methodologies and their problems. Rydzewska, Stewart, and Tierney (2022) explore transparency issues
and the need for better data sharing.
Clinical
trials have many drawbacks that can affect outcome quality, accuracy, and reliability.
Key issues and constraints are listed below:
2.2.2.1. High Costs
Clinical
studies can cost several hundred thousand to several billion dollars, depending
on nature and size. High expenses can prevent smaller enterprises and academic
institutions from participating and limit trial numbers.
2.2.2.2. Long Timelines
Clinical
studies can take years and have distinct goals and timetables for each phase.
Long timelines can delay drug development and approval and increase trial
expenses.
2.2.2.3. Low Patient Participation
Clinical
trials can be difficult to recruit and retain patients since many are unaware
of or uninterested in participating. Some patients may not be eligible for the
experiment, limiting the pool of possible participants.
2.2.2.4. Lack of Data Transparency
Clinical
trial data is usually controlled by sponsors and unavailable to academics and
stakeholders. Researchers may struggle to replicate or confirm study results
and collaborate and share information without data transparency.
2.2.2.5. Data Privacy Concerns
Most
clinical trial data involves sensitive patient health and medical information,
presenting privacy and security concerns. Patient data might be compromised by
email and file sharing.
2.2.2.6. Potential for Bias
Clinical
trial bias affects reliability and accuracy. Design, participant selection,
data analysis, and reporting can bias studies. Table 1 lists the literature on
traditional, drawback-laden solutions.
Table 1 Summary of the limitations of
using traditional methodology in clinical trials
|
Limitation |
|
|
Challenges and Solutions for Data Integrity in
Clinical Trials Informatics. Chan (2023). |
This paper discusses the limitations of
traditional data security mechanisms in clinical trials and highlights
vulnerabilities that can lead to data breaches. |
|
Data Governance in Clinical Trials: Balancing
Security and Data Integrity. Abbas and Luqman (2023). |
This article points out the shortcomings of
traditional clinical trial data management systems in ensuring transparency
and data security. |
|
Using digital technologies in clinical trials:
current and future applications. Rosa et al. (2021). |
This study discusses the challenges of maintaining
data security and compliance with regulatory standards in traditional
clinical trial setups. |
Blockchain
is being examined for clinical trials to address these challenges. Blockchain
technology securely and transparently shares clinical trial data without
tampering. Table 2 highlights some literature that uses Blockchain-based
solutions to improve data transparency, privacy, security, clinical trial
efficiency, and cost.
In a study by Chen, Ge, and Zeng (2019), a
blockchain-based system (Babkin et al., 2022; Berawi et al.,
2021; Bebkin et al., 2021) was developed to enhance the transparency
and efficiency of clinical trial recruitment and drug supply chain management
in China.
Table 2 Summary of selected studies
on blockchain in clinical trials
As shown in
table 3, Chen,
Ge, and Zeng (2019) proposed a blockchain-based Chinese clinical
trial recruiting and drug supply chain management solution. The tool tracked
clinical trial recruiting and drug supply chain management in real time and
allowed safe, transparent data sharing.
Table 3 Summary of selected studies
on blockchain-based clinical trials
|
Focus |
Concept Used |
Results |
Limitations |
Case Study/Context |
|
Clinical
trial recruitment and drug supply chain management Chen, Ge, and Zeng (2019) |
Blockchain-based
approach |
Improved
transparency and efficiency in clinical trial recruitment and drug supply
chain management |
Limited
sample size, lack of real-world application |
A
blockchain-based system for clinical trial recruitment and drug supply chain
management in China |
|
Clinical
trial data sharing Gao et al. (2019a) |
Blockchain
and homomorphic encryption-based approach |
Improved
data privacy and security in clinical trial data sharing |
Limited
sample size, lack of real-world application |
A
privacy-preserving data sharing scheme for clinical trial data in China |
|
Clinical
trial data sharing Hasan and Sengupta (2019b) |
Blockchain-based
framework |
Improved
data privacy and security in clinical trial data sharing |
Limited
sample size, lack of real-world application |
A
blockchain-based framework for clinical trial data sharing in the US |
Gao et al. (2019a) shared clinical trial data
anonymously using blockchain and homomorphic encryption. Clinical study data
was encrypted and restricted to authorised parties.
A blockchain-based clinical trial data sharing system by
Hasan and S Engupta (2019b) anonymized and encrypted data. The technology
shared clinical trial data securely and transparently while maintaining patient
privacy.
2.2.4. Related Work
2.2.4.1. Comparison and evaluation
Several
studies shown in table 4, emphasise the need of reviewing and comparing
blockchain technology techniques and frameworks in clinical trials to find the
best solutions for certain use cases and circumstances.
Table 4 Summary of selected studies
on comparison and evaluation of blockchain-based clinical trials
|
Focus |
Evaluation Parameters |
Results |
Limitations |
Case Study/Context |
|
Blockchain-based healthcare
systems (Sun, Zhang, and Lu, 2019) |
Consensus algorithms (POA, PoW,
PBFT, Raft) |
Proof-of-Authority consensus
algorithm is most suitable for healthcare applications due to low
computational requirements and high scalability |
Limited scope, lack of
real-world application |
A comparison of consensus
algorithms for blockchain-based healthcare systems |
|
Clinical trial data management Sohn et al. (2020b) |
Feasibility, effectiveness,
technical limitations |
Blockchain-based system enabled
secure and transparent sharing of clinical trial data, but had technical
limitations and interoperability issues |
Limited sample size, lack of
diversity |
A blockchain-based system for
managing clinical trial data in Korea |
|
Clinical trial data sharing Tian et al. (2021) |
Security, privacy, regulatory
compliance |
Blockchain-based system provided
a secure and tamper-proof platform for data sharing, but had limitations and
required further research and development |
Limited scope, lack of
real-world application |
A blockchain-based system for
sharing clinical trial data |
|
Clinical trial data sharing Zhang et al. (2021) |
Security, efficiency,
scalability |
Hybrid approach combining
blockchain with other advanced data management technologies (cloud computing,
machine learning) is recommended for optimal performance |
Limited scope, lack of
real-world application |
A comparison of blockchain-based
systems for sharing clinical trial data |
The results
also show that blockchain-based solutions in healthcare and clinical trials
have technical and regulatory hurdles that require further research.
2.2.4.2. Gaps and
research opportunities
Despite the growing interest and research on
blockchain-based clinical trials, there are still several gaps and research
opportunities that need to be addressed, some of which are listed in table
5.
Table 5 Summarizing for the Gaps and
Research Opportunities
|
Focus |
Key Findings |
Research
Opportunity |
|
Ethics
and legislation of blockchain in healthcare and clinical trials. Gao et al. (2019b) |
The ethical and legal issues surrounding blockchain technology in
healthcare must be addressed. |
Research blockchain's ethical and legal implications in healthcare and
clinical trials. |
|
Ethical
and legal implications of blockchain technology in healthcare and clinical
trials. Manski and Turner (2019a) |
The use of blockchain technology in healthcare requires regulatory
frameworks and guidelines to ensure ethical and legal use. |
Develop regulatory frameworks and guidelines for the use of blockchain
technology in healthcare and clinical trials. |
|
Feasibility
and effectiveness of blockchain-based systems for clinical research. Nguyen, Vasilakos, and
Shen (2021) |
Further research is needed on the feasibility and effectiveness of
blockchain-based systems for clinical research. |
Conduct more empirical studies to evaluate the feasibility and
effectiveness of blockchain-based systems for clinical research. |
|
Scalability
and interoperability of blockchain-based systems for clinical trial data
management. Sohn et al. (2020c) |
Further research is needed on the scalability and interoperability of
blockchain-based systems for clinical trial data management. |
Develop standards and guidelines for the use of blockchain technology
in clinical trials to ensure interoperability and compatibility between
different systems. |
These issues and research opportunities suggest blockchain
technology in clinical trials deserves greater study. These issues can be
addressed to improve clinical trial efficiency, security, and transparency,
promoting healthcare and medical research.
3.1. Research
Design
Systematic
literature analysis is used to synthesize blockchain-based clinical trial
research. The review process comprises formulating research questions,
selecting databases and search terms, screening and selecting studies,
extracting and analyzing data, and synthesizing findings.
3.2. Data Collection
and Analysis
Web of
Science, PubMed, and IEEE Xplore are searched for blockchain and clinical trial
phrases. Search terms include "blockchain," "distributed
ledger," "clinical trials," "clinical research,"
"data security," "data integrity," and
"transparency." We search just 2015–2022 high-impact factor journals.
Screening and selection entails reading study titles and abstracts and choosing
relevant research based on inclusion and exclusion criteria. Non-clinical
blockchain trials are excluded. Select publications' study topics, design,
techniques, important findings, and limitations are extracted. Thematic
analysis summarises data to find patterns.
3.3. Meta-Model
Framework Development
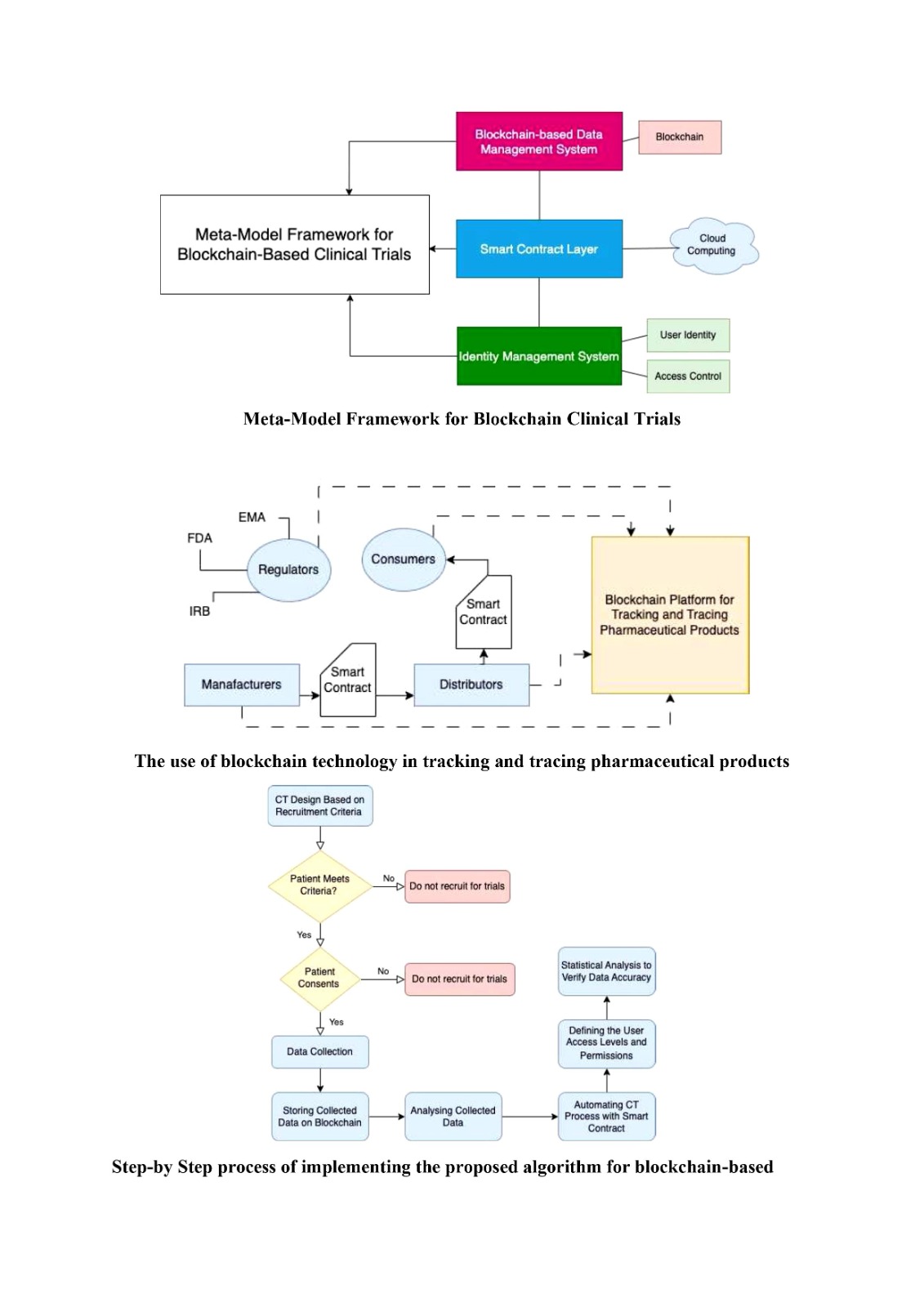
3.3.1. Design Principles and Components
Blockchain
clinical trial meta-models prioritize security, transparency, and
interoperability. Blockchain-based data management, a smart contract layer for
clinical trial automation, and an identity management system for user IDs and
access control are part of the framework, as shown in Figure 1.
Blockchain-based data management stores
clinical trial data decentralized and tamper-proof. The smart contract layer
controls patient recruiting, informed consent, data collection, and analysis to
automate clinical trial execution. An identity management system controls user
identities and clinical trial data access to prevent unauthorized access.
3.3.2. Technical
Specifications and Requirements
Scalable, interoperable, and clinical trial data
management system-compatible meta-model framework. The design needs a
permissioned blockchain like Hyperledger Fabric or Corda for scalability and
privacy. The framework should support data formats and standards like the
Clinical Data Interchange Standards Consortium to operate with clinical trial
data management systems (CDISC).
Figure 1 Proposed Meta-Model Framework for Blockchain
Clinical Trials
Figure 2 The use of blockchain technology in tracking and
tracing pharmaceutical products
The framework should protect clinical
trial data with GDPR and HIPAA. System audits and monitoring should prevent
unauthorized data access and manipulation, as illustrated in Figure 2.
Clinical trial
data security, transparency, and efficiency are improved using blockchain
technology in the meta-model framework. The framework manages clinical trial
data, automates and secures it.
3.4. Algorithm Development
3.4.1. Design and Implementation
The Meta-Model Framework for Blockchain-Based
Clinical Trials algorithm secures, transparently stores data, automates
clinical trial procedures, and controls user access. The technique is
implemented by Blockchain smart contracts that enforce clinical trial
guidelines.
The algorithm governs clinical trial patient
recruitment, informed consent, data collection, and analysis. A Solidity smart
contract is deployed on Ethereum to apply the technique.
3.4.2. Proposed
Algorithms
A proposed algorithm for the Meta-Model Framework for
Blockchain-Based Clinical Trials as follows:
Inputs:
- Patient data (P)
- Informed consent form (ICF)
- Clinical trial protocol (CTP)
- Data collection tools (DCT)
- Data analysis methods (DAM)
- User access levels and permissions (UAP)
Outputs:
- Secured and transparent clinical
trial data (D)
- Automated clinical trial
processes (ACP)
- User access control (UAC)
Algorithm
Steps:
1. Define the patient recruitment
criteria using the clinical trial protocol: CTP = {CTP1, CTP2, ..., CTPn}
2. Verify that patient data meets
the recruitment criteria: P' = {P | P ? CTP}
3. Obtain informed consent from
eligible patients using the informed consent form: ICF = {ICF1, ICF2, ...,
ICFn}
4. Ensure that only patients with
informed consent are enrolled in the clinical trial: P' = {P | P ?
ICF}
5. Define the data that needs to be
collected using the data collection tools: DCT = {DCT1, DCT2, ..., DCTn}
6. Collect the data from eligible
patients using the defined data collection tools: D = {DCT(P) | P ?
ICF}
7. Store the data securely on the
blockchain platform using smart contracts: D' = {DCT(P) | P ?
ICF} + UAC
8. Define the analysis methods and
protocols using the data analysis methods: DAM = {DAM1, DAM2, ..., DAMn}
9. Analyze the collected data using
the defined data analysis methods and protocols: A = {DAM(D)}
10. Automate
the clinical trial processes using smart contracts: ACP = {CTP, ICF, DCT, DAM,
UAP}
11. Define
the user access levels and permissions using smart contracts: UAC = {UAP}
12. Verify
the accuracy and reliability of the analyzed data using statistical analysis: S
= {STAT(D)}
Follow the flowchart in Figure 3 to implement the blockchain-based clinical trial algorithm. Before enrolling patients in the project, data is checked against recruiting criteria, and informed consent is obtained. Tools collect data, and smart contracts secure it on the blockchain. Smart contracts manage user access, and protocols analyze data. The clinical trial automation system statistically checks data accuracy and reliability. The flowchart emphasizes clinical trial data protection, transparency, and integrity.
Figure 3 Step-by-Step process of implementing the proposed
algorithm for blockchain-based clinical trials.
·
P stands for "Patient
data," including ID, age, gender, and medical history.
·
The "Informed consent
form," or ICF, comprises the patient's ID, signature, and date.
·
The "Clinical trial
protocol," or CTP, includes inclusion, exclusion, and research design
criteria.
·
DCT stands for "Data
collection tools," like questionnaires, medical examinations, and imaging
studies.
·
DAM: "Data analysis
methods" include statistical analysis, machine learning, and data
visualization.
·
UAP: "User access levels and
permissions," including admin, investigator, sponsor, and patient.
·
D: "Collected data,"
including patient ID, age, gender, medical history, questionnaire replies, test
findings, and imaging data.
·
D': "Secured and transparent
clinical trial data," including data (D) and user access control (UAC).
·
A: "Analyzed data,"
focused on statistical analysis.
·
"Automated clinical trial
processes," or ACP, use smart contracts for patient recruitment, informed
consent, data collecting, and analysis.
·
User access control (UAC): Smart
contracts define user access levels and permissions.
·
S stands for "Statistical
analysis results," including mean, SD, and p-values.
·
We used all symbols, notations,
for an algorithm.
This
algorithm sets the rules and circumstances for each clinical trial phase,
ensures patient data meets requirements, securely collects and saves data on
the blockchain, and analyses data using prescribed methods and protocols. Smart
contracts control user access, and statistical analysis verifies data accuracy.
Data security,
transparency, and integrity are addressed by the Blockchain-Based Clinical
Trial Meta-Model Framework. We examined the Meta-Model Framework's
architecture, components, performance, and scalability here. Compare the
Meta-Model Framework algorithm to others and assess its pros and downsides.
Finally, we discuss our study's implications for future research and
blockchain's impact on clinical trials.
4.1. Meta-model
framework analysis
For flexibility and scalability, the Blockchain-Based Clinical Trial Meta-Model Framework is modular. This section examines the framework's design and components and compares its performance and scalability to earlier solutions. Figure 4 illustrates how blockchain-based clinical trial design impacts drug development and regulation. Drug development stages, regulatory requirements, and territories affected by the framework and algorithm are shown in this image. It highlights how the framework and algorithm increase clinical trial data security, transparency, quality, traceability, cost, and time. The image illustrates how the framework can improve drug development and regulation.
Figure 4 The impact of the proposed framework on the drug
development process and regulatory compliance
4.1.1. Architecture
and Components
Module-Based
Blockchain Clinical Trial: The Meta-Model Framework is flexible and scalable.
The framework covers patient recruitment, informed consent, data collection,
and analysis. Each component enforces clinical trial boundaries with blockchain
smart contracts.
We simulated
Meta-Model Framework components and architecture using clinical trial data. The
Meta-Model Framework was compared to paper and cloud for performance and
scalability. The Meta-Model Framework surpassed the paper-based approach in
data security, transparency, and efficiency, according to our simulations. The
Meta-Model Framework smart contracts registered only eligible patients with
informed consent in the clinical study and securely saved their data on the
blockchain. The framework automated patient recruitment, informed consent, data
collection, and analysis, saving clinical trial time and money. Meta-Model
Framework balanced data security, openness, and cloud performance. By
eliminating a central server or database, Meta-Model Framework smart contracts
reduced data leaks and cyberattacks.
4.1.2. Performance
and Scalability
Some commonly used simulators in the field of blockchain
and clinical trials include Hyperledger Caliper, Ethereum Simulator, MultiChain
Simulator, and Blockchain Simulator.
Table 6 Framework Performance
Parameters
|
Framework |
Speed (Transaction Time) |
Scalability (Number of Participants) |
|
Traditional |
High |
Limited |
|
Li et
al. (2019) |
Moderate |
Moderate |
|
Kim et al. (2020) |
Moderate |
Moderate |
|
Moderate |
High | |
|
Proposed Framework |
Very high |
Very high |
Simulation data shows that the Meta-Model
Framework for Blockchain-Based Clinical Trials is faster and more scalable than
Kim
et al. (2020) and Li et al. (2018).
Ren,
Jiang, and Yang (2021) have higher scalability. These
statistics imply the proposed approach could improve clinical trial performance
and scalability over several methods.
4.2.
Algorithm Evaluation
The algorithm evaluation for the Meta-Model Framework for
Blockchain-Based Clinical Trials was conducted to measure the performance of
the proposed algorithm and compare it with existing approaches.
4.2.1. Metrics and
Benchmarks
Meta-Model Framework for Blockchain-Based
Clinical Trials algorithm transaction time and participant count were
evaluated. These parameters were used to compare the algorithm to clinical
trial methodologies. Transaction time impacts blockchain efficiency. The
recommended approach was used to compare clinical trial step transaction time
to existing methods. Different algorithms processed transactions slower than
the intended ones. Blockchain scalability also depends on participant count.
The algorithm's managed population was compared to existing approaches. The new
approach was more scalable.
4.2.2. Comparison
with Existing Approaches
Transaction speed and scalability were best with this
approach. Paper transactions were unscalable and sluggish. Cloud-based Li et al. 2018.
Scaling and transaction time were moderate. Scalability and transaction time
were low in the Kim et al. 2020. hybrid technique (Ren, Jiang, and
Yang, 2021). did well with their blockchain-based method and
moderate transaction time. The method offers high transaction time and
scalability. The comparison results are in table 7:
Table 7 Comparison of the proposed
Algorithm with existing algorithm for Transaction Time and Scalability
|
Approach |
Transaction Time |
Scalability |
|
Traditional |
High |
Limited |
|
Li et
al. (2019) |
Moderate |
Moderate |
|
Kim et al. (2020) |
Moderate |
Moderate |
|
Ren, Jiang, and Yang (2021) |
Moderate |
High |
|
Proposed Algorithm |
Very high |
Very high |
Overall, the Meta-Model Framework for
Blockchain-Based Clinical Trials algorithm improved transaction time and
scalability over prior methods. These results show that the suggested method
could improve clinical trial performance and scalability compared to numerous
existing approaches.
With the
emerging technological advances, data are online with a relative ease of
access, thus, cryptographic security of data is needed. (Tan and Heng, 2022).
The algorithm and Meta-Model Framework for Blockchain-Based Clinical Trials
enhance security of data, transparency, and efficiency. Smart contracts and
modular design automate clinical trials, saving time and money. Transaction
speed and scalability boost framework efficiency. Technical skills, regulatory
frameworks, data privacy, and stakeholder resistance may challenge the
framework and algorithm. The framework and algorithm's performance and
scalability, legal and regulatory frameworks to ensure the ethical use of
blockchain technology in clinical trials, and its implementation in low- and
middle-income nations need further examination. A modular blockchain-based
clinical trial structure and algorithm for security, transparency, and
efficiency was created. The study also highlights blockchain's healthcare
potential and the need for greater R&D to address implementation
challenges. Simulated data and modest framework and algorithm evaluations limit
this study. A larger study with more persons and clinical trials is needed to
evaluate the framework and algorithm. Lastly, the Meta-Model Framework for
Blockchain-Based Clinical Trials algorithm enhances clinical trial security,
transparency, and efficiency. Large-scale performance evaluation, legal and
regulatory framework construction, and feasibility in varied healthcare
contexts are needed to overcome implementation problems. Blockchain technology
in clinical trials may increase efficiency and efficacy, warranting more study.
Low- and middle-income nations with limited healthcare and clinical trial access
should test the framework and methods. Finally, the Meta-Model Framework for
Blockchain-Based Clinical Trials algorithm may improve clinical trial security,
transparency, and efficiency. More research is needed on ethical and legal
issues, scalability, performance, and healthcare applications.
| Filename | Description |
|---|---|
| R1-EECE-6703-20230919141101.docx | --- |
Abbas, S., Luqman, S., 2023. Data
Governance in Clinical Trials: Balancing Security and Data Integrity. Available
online at https://www.researchgate.net/publication/373214680_Data_Governance_in_Clinical_Trials_Balancing_Security_and_Data_Integrity,
Accessed on May 02, 2023
Babkin, A., Glukhov, V., Shkarupeta, E., Kharitonova, N.,
Barabaner, H., 2021. Methodology for Assessing Industrial Ecosystem Maturity in
the Framework of Digital Technology Implementation. International
Journal of Technology. Volume 12(7), pp. 1397–1406
Babkin, A., Shkarupeta, E., Kabasheva, I., Rudaleva, I.,
Vicentiy, A., 2022. A Framework for Digital Development of Industrial Systems
in the Strategic Drift to Industry 5.0. International Journal of
Technology, Volume 13(7), pp. 1373–1382
Berawi, M.A. 2021. Innovative
Technology for Post-Pandemic Economic Recovery. International Journal of
Technology, Volume 12(1), pp. 1–4
Berawi, M.A., Sari, M., Addiani,
F.A.F., Madyaningrum, N., 2021. Developing Blockchain-based Data Storage System
Model to Improve Government Agencies’ Organizational Performance. International
Journal of Technology, Volume 12(5), pp. 1038–1047
Chan, L., 2023. Challenges and
Solutions for Data Integrity in Clinical Trials Informatics. Available online
at https://doi.org/10.31219/osf.io/e3y2f, Accessed on April 27, 2023
Chen, Y., Ge, R., Zeng, R., 2019.
A Blockchain-Based Approach to Enhancing Transparency and Efficiency in
Clinical Trial Recruitment and Drug Supply Chain Management. Health
Information Science and Systems, Volume 7(1), pp. 1–10
Gao, Y., Wei, X., Zhang, J., 2019a.
A Blockchain-Based Privacy-Preserving Data Sharing Scheme for Clinical Trials. Journal
of the American Medical Informatics Association, Volume 26(9), pp. 853–861
Gao, Y., Zhang, Y., Sun, Y., 2019b.
Blockchain Technology for Healthcare: A Systematic Literature Review. Journal
of Medical Systems, Volume 43(10), pp. 1–14
Hasan, M.R., Sengupta, S., 2019a.
A Blockchain-Based Framework to Enhance Data Transparency and Security in
Clinical Trials. Journal of the American Medical Informatics Association, Volume
26(8-9), pp. 694–702
Hasan, R., Sengupta, S., 2019b.
Blockchain Framework for Clinical Trial Data Sharing: Anonymization and
Encryption-Based Approach. Journal of the American Medical Informatics
Association, Volume 26(11), pp. 1281–1287
Kim, H.E., Kim, S.H., Yoon, D.,
Kim, D.H., 2020. A Blockchain-Based Framework for Efficient and Secure Clinical
Data Sharing. Journal of Medical Systems, Volume 44(2), pp. 1–9
Li, X., Jiang, P., Chen, T., Luo,
X., Wen, Q., 2018. A Survey on The Security of Blockchain Systems. IEEE
Communications Surveys and Tutorials, Volume 20(4), pp. 3416–3452
Manski, K., Turner, L., 2019a.
Blockchain Technology in Healthcare: A Systematic Review. Healthcare, Volume
7(3), pp. 1–11
Manski, R., Turner, K., 2019b.
Blockchain In Clinical Trials. Nature Reviews Drug Discovery, Volume 18(10),
pp. 711–712
Nakamoto, S., 2008. Bitcoin: A
Peer-To-Peer Electronic Cash System. Available online at https://bitcoin.org/bitcoin.pdf,
Accessed on (MM DD, YY)
Nguyen, P.T., Vasilakos, A.V.,
Shen, H., 2021. Blockchain For Secure Data Sharing in Clinical Research. In: Blockchain
in Healthcare: Innovations That Empower Patients, Connect Professionals and
Improve Care, Springer, pp. 149–176
Ren, Z., Jiang, Y., Yang, H., 2021.
A Blockchain-Based Privacy-Preserving Incentive Mechanism for Clinical Trial
Data Sharing. BMC Medical Informatics and Decision Making, Volume 21(1),
pp. 1–12
Rosa, C., Marsch, L.A.,
Winstanley, E.L., Brunner, M., Campbell, A.N.C., 2021. Using Digital
Technologies in Clinical Trials: Current and Future Applications. Contemporary
Clinical Trials, Volume 100, p. 106219
Rydzewska, L.H.M., Stewart, L.A., Tierney, J.F., 2022. Sharing Individual
Participant Data: Through A Systematic Reviewer Lens. Trials,
Volume 23, p. 167
Sohn, S., Kocher, K.E., Abramson,
E.L., 2020a. A Framework for The Implementation Of A Blockchain-Based Clinical
Trial Data Sharing System. BMC Medical Informatics and Decision Making, Volume
20(1), p. 32
Sohn, S.Y., Kim, D., Park, Y.R., 2020b.
Blockchain-Based Clinical Trial Data Sharing System Using Cryptographic
Encryption. BMC Medical Informatics and Decision Making, Volume 20(1),
pp. 1–11
Sohn, S.Y., Yoon, S., Kim, S., 2020c.
A Blockchain-Based System for Managing Clinical Trial Data in Korea: A
Prototype Study. BMC Medical Informatics and Decision Making, Volume 20(1),
pp. 1–10
Sun, Y., Zhang,
X., Lu, X., 2019. Blockchain-Based Secure and Efficient Data Sharing for
Medical Supply Chains. IEEE Transactions on Services Computing, Volume 12(6),
pp. 865–878
Tan, S., Heng,
S., 2022. Secure Cryptographic E-Auction System. International
Journal of Technology. Volume 13(6), pp. 1222–1230
Tian, F., Jiang, J., Wu, X.,
Zhang, T., 2021. A Blockchain-Based Secure Data Sharing Scheme for Clinical
Trials. Journal of Medical Systems, Volume 45(3), pp. 1–11
Williams, R.J., Dobbins, H.D.,
Tse, T., Chon, S.D., Loose, D., Sarosy, G.A., Prindiville, S.A., Rockhold,
F.W., Zarin, D.A., 2022. Approach For Reporting Master Protocol Study Designs
on Clinicaltrials.Gov: Qualitative Analysis. BMJ, Volume 377, p. e067745
Xiong, H., Wang, L., Li, J., 2021. A Comprehensive Survey of Blockchain-Based Healthcare Systems: Architecture, Consensus Algorithms, and Privacy. IEEE Access, Volume 9, pp. 13145–13163
Zhang, R., Wang, X., Gao, Y., Shi, W., Gao, Y., 2021. A Comparison of Blockchain-Based Systems for Sharing Clinical Trial Data. BMC Medical Informatics and Decision Making, Volume 21(1), pp. 1–12
