Effect of Multi-walled Carbon Nanotube and Polyethylene Glycol Addition in Nanofluid Quench Medium for Steel Heat Treatment Application
Corresponding email: wahyuaji.np@gmail.com
Published at : 05 Feb 2024
Volume : IJtech
Vol 15, No 2 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i2.6690
Putra, W.N., Ariati, M., Suharno, B., Noviyanto, A., Riko, I.M., 2024. Effect of Multi-walled Carbon Nanotube and Polyethylene Glycol Addition in Nanofluid Quench Medium for Steel Heat Treatment Application. International Journal of Technology. Volume 15(2), pp. 364-372
| Wahyuaji Narottama Putra | Department of Metallurgical & Materials Engineering, Faculty of Engineering, Universitas Indonesia, Kampus Baru UI, Depok, 16424, Indonesia |
| Myrna Ariati | Department of Metallurgical & Materials Engineering, Faculty of Engineering, Universitas Indonesia, Kampus Baru UI, Depok, 16424, Indonesia |
| Bambang Suharno | Department of Metallurgical & Materials Engineering, Faculty of Engineering, Universitas Indonesia, Kampus Baru UI, Depok, 16424, Indonesia |
| Alfian Noviyanto | 1. Nano Center Indonesia, Jl. Puspiptek, South Tangerang, Banten, 15314, Indonesia, 2. Department of Mechanical Engineering, Mercu Buana University, Kebun Jeruk, Jakarta, 11650, Indonesia |
| I Made Riko | Institute of Materials Research and Engineering, A*Star Singapore, 2 Fusionopolis Way, 138634, Singapore |
In the steel heat treatment industry, quenching is a critical stage for
enhancing the characteristics of steel. However, the lack of adherence to
appropriate procedures, especially in quenchant selection, can lead to cracks
or distortion. Quenchant selection is based on the required cooling rate for
the specific steel being quenched. Cooling rate of the quenchant is primarily
determined by the thermal conductivity of the fluid. This conductivity can be
modified by dispersing stabilized solid particles, typically in the nano-scale
range, thus forming a nanofluid. It is important to note that a higher
conductivity will increase the heat transfer characteristic and vice versa,
hence, cooling rate can be controlled by adjusting the amount of the dispersed
particle. In this study, the dispersed particle and surfactant used was
multi-walled carbon nanotube (MWCNT) and polyethylene glycol (PEG),
respectively. The concentrations of the dispersed particle were 0.1, 0.3, and
0.5 weight%. Furthermore, the surfactant was added at 3 – 30% on each particle
variation. The results showed that the highest thermal conductivity of 0.68
W/mK was achieved at 0.5% MWCNT and 5% PEG. This translated into better steel
properties, as it led to a hardness of 48 in Hardness Rockwell C-scale (HRC)
compared with the water-quenching technique. A higher percentage of PEG
surfactant decreases the thermal conductivity of the quenching medium and steel
hardness. This decrease was attributed to the high viscosity of the medium. In
conclusion, adjusting the particle and surfactant concentration allows for the
optimal quenching medium, offering enhanced steel properties.
Heat Treatment, MWCNT, Nanofluid, PEG, Quench Medium, S45C Carbon Steel
Nanofluid is a fluid containing nano-sized particles suspended in a base
fluid. These nanoparticles serve to improve thermal conductivity. It is
important to note that solid particles generally have higher conductivity
compared to any fluid (Ali and Salam, 2020).
The extensive surface area in nanoparticles contributes to even higher
conductivity. Therefore, nanofluid is commonly used in heat transfer-intensive
processes, such as coolant systems (Septiadi et
al., 2020), or quenching mediums in heat treatment (Radhiyah and Nurziela, 2020; Yahya et al., 2018; Babu, Arularasan, and
Ramkumar, 2017).
Several types of
solid particles can be added to nanofluid, including metals, metal oxides such
as CuO, TiO2, and Al2O3, as well as
carbon-based particle (Arularasan and Babu, 2021;
Ramadhani et al., 2019; Xia et
al., 2014). Carbon-based particle, specifically advanced types such
as MWCNTs, has garnered increased attention due to the significantly higher
thermal conductivity (Chen, Zeng, and Yuan, 2017).
Therefore, a small addition of this particle has the potential to substantially
increase the thermal conductivity of nanofluid (Hamidi
and Putra, 2023).
In the base fluid,
nanoparticles were dispersed, but a challenge, namely agglomeration, arises
with the particle dispersion (Ilyas, Pendyala, and
Marneni, 2014). Fine particles are prone to easy agglomeration, leading
to inhomogeneous nanofluid. This problem also impacted the stability of
nanoparticles, as they tend to settle at the bottom of nanofluid container (Ilyas, Pendyala, and Marneni, 2014). To overcome
the challenge, a surfactant was added to improve the dispersion inside the
fluid (Jehhef and Siba, 2019; Adiwibowo, Ibadurrohman,
and Slamet, 2018). Several types of these surfactants were categorized
as anionic, cationic, non-ionic, and zwitterionic. The classification was based
on the charge type of the molecule. Surfactants modify the surface of
nanoparticles by decreasing the surface tension, thereby enhancing stability
and dispersion, leading to higher thermal conductivity (Qadariyah
et al., 2022; Asadi et al., 2017; Paramashivaiah
and Rajashekhar, 2016; Kusrini et al., 2019).
Nanofluid
application is primarily applied in heat transfer fluid due to the superior
thermal conductivity. A specific application of the heat transfer fluid is in
the quenching medium during the heat treatment of steel (Agboola et al., 2020; Kresnodrianto et al., 2018; Ikubanni
et al., 2017). Improving steel properties, particularly hardness,
can be achieved by quenching it from the austenitizing temperature,
transforming the microstructure from Austenite to the Martensite phase. The
cooling rate plays a crucial role in the transformation, with a slower rate
resulting in a phase other than Martensite.
Rapid cooling
leads to cracks and distortion in the steel, necessitating the need for
appropriate quench medium with better cooling rate (Fredj
et al., 2017). The use of nanofluid offers a distinct advantage
as cooling rate can be adjusted based on the quantity of nanoparticles added to
the base fluid. This adjustability makes the quenchant highly versatile in the
heat treatment industry.
This study aimed
to provide a more comprehensive experimental analysis of the effect of
combining MWCNT and PEG in nanofluid, which is to be used specifically as a
quenchant in a steel heat treatment process. The difference in the thermal
conductivity of the quenchant was compared directly to the hardness of the
quenched steel. A direct relationship between the thermal conductivity and
cooling rate could be used to design a better quenchant. In summary, a
nanofluid with an adjustable cooling rate may provide an appropriate solution
in a thermal-sensitive heat treatment process to prevent defects.
MWCNT used in this study was a laboratory grade
purchased from Sigma Aldrich. According to the datasheet, MWCNT particle had a
diameter ranging from 50 – 90 nm, with an average of 65 nm. To explore deeper
into the particle characteristics, a Scanning Electron Microscope (SEM),
specifically FEI Inspect F50 with integrated Energy Dispersive Spectroscopy
(EDS) from EDAX, was applied for observation. Nanotube was used to synthesize a
nanofluid with distilled water as the base fluid. Finally, the particle
concentration was varied at 0.1, 0.3, and 0.5 % w/v.
To
improve the dispersion of MWCNT in the base fluid, Polyethylene Glycol (PEG)
surfactant was used as a stabilizer. PEG, purchased from Sigma Aldrich as a
liquid with an average molecular weight of 200, was added at concentrations
ranging from 3 – 30 % v/v for each MWCNT percentage variation. To study
nanofluid characteristics, zeta potential and thermal conductivity were
conducted. Zeta potential was performed using the Horiba SZ-100 series machine
to observe the stability of the particle in the fluid. Meanwhile, KD2 Pro
Thermal Properties Analyzer was adopted to examine the thermal conductivity
based on the transient heat transfer principle.
The
synthesized nanofluid was used as a quench medium in the steel heat treatment
process to enhance the characteristic, particularly hardness. A medium carbon
steel S45C was used as the sample for the heat treatment. The chemical
composition was verified using Optical Emission Spectroscopy (OES) with the
Foundry-Master Xpert equipment. The dimension of the steel is shown in Figure
1a. This sample was austenized at 900°C for 60 minutes to achieve a complete
Austenite phase. However, to prevent cracking or distortion due to thermal
shock, preheating at 540°C was conducted (Mochtar, Putra, and Abram, 2023). The complete thermal treatment
cycle is presented in Figure 1b. After austenitization, the steel was quenched
in nanofluid to obtain a Martensite phase. Microstructure observation and
hardness test were performed to confirm the phase transformation in the sample.
In this study, the microstructure was observed using Olympus
Inverted Metallurgical Microscope BX41M-LED. The steel sample was prepared
beforehand by etching in 2% Nital solution. The etchant duration was varied
from 3 – 10 seconds. For hardness measurement, Rockwell and Vickers hardness
tests were performed with the application of Qualirock Digital Hardness Tester
and Micromet 5100 series micro-indentation tester, respectively.
Figure 1 (a) Steel sample
dimension, (b) Thermal cycle for steel heat treatment
3.1. Preliminary
Observation on the Multiwalled Carbon Nanotube and S45C Steel Sample
MWCNT was examined using SEM to observe the
morphology. Figure 2 presents the electron microscope imaging of MWCNT
particle, showing a length of more than 1 .
Figure 2 Electron microscope image of Multiwalled Carbon Nanotube
The sample
used for quenching was S45C medium carbon steel. Furthermore, the chemical composition was verified
using OES and the result is presented in Table 1.
Table 1 S45C chemical composition by OES
|
Element |
Weight % |
|
Fe |
98.2 |
|
C |
0.42 |
|
Si |
0.26 |
|
Mn |
0.68 |
|
P |
0.008 |
|
S |
0.006 |
Based on the result, the steel sample
conformed to the S45C standard (Otai, 2015)
3.2. Multiwalled
Carbon Nanotube Stability of Nanofluid in Various Surfactant Concentrations
Zeta potential was used to assess the
stability of MWCNT inside nanofluid. The measurement specifically targeted the
concentration of 0.5% MWCNT, with the addition of 3, 7, 10, and 30% of
surfactant. Table 2 shows the result of the measurement.
Table 2 Zeta potential measurement of nanofluid
|
MWCNT (%) |
PEG (%) |
Zeta Potential (mV) |
|
0.5 |
3 |
-37.2 |
|
7 |
-25.5 | |
|
10 |
-23.4 | |
|
30 |
-23.2 |
The result showed that the
introduction of additional PEG enhanced stability by increasing zeta potential
to -37.2 mV. Nanofluid with a zeta potential of more than ± 30 mV was
considered to the moderately stable (Ghadimi, Saidur,
and Metselaar, 2011). On the other hand, less than ± 30 mV implied
suboptimal stability, potentially leading to sedimentation. The augmentation of
PEG contributes to stability improvement by modifying the surface tension on
the particle. During absorption, the particle will have lower surface tension
and create a layer to avoid agglomeration with similar particles. Therefore,
the addition of 3% PEG yielded the most favorable zeta potential in this
investigation. A decrease in stability was observed with higher PEG
concentration, attributable to the formation of micelles from the bulk
surfactant (Zhang et al., 2021; Paramashivaiah and Rajashekhar, 2016). The formation of micelle reduces the ability to
modify the surface tension of the particle, resulting in lower stability.
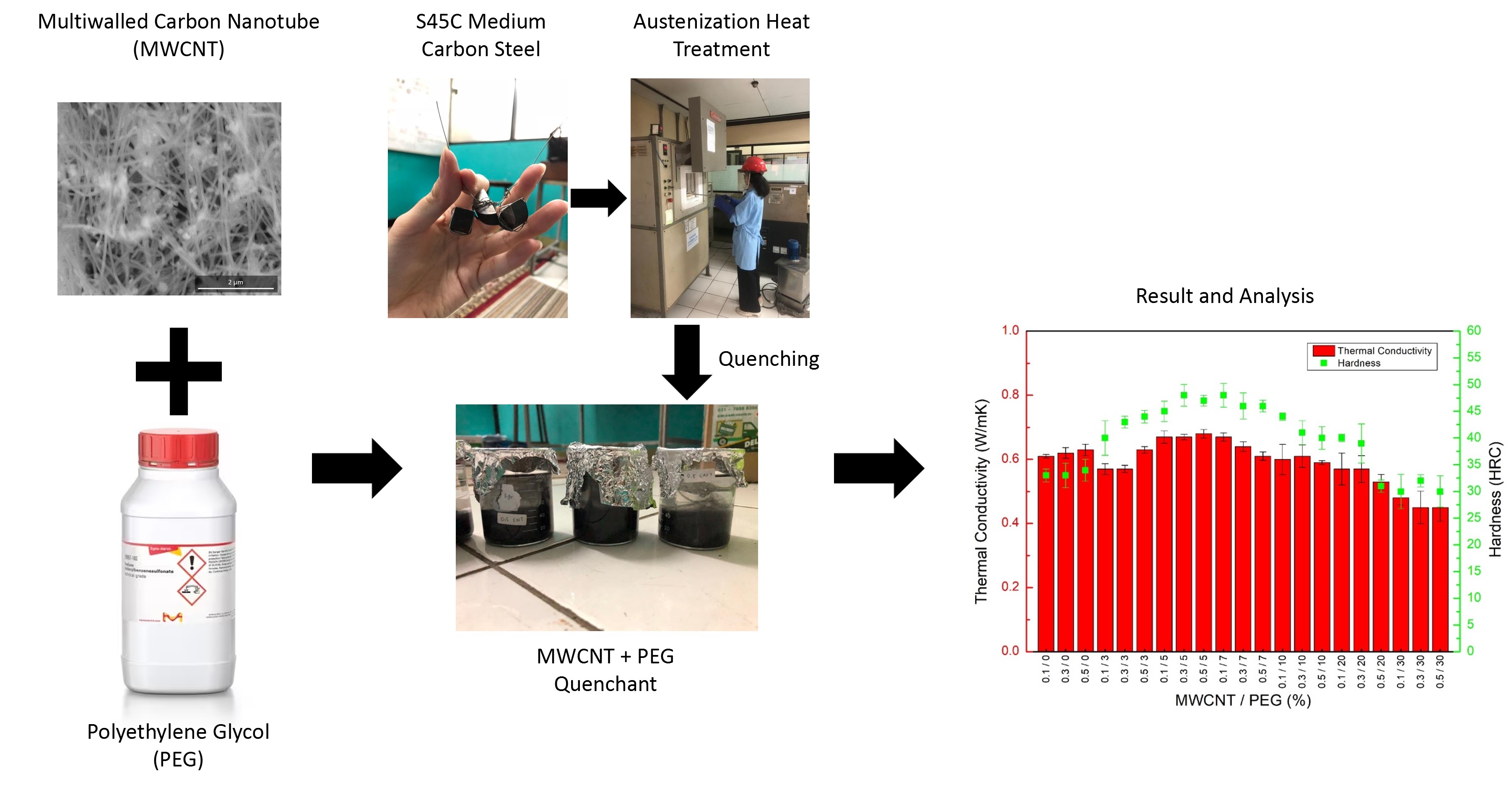
An examination of the effect of additional MWCNT and surfactant on thermal conductivity was also conducted. Figure 3a shows the measurement result on all nanofluid variables. The inclusion of MWCNT increased the thermal conductivity of the quenchant, even without surfactant. Increasing the percentage of nanotube and PEG could enhance conductivity. The highest thermal conductivity of 0.68 W/mK was obtained after 0.5% MWCNT and 5% PEG were added. For comparison, distilled water had a conductivity of 0.59 W/mK, which was improved by approximately 15%. The results suggested that the optimum surfactant concentration was 5%.
Figure 3 (a) Thermal conductivity measurement result,
(b) Steel hardness measurement result
The thermal conductivity
decreased at a higher percentage of PEG surfactant. The poorest conductivity
was observed after the addition of 30% PEG, yielding a value of 0.45 W/mK, even
lower than distilled water. This decline could be attributed to the formation
of surfactant micelles, as explained previously. Higher surfactant
concentration may compromise stability and create agglomeration of MWCNT. This
results in an inhomogeneous nanofluid and reduces the effective transfer of
heat.
3.4.
Improvement of S45C Steel Hardness after Quenching Process in Various
MWCNT-based Nanofluid Quenchant
Nanofluid was used as a
medium to quench austenized S45C medium carbon steel. After quenching, the
sample hardness was measured and presented in Figure 3b. The hardness value
trend was similar to the thermal conductivity. The variable with the highest
thermal conductivity (0.5% MWCNT and 5% PEG) also had the highest hardness at
48 Rockwell hardness C scale (HRC). For comparison, S45C steel sample had a
value of 12 HRC before any heat treatment process, then escalated to 43 HRC
after being quenched with distilled water. Therefore, the increase in hardness achieved
through nanofluid was up to 11% compared to distilled water quenching. S45C
steel sample after being treated with nanofluid was nearly identical to the
expensive high alloy tool steel SKD 61 (Kosasih, Priadi,
and Suliyanti, 2023).
The same trend was followed
for the sample with higher surfactant concentrations. Hardness decreased quite
significantly after the addition of 20% and 30% surfactant. The decrease has a
strong relation with the lower thermal conductivity. In heat treatment, a high
cooling rate was needed for the transformation from the Austenite to the
Martensite phase. Therefore, a quench medium with high thermal conductivity was
essential to obtain a high cooling rate and vice versa (Aziz
and Zaharudin, 2020). Based on the result, it was concluded that higher
thermal conductivity could provide a faster cooling rate, resulting in higher
steel hardness.
3.5. Microstructure
Evolution of S45C Steel after Quenching Process
Microstructure
observation was conducted to support the steel hardness result. Figure 4a and 4b shows the microstructure before and
after heat
treatment, respectively.
Figure 4 Microstructure of S45C (a) before heat
treatment, (b) after quenched in distilled water.
In Figure 4b, distinguishing between Bainite and Martensite
phases in microstructure after quenching proved challenging. A Vickers Hardness was used to
discriminate between the two phases, based on their hardness, as shown in Table
3. Microhardness result suggested that the brighter area was the Martensite
phase, while the darker
area was the Bainite phase. This interpretation was in line with a
similar microstructure
reported by
Nishimoto (Nishimoto et
al., 2022).
Table 3 Vickers microhardness results in
quenched microstructure
|
Area |
Vickers Hardness Number (HV) |
|
Brighter phase |
743 ± 15 |
|
Darker phase |
563 ± 17 |
For comparison, the microstructure of the
steel with the lowest and highest hardness after quenched in nanofluid are
presented in Figure 5a and 5b, respectively. Figure 5a showed that the
microstructure was dominated by Ferrite and pearlite phases, with some
Martensite phases in certain areas. Due to the presence of Ferrite phase,
hardness was low. Meanwhile, Figure 5b showed only Martensite and Bainite
phases, contributing to significantly higher steel hardness. Comparing Figure 4b
(quenched in distilled water) and 5b (quenched in nanofluid), it was observed
that the amount of Martensite was slightly higher in 5b, hence, hardness was
also higher.
Figure 5 Microstructure of S45C quenched in nanofluid
with (a) 0.5% MWCNT and 30% PEG, (b) 0.3% MWCNT and 5% PEG
In conclusion, the integration of MWCNT as nanoparticle and PEG
surfactant improved the performance of quenching medium by enhancing thermal
conductivity. The superior thermal conductivity of nanoparticles contributed to
enhanced nanofluid conductivity. The results showed that in the absence of a
surfactant, the inclusion of MWCNT alone elevated water conductivity to 0.63
W/mK. Significantly, the introduction of 5% PEG concentration led to further
refinement, thereby increasing the value to 0.68 W/mK. This refinement resulted
from the improved dispersion and stabilization of MWCNT particles facilitated
by PEG. Zeta potential analysis showed that the addition of 3% surfactant led
to an increase, reaching -37.2 mV. This showed a moderate level of nanofluid
stability. The pursuit of higher thermal conductivity translated into a faster
cooling rate and increased steel hardness. Experimental results substantiated
this notion, as steel sample subjected to quenching in nanofluid with high
conductivity showed hardness level of 48 HRC. The excessive addition of
surfactant adversely affected thermal conductivity and steel hardness
post-quenching. At higher concentrations, micelle formation occurred,
diminishing the efficacy of modifying surface tension and leading to particle
agglomeration. Consequently, thermal conductivity was reduced to 0.45 W/mK, and
steel hardness decreased to a minimum of 30 HRC due to a slower cooling rate.
This study explored the development of an adaptable cooling rate quenchant
based on nanofluid, offering a potential solution for steel heat treatment
industry, particularly for products sensitive to thermal variations. The determination
of an optimal ratio between particle and surfactant concentration was a
critical factor in achieving the desired objectives.
The authors are grateful to Mr. Eddie Susanto and Ms.
Edela Uswah Dien-Muhammad for the significant assistance provided on the
technical aspect of this study.
Adiwibowo, M.T., Ibadurrohman, M., Slamet, S.,
2018. Synthesis of Zno Nanoparticles and their Nanofluid Stability in the
Presence of a Palm Oil-Based Primary Alkyl Sulphate Surfactant for Detergent
Application. International Journal of
Technology, Volume 9(2), pp. 307–316
Agboola, O.O., Ikubanni, P.P., Adeleke, A.A.,
Adediran, A.A., Adesina, O.S., Aliyu, S.J., Olabamiji, T.S., 2020. Optimization
of Heat Treatment Parameters of Medium Carbon Steel Quenched in Different Media
using Taguchi Method and Grey Relational Analysis. Heliyon, Volume 6(7)
Ali, A.R.I., Salam, B., 2020. A Review on Nanofluid:
Preparation, Stability, Thermophysical Properties, Heat Transfer Characteristics
and Application. SN Applied Sciences, Volume 2(10), p. 1636
Arularasan, R., Babu, K., 2021. Thermally Annealed
Biochar Assisted Nanofluid as Quenchant on the Mechanical and Microstructure Properties
of AISI-1020 Heat-treated steel—a Cleaner Production Approach. Biomass
Conversion and Biorefinery, pp. 1–10
Asadi, A., Asadi, M., Siahmargoi, M., Asadi, T.,
Gholami Andarati, M., 2017. The effect of surfactant and sonication time on the
stability and thermal conductivity of water-based nanofluid containing Mg(OH)2
nanoparticles: An experimental investigation. International Journal of Heat
and Mass Transfer, Volume 108, pp. 191–198
Aziz, R.A., Zaharudin, R., 2020. Thermal
Conductivity and Stability Studies of Cooking and Waste Cooking Oil as a Based
Fluid of TiO2 Nanofluid for Carbon Steel Quenching Process. Journal
of Advanced Research in Fluid Mechanics and Thermal Sciences, Volume 78, pp.
22–33
Babu, K., Arularasan, R., Ramkumar, S.S., 2017.
Quenching Performance of AISI 1010 in CNT Nanofluids. Materials Today:
Proceedings, Volume 4, pp. 11044–11049
Chen, S., Zeng, X., Yuan, Q., 2017. Effect of Carbon
Nanotube Concentration on Cooling Behaviors of Oil-based Nanofluids During the Immersion
Quenching. Journal of Shanghai Jiaotong University (Science), Volume 22,
pp. 395–401
Fredj, E.B., Nanesa, H.G., Shahriari, D., Morin,
J.-B., Jahazi, M., 2017. Effect of Cooling Rate on Phase Transformation and Microstructure
Evolution in a Large Size Forged Ingot of Medium Carbon Low Alloy Steel. In:
TMS 2017 146th Annual Meeting and Exhibition Supplemental
Proceedings, pp. 413–423
Ghadimi, A., Saidur, R., Metselaar, H.S.C., 2011.
A Review of Nanofluid Stability Properties and Characterization in Stationary Conditions.
International Journal of Heat and Mass Transfer, Volume 54 (17-18), pp. 4051–4068
Hamidi, R.R., Putra, W.N., 2023. Effect of Polyethylene
Glycol Addition as Surfactant on Thermal Conductivity of Carbon Nanotube Based Nanofluids.
In: AIP Conference Proceedings, Volume 2538(1), p. 030001
Ikubanni, P.P., Adediran, A.A., Adeleke, A.A.,
Ajao, K.R., Agboola, O.O., 2017. Mechanical Properties Improvement Evaluation
of Medium Carbon Steels Quenched in Different Media. International Journal
of Engineering Research in Africa, Volume 32, pp. 1–10
Ilyas, S.U., Pendyala, R., Marneni, N., 2014.
Preparation, Sedimentation, and Agglomeration of Nanofluids. Chemical
Engineering & Technology, Volume 37(12), pp. 2011–2021
Jehhef, K.A., Siba, M.A.A.A., 2019. Effect of
Surfactant Addition on the Nanofluids Properties: A Review. Acta Mechanica
Malaysia, Volume 2(2), pp. 01–19
Kosasih, R., Priadi, D., Suliyanti, M.M., 2023.
Coating Material Development by Pulsed Laser Deposition for JIS SKD61 Steel
Insert Pins Used in Aluminum Casting Industry. International Journal of
Technology, Volume 14(4), pp. 833–842
Kresnodrianto, Harjanto, S., Putra, W.N.,
Ramahdita, G., Yahya, S.S., Mahiswara, E.P., 2018. Characterization of Water-based
Nanofluid for Quench Medium. In: IOP Conference Series: Materials
Science and Engineering, Volume 348(1), p. 012009
Kusrini, E., Putra, N., Siswahyu, A., Tristatini,
D., Prihandini, W.W., Alhamid, M.I., Yulizar, Y., Usman, A., 2019. Effects of
Sequence Preparation of Titanium Dioxide–Water Nanofluid using
Cetyltrimethylammonium Bromide Surfactant and TiO2 Nanoparticles for
Enhancement of Thermal Conductivity. International Journal of Technology, Volume
10(7), pp. 1453–1464
Mochtar, M.A., Putra, W.N., Abram, M., 2023.
Effect of Tempering Temperature and Subzero Treatment on Microstructures, Retained
Austenite, and Hardness of AISI D2 Tool Steel. Materials Research Express,
Volume 10(5), p. 056511
Nishimoto, M., Muto, I., Doi, T., Kawano, K.,
Sugawara, Y., 2022. Effect of Quenching in Aqueous Polyvinylpyrrolidone Solutions
on the Microstructure and Pitting Corrosion Resistance of AISI 1045 Carbon Steel.
Materials and Corrosion, Volume 74(5), pp. 724–733
Otai, 2015. JIS G4051 S45C Steel For Machine
Structural Use. Available Online at: https://www.astmsteel.com/product/jis-s45c-steel-machine-structural/,
Accessed on August 01, 2023
Paramashivaiah, B.M., Rajashekhar, C.R., 2016.
Studies on the Effect of Various Surfactants on Stable Dispersion of Graphene Nanoparticles
in Simarouba Biodiesel. In: IOP Conference Series: Materials Science and
Engineering, Volume 149(1), p. 012083
Qadariyah, L., Sahila, S., Sirait, C., Purba,
C.P.E., Bhuana, D.S., Mahfud, M., 2022. Surfactant Production of Methyl Ester
Sulfonate from Virgin Coconut Oil using Aluminum Oxide with Microwave
Assistance. International Journal of Technology, Volume 13(2), pp. 378–388
Radhiyah, A.A., Nurziela, K., 2020. Effect of TiO2
Nanofluid and Hybrid TiO2 Nanofluid on Mechanical Properties of
Steels for Automotive Applications. In: Journal of Physics: Conference
Series, Volume 1529(5), p. 052036
Ramadhani, C.A., Putra, W.N., Rakhman, D.,
Oktavio, L., Harjanto, S., 2019. A Comparative Study on Commercial Grade and
Laboratory Grade of TiO2 particle in Nanofluid for Quench Medium in
Rapid Quenching Process. In: IOP Conference Series: Materials Science
and Engineering, Volume 622(1), p. 012017
Septiadi, W.N., Prasetia, K.W.T., Murti, M.R.,
Sukadana, I.G.K., Rahman, F., Putra, G.J.P., Marianti, K.M., 2020. Investigated
on Thermal Design of a Computer Cooling System with the Effective Length of a
Cascade Heat Pipe. International Journal of Technology, Volume 11(7),
pp. 1388–1396
Xia, G., Jiang, H., Liu, R., Zhai, Y., 2014.
Effects of Surfactant on the Stability and Thermal Conductivity of Al2O3/de-ionized
Water Nanofluids. International Journal of Thermal Sciences, Volume 84, pp.
118–124
Yahya, S.S., Harjanto, S., Putra, W.N., Ramahdita, G., 2018. Characterization and Observation of Water-Based Nanofluids Quench Medium With Carbon Particle Content Variation. In: AIP Conference Proceedings, Volume 1964,(1) pp. 1–10
Zhang, J., Ge, D., Wang, X., Wang, W., Cui, D., Yuan, G., Wang, K., Zhang, W., 2021. Influence of Surfactant and Weak-Alkali Concentrations on the Stability of O/W Emulsion in an Alkali-Surfactant–Polymer Compound System. American Chemical Society (ACS) Omega, Volume 6(7), pp. 5001–5008
