Performance of In-Situ Stirring Batch Reactor Transesterification of Nannochloropsis sp Microalgae into Biodiesel
Corresponding email: yustiawulandari_che@itats.ac.id
Published at : 10 Jul 2024
Volume : IJtech
Vol 15, No 4 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i4.6678
Mirzayanti, Y.W., Marlinda, L., Irawan, H., Al Muttaqii, M., Ma'sum, Z., Asri, N.P., Chern, J.-M., 2024. Performance of In-Situ Stirring Batch Reactor Transesterification of Nannochloropsis sp Microalgae into Biodiesel. International Journal of Technology. Volume 15(4), pp. 859-869
| Yustia Wulandari Mirzayanti | Department of Chemical Engineering, Faculty of Technology Industrial, Institut Teknologi Adhi Tama Surabaya, Surabaya 60117, Indonesia |
| Lenny Marlinda | Department of Industrial Chemistry, University of Jambi, Jambi 36361, Indonesia |
| Hery Irawan | Department of Mechanical Engineering, Faculty of Technology Industrial, Institut Teknologi Adhi Tama Surabaya, Surabaya 60117, Indonesia |
| Muhammad Al Muttaqii | Research Center for Chemistry, Badan Riset dan Inovasi Nasional, South Tangerang 15314, Indonesia |
| Zuhdi Ma'sum | Department of Chemical Engineering, University of Tribhuwana Tungga Dewi, Malang 65144, Indonesia |
| Nyoman Puspa Asri | Department of Food Technology, University of Ciputra, Surabaya, 60219, Indonesia |
| Jia-Ming Chern | Department of Chemical Engineering and Biotechnology, Tatung University, Taipei, 10491, Taiwan |
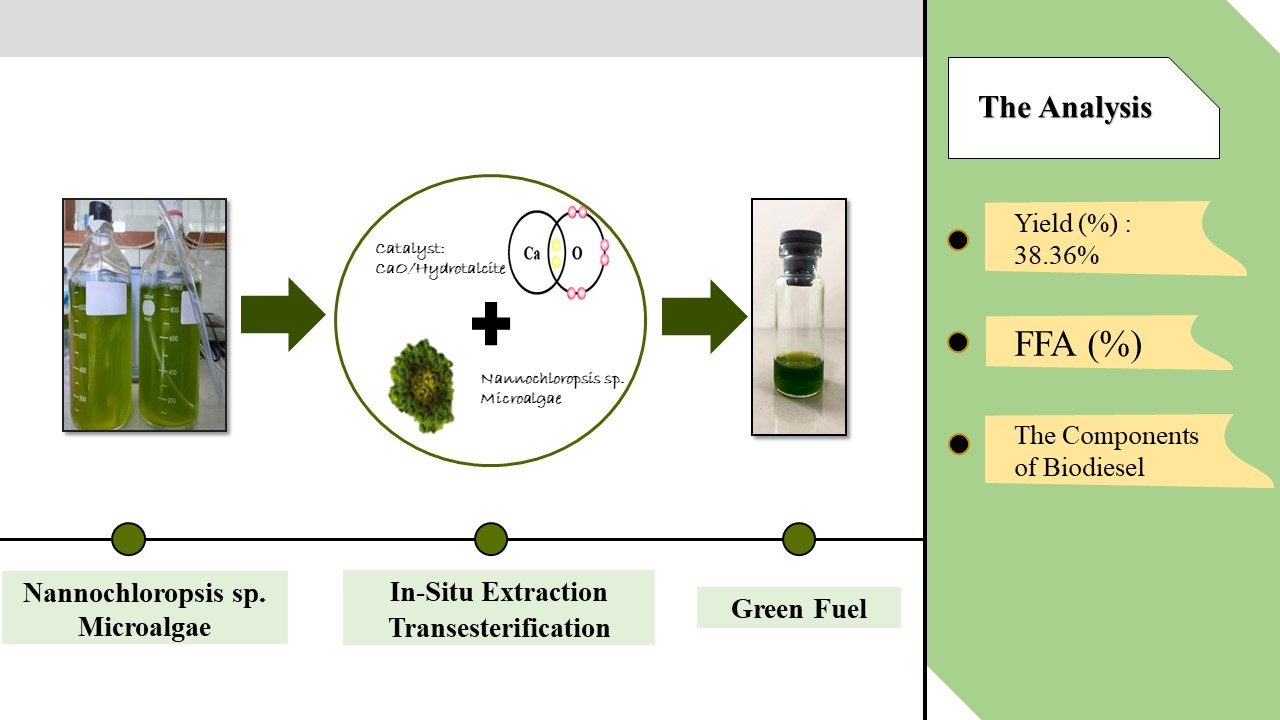
In-situ
stirring batch reactor transesterification is a widely used method for the
conversion
of various biomass into biodiesel,
particularly Nannochloropsis sp
microalgae. Therefore,
this study aims to investigate the effect of CaO/Hydrotalcite catalyst loading
ratio and stirring rate on in situ stirring batch reactor
transesterification of Nannochloropsis sp microalgae to produce
biodiesel. In-situ transesterification was carried out using stirring
with a four-blade paddle (50 w), while CaO/Hydrotalcite catalyst preparation
was performed using incipient wetness impregnation. Nannochloropsis sp
microalgae were then converted into biodiesel with varying loading ratio of
catalyst (1:1, 3:1, and 5:1 %wt/wt) and stirring speed of agitation (50, 100,150, 200, and 250 rpm) for 4 hours
(60oC). Subsequently, biodiesel obtained from the process was
analyzed using gas chromatography (GC). The results showed that the surface
area of CaO/Hydrotalcite was 45.756 m2/g. The conversion yields of Nannochloropsis
sp microalgae to biodiesel with CaO/Hydrotalcite reached 38.36% with an
agitation rate of 250 rpm. Based on the fatty acids analyzed using GC methods,
the products predominantly contained olefine (61.49%) and gasoline (38.51%).
Biodiesel; CaO/Hydrotalcite; In-Situ transesterification; Nannochloropsis sp.
Renewable energy, such
as biofuels, is a valuable alternative to fossil fuels due to its beneficial
properties. In addition, biofuels
are classified into generations based on the raw materials used during their
synthesis, including edible and non-edible oils, biomass, lignocellulose, and
residues (Lomeu et al., 2023;
According to previous studies, biodiesel is predominantly produced using transesterification
method, which comprises mixing lipid (triglyceride) and alcohol, with catalysts being used to facilitate the
synthesis of ester and glycerol. High free fatty acids (FFA) cannot be directly
tranesterified using an alkaline catalyst, which gives low yield and low
quality of biodiesel
Several studies have explored the conventional conversion of Nannochloropsis
sp. microalgae to biodiesel using extraction with solvents, followed by
transesterification process (
Heterogeneous catalyst are gaining more interest because its
have advantages over homogeneous catalysts (Aisyah et al., 2023; Rahma and Hidayat, 2023; Cercado,
Ballesteros, and Capareda, 2018). Based on previous studies, calcium oxide (CaO)
is a base heterogeneous catalyst with good potential for chemical reaction,
particularly transesterification reaction
The novelty in this current
study lies in the use of a metal catalyst (CaO/Hydrotalcite) to convert Nannochloropsis
sp microalgae into biodiesel using in-situ transesterification method as
well as exploring the effectiveness of the agitation rate parameters on reactor
batch in-situ transesterification. CaO is an alkali metal oxide with various
advantages, while hydrotalcite is a heterogeneous catalyst that can accelerate
transesterification reaction. The investigation primarily focuses on the
performance of the combination of CaO metal and hydrotalcite as catalyst. Reactor's design was in-situ
stirred batch reactor with a unique design comprising a four-blade paddle. Catalyst
used was an alkali metal, which comprised the combination of CaO/Hydrotalcite
heterogeneous catalyst and methanol as a solvent and a reactant in in-situ
transesterification process, with n-hexane serving as a co-solvent. Therefore, this study aims to determine the
effect of CaO/Hydrotalcite loading ratio and stirring rate in converting Nannochloropsis
sp. microalgae.
This section described the principal materials
and the methodologies used in the experimental procedures.
2.1. Material, Equipment, and Apparatus
Nannnochloropsis sp
microalgae were obtained from the Brackish Water Aquaculture Fisheries at
Jepara, Central Java, Indonesia. Meanwhile, calcium carbonate, methanol,
n-hexane (Merck & Co, 99%), and hydrotalcite synthetic (Sigma Aldrich, 99%)
were purchased from an Indonesian supplier. The major equipment was in-situ
stirred batch reactor (material: stainless steel type 304), vertical
cylindrical shape equipped with a stirrer. The top and bottom covers were
flange-shaped, with a total tank volume of 20 liters and a stirrer of 50 w
(four-blade paddle) (Figure 1a). Figure 1b illustrated a stirred tank batch
reactor with dimensions of outside diameter (do) = 7.48 in; cylinder
height (Ls) = 7.48 in; cylinder thickness (ts) = 3/16 in;
and leg support (l) = 20 in (Scale 1:1). The heating circulation
had a maximum speed of 500 rpm, a maximum temperature of 250oC, and
H of 20.5 in (scale = 1:1).
2.2.
Synthesis and Characterization of
CaO/Hydrotalcite
The incipient wetness impregnation method was
used for the synthesis of the CaO/Hydrotalcite catalyst, which was placed in an
oven for 12 hours at 100oC. The calcium carbonate (CaCO3)
was calcined into CaO for 3 hours at 900oC and then weighed with
loading ratio of CaO to hydrotalcite (1:1, 3:1, and 5:1 wt/wt). Subsequently,
CaO was dissolved in distilled water, and the solution was sprayed onto
hydrotalcite slowly and evenly until it seeped into hydrotalcite pores at room
temperature. The sample was dried at 100oC for 12 h and treated in
the furnace at 900oC for 3 hours. The final product was
CaO/Hydrotalcite catalyst powder, which was characterized using X-ray
diffraction (XRD), X-ray Fluorescence (XRF), and Brunaur Emmett and Teller (BET) to investigate the surface area. The characterization techniques
of XRD investigations were performed using instruments possessing Cu K-Alpha
source, 40 kV and 30 mA generator settings, and no beam monochromator.
Figure 1 a) In Situ stirred batch
reactor transesterification, b) stirred tank batch reactor, and c) heat
circulation
2.3.
In-Situ Transesterification Reaction
The experimental setup consisted of in-situ stirred batch reactor, as
shown in Figure 1. The weighed amount of Nannochloropsis sp microalgae
biomass was mixed with CaO/Hydrotalcite catalyst (10%wt of microalgae) and
methanol. The mixture obtained was then blended for 4 hours at 60oC
with varying agitation rates of 50, 100, 150, 200, and 250 rpm. After the
reaction had occurred, it was cooled to separate biodiesel from glycerol and
suspended solids. Subsequently, the filtered residue was washed
using 30 mL of a mixture of methanol-n-hexane (1:1 v/v) to obtain fatty acids
methyl ester (FAME). The filtrate was separated by adding n-hexane with a 1:1
(v/v) ratio, shaken, and allowed to stand until 2 layers formed for 30 minutes.
The bottom layer was then removed from the separating, and n-hexane was added
with a 1:1 (v/v) ratio. The solution was shaken and allowed to stand until 2
layers formed for 8 hours. The top layer was washed to remove any catalyst and
glycerol in the mixture. The product obtained was distilled (70oC)
to separate FAME and solvent. Gas Chromatography-Mass Spectrometry
(GC-MS) was used to analyze biodiesel for detected fatty acids in higher yields
in all variables. The peak area from GC-MS data could be read by the
normalization method based on the peak area per percentage of identified
components used to measure the GC-MS results. All hydrocarbon components were
identified using Wiley275 and NIST02 mass spectral data libraries. Hydrocarbon
components with a probability equal to or above 80% were considered in line
with previous studies (Marlinda
et al., 2022; Mirzayanti et al., 2020b). This current study optimized the process variables for the highest
yield, namely the effectivity of the speed
of stirring conversion to crude biodiesel and loading of CaO/Hydrotalcite
catalyst by microalgae, density, acid number, and %FFA. The limitation
of this report was that it solely focused on the effect of the CaO and
hydrotalcite loading ratio and stirring speed on yield and FFA. The best yield
obtained was subjected to GC-MS testing to determine the content of hydrocarbon
compounds produced.
2.4. Analysis of Crude Biodiesel
Analysis and determination of the %yield of crude biodiesel was calculated with the formulations presented below (Equation 1)
where
MB is the mass
of crude biodiesel (g); and MA is microalgae (g) mass. Compound analysis
biodiesel components (ASTM D4007) were analyzed using GC-MS.
3.1. Characteristics of
Nannochloropsis sp Microalgae
Characteristics
of lipid of liquid Nannochloropsis sp microalgae biomass are presented in Table 1.
The results showed that the highest fatty acids content in Nannochloropsis sp microalgae was oxygenated compounds
(51.80%), followed by carboxylic acid (46.61%). In addition, oxygenated compounds were hydrocarbons containing at least 1 oxygen
atom as a part of their chemical structure, and are commonly referred to as
fuels. The 4 general classes of hydrocarbons included
alkanes, alkenes, alkynes, and arenes. Carboxylic acid, in organic
chemistry, is an organic acid containing a carboxyl group (C(=O)OH) attached to
an R-group. This compound was characterized by its carbon (C) atom being bonded to an
oxygen (O) atom by a double bond as well as to a hydroxyl group (?OH) by a
single bond. Based on Table 1, Nannochloropsis sp microalgae could be
used as raw materials for biodiesel.
Table 1
Fatty acid content in Nannochloropsis sp microalgae
|
No |
Component |
%area |
|
1 |
Alkenes/olefins |
1.59 |
|
2 |
Oxygenated compounds |
51.80 |
|
3 |
Carboxylic acid |
46.61 |
The
total lipid content of microalgae biomass largely depended on the specific
cultivation technology. Microalgae culture condition, nutrients, and light intensity could be
optimized to increase the oil content, leading to enhanced biodiesel
production. In addition, the growth rate of this organism and maximum biomass
production depended on their culture systems (light, temperature, pH, salinity,
consumption of O2, CO2, nutrients, and toxic chemicals) (Medipally et al., 2015). According to previous
studies, nutrients for microalgae growth included nitrogen (N) and/or
phosphorus (P) (Kazemifard et al.,
2019). Nannochloropsis sp microalgae could serve as biodiesel due to
the abundance of its oleic acid content (73.40%) among the remainder of the
MUFA composition (Kanagesan
et al., 2020). The
specific growth rate of Nannochloropsis
sp microalgae strains generally ranged from 0.11 to 0.21 per day, with
total lipid content of 37-60% of dry weight (DW) (Ma et al., 2016).
3.2. Catalyst Characterization
Figure
2 showed a series of XRD diffractions of CaO/Hydrotalcite. The initial
incipient wetness impregnation at 900oC in 3 hours predominantly
contained CaO and MgO with few amounts of Al2O3. In
addition, the XRD pattern of CaO/Hydrotalcite catalyst had related curves in terms of
uniformity. The peaks obtained were compared with the Joint Committee on Powder
Diffraction Standards (JCPDS) file. From Figure 3, the principal components of
CaO/Hydrotalcite catalyst
included CaO, Ca2Al, and MgO. Diffraction 2? from JCPDS for
using CaO was 32.2o, 37.3o, and 53.8o.
Therefore, the pattern in Figure 2 showed the intensified and narrow peaks at 2 =
17.88o, 28.47o, 33.92o, 42.88o,
47.07o, and 50.60o, as reported in a previous study (Mohamad
et al., 2018). Catalyst characterization
also used XRF analysis to determine the chemical compositions. The elemental
chemical compositions of catalyst in this study are presented in Table 2.
According to the XRF, the CaO content of the various ratio of CaO with hydrotalcite
(1:1, 3:1, and 5:1 %wt/wt) increased gradually. The highest amount of CaO was
obtained at ratio of 5:1 %wt/wt (84.13 %m/m), as shown in Table 2. The results
suggested that the decomposition of carbonates was achieved at 900oC
for approximately 3 hr. In a previous study, the majority of variation ratio
catalyst above 50% were CaO species (Amusan et al., 2019). The total content of MgO and Al2O3
was below 50% because hydrotalcite was decomposed into metal oxides when
calcined at 900oC
for 3 hours (Amusan et al., 2019; Mohamad et al., 2018).
The surface area of CaO/Hydrotalcite particles synthesized to catalyst (obtained by calcination at 900oC for 3 h) from BET and BJH was 45.756 m2/g. Furthermore, the surface area of the obtained catalyst showed good activity for converting Nannochloropsis sp oil into biodiesel. This result was consistent with a previous study, which distributed CaO or K2CO3 in various support materials (Manurung et al., 2023; Zhang et al., 2019). Navajas et al. (2018) also reported the best results for hydrotalcite activity as a heterogeneous methanolysis catalyst. These outcomes were attributed to the high basicity properties of the solid that was subjected to the rehydration process after calcination with a surface area of 12 and 66 m2/g. Apart from the strong basic sites, the presence of interlayer hydroxide anions at the accessible crystal edges enhanced catalyst's activity. However, hydrotalcite synthesized using a variety of processes had a surface area of 131-153 m2/g at pH 10 and 150oC (Navajas et al., 2018). This result was better than previous studies, which obtained an average value of 34.6 m2/g (Anr et al., 2017). The CaO/Hydrotalcite area was more significant compared to a previous study at 4.34 m2/g (Win and Khine, 2017).
Figure 2 XRD diffractogram of CaO/Hydrotalcite calcined at
900oC for 3 h (5:1 %wt/wt)
Table 2 XRF
analysis of CaO/Hydrotalcite at 900oC for 3 hours
|
No |
Component |
%Massa (m/m) | ||
|
1:1 |
3:1 |
5:1 | ||
|
1 |
CaO |
55.05 |
66.80 |
84.13 |
|
2 |
MgO |
32.33 |
24.08 |
11.76 |
|
3 |
Al2O3 |
12.35 |
8.90 |
4.00 |
|
4 |
SiO2 |
0.17 |
0.11 |
0.02 |
|
5 |
Na2O |
0.08 |
0.10 |
0.08 |
|
6 |
Fe2O3 |
0.01 |
0.01 |
0.00 |
|
7 |
SO3 |
0.01 |
0.01 |
0.01 |
3.3. Catalytic Performance for
Biodiesel Production
In-situ
stirring batch reactor transesterification reaction was performed using
CaO/Hydrotalcite, and the reaction conditions were investigated by varying the
different parameters. The effect of stirring speed and CaO loading on
transesterification was also studied in the reaction.
3.3.1. Effect
of stirring rate and CaO loading on %yield crude biodiesel
Figure
3a presents stirring speed of the different CaO/Hydrotalcite ratio of
CaO/Hydrotalcite catalyst. In addition, the highest yield of crude biodiesel at
250rpm was obtained with a 1:1 wt/wt ratio of catalyst (38.36%). A high mixing
must be used during in situ transesterification to obtain high FAME and
yield (Salam, Velasquez-Orta, and Harvey, 2016). The results showed that the yield increased with an increase in mixing
speed. The process showed that the mixing speed at 250 rpm was 38.36%. Figure 3 also showed the presence of an anomaly in the decrease of
%yield crude biodiesel of CaO/Hydrotalcite catalyst (1:1 %wt/wt; 150 rpm and
3:1 %wt/wt; 100 and 200 rpm). Loading ratio of CaO/Hydrotalcite catalyst at 1:1
produced the highest yield. This was due to the best metal composition in the
1:1 %wt/wt composition. When catalyst loading ratio was increased (5:1 %wt/wt),
the results showed a decrease in
%yields, as reported in previous studies (Koech, Kumar, and Siagi, 2020). The outcome observed was caused by interaction
with other molecules/compounds, leading to increased by-products. The high
catalyst concentration led to a corrosive nature, which hindered
transesterification reaction.
Figure 3 Effect of stirring speed (a) on %yield crude biodiesel based on
the effect of CaO/Hydrotalcite loading ratio (b) on FFA crude biodiesel based
on the effect of CaO/Hydrotalcite loading ratio
3.3.2. Effect of stirring rate and CaO loading on %FFA crude
biodiesel
Figure 3b
showed that the lowest FFA in loading ratio CaO/Hydrotalcite of 1:1 %wt/wt (250
rpm) was 1.39%. In addition, the higher stirring speed, the higher the level of
decrease in %FFA. The results also showed that stirring rate had a significant
effect on the FFA of crude biodiesel, where the higher stirring rate, the
smaller the FFA observed. The acid number obtained affected the quality of
biodiesel, and the higher the acid number in biodiesel, the lower its quality.
This parameter could also affect the shelf life and the level of corrosiveness
of the engine. The results showed that high acid numbers created deposits in the fuel
system and reduced the quality of fuel system components.
3.4. Properties of
Nannochloropsis sp Microalgae Biodiesel
Table 3 presented the physicochemical characteristics of Nannochloropsis sp microalgae biodiesel. The properties showed that Nannochloropsis sp microalgae could be used as biodiesel raw material. According to a previous study, biodiesel obtained from this species had a density quality of 0.839 g/m3 (Koech, Kumar, and Siagi, 2020; Salam, Velasquez-Orta, and Harvey, 2016). The results in Table 3 confirmed the good characteristics of Nannochloropsis sp microalgae for conversion into biodiesel.
Table 3 Physicochemical properties Characteristics of Nannochloropsis
sp Microalgae
Biodiesel
|
No |
Properties |
Test method |
Standard |
Values of algae Biodiesel |
|
1 |
Density (g/cm3) |
SNI 7182:2015 |
0.850 – 0.890 |
0.869 |
|
2 |
Acid value (KOH/g) |
ASTM D 664 |
max. 0.5 |
0.315 |
|
3 |
Free Fatty Acid value |
- |
max. 2 |
1.39 |
Based on the results of data
processing of GC-MS, Nannochloropsis
sp microalgae biodiesel using CaO/Hydrotalcite consisted of
toluene (61.49%area) and Octane (38.51%), as shown in Table 4. Toluene was a
hydrocarbon compound belonging to the olefin/alkenes group with a closed chain/aromatic
type. Several studies had shown that it had the molecular formula of C6H5CH3
and was a derivative of benzene compounds, namely methylbenzene. Meanwhile,
octane was a hydrocarbon compound with an open/aliphatic chain type in the
Alkane group. According to previous reports, it had the molecular formula of C8H18
was a compound belonging to the alkane group. Petroleum fractions were
classified based on the amount of carbon in the hydrocarbon bond chain where C4-C12
was included in gasoline, C10-C16 was kerosene, and C12-C22
was categorized as biodiesel. Olefins contained in biofuels were C7
hydrocarbons, and constituents were hydrocarbons with the number of carbon
chains C8 included in one of the gasoline, which had a range of
carbon numbers C4-C12 (Salam, Velasquez-Orta, and Harvey, 2016).
Transesterification process in the presence of CaO occurred through various steps. Protons (H+) were extracted from CH3OH at the CaO base site to form CH3O-. In triglyceride compounds, the carbon atom of the carbonyl functional group was attacked by CH3O- to produce the alkoxy carbonyl compound, which was rearranged into FAME (the desired product) and the diglyceride anion. Subsequently, the diglyceride anion attacked the calcium hydroxide cation to form diglyceride and CaO, leading to the recovery of catalyst. Based on the results and previous studies, CaO/Hydrotalcite catalyst was a solution to obtain catalyst system that combined the characteristics of a bimodal pore distribution and micropores. In addition, it provided basic sites and high surface density along with the presence of a suitable macro or mesopore network responsible for triglyceride transport. This allowed rapid access to the active site from the bulk reaction medium and removal of products (glycerol and FAME) from catalyst (Nisar et al., 2021). The reaction mechanism comprising the use of CaO is presented in Figure 4.
Figure 4 A chemical reaction
mechanism involving CaO
Table 4 Nannochloropsis sp microalgae biodiesel compounds using GC-MS analysis
|
No |
Compounds |
Retention Time |
%area |
|
1 |
Toluene |
2.876 |
61.49 |
|
2 |
Octane |
3.181 |
38.51 |
In conclusion, Nannochloropsis
sp microalgae was subjected to in situ extraction-transesterification with
methanol (solvent) and n-hexane (co-solvent) using CaO/Hydrotalcite catalyst.
The highest yield obtained was 38.36%, with an FFA content of 1.39% (250 rpm;
acid number 7.63 mg NaOH/g sample, and density 0.908 g/cm3). The
GC-MS analysis showed the presence of toluene-like olefin (61.49%) and
octane-like gasoline (38.51%)s group, showing the effectiveness of CaO/Hydrotalcite
catalyst in converting Nannochloropsis sp microalgae to biodiesel using in
situ stirring batch reactor transesterification.
The authors are grateful to the Ministry of Education, Culture, Research, and Technology for supporting
this study in the Regular Fundamental Research Project. The number of contract
was 183/E5/PG.02.00.PL/2023; 074/SP2H/PT/LL7/2023.
Aisyah, A.N., Ni’maturrohmah, D., Putra, R.,
Ichsan, S., Kadja, G.T.M., Lestari, W.W., 2023. Nickel Supported on MIL-96(Al)
as an Efficient Catalyst for Biodiesel and Green Diesel Production from Crude
Palm Oil. International Journal of Technology, Volume 14(2), pp. 276–289
Amusan,
O.O., Louis, H., Hamzat, A.T., Oyebanji, O.F., Alagbe, A.T., Magu, T.O., 2019.
Asian Journal of Nanoscience and Materials Synthesis and Characterization of
CaO Catalyst Obtained from Achatina Achatina and Its Application in Biodiesel
Production. Asian Journal of Nanoscience and Materials, Volume 2, pp. 271–277
Asri, N.P., Saraswati, R., Hindarso, H.,
Suprapto, Mirzayanti, Y.W., Yogaswara, R.R., 2021. Study of Catalyst Support
Utilization on Zno-Based Solid Catalyst to Its Activity at Transesterification of
Kesambi (Schleichera oleosa) Oil. IOP Conference Series: Materials Science
and Engineering, Volume 1034(1), p. 012059
Budianto, A., Pambudi, W.S., Sumari, S., Yulianto,
A., 2018. PID Control Design for Biofuel Furnace Using Arduino. Telkomnika
(Telecommunication Computing Electronics and Control), Volume 16(6), pp. 3016–3023
Cercado,
A.P.I., Ballesteros, F.C., Capareda, S.C., 2018.
Biodiesel From Three Microalgae Transesterification Processes Using Different
Homogenous Catalysts. International Journal of Technology, Volume 9(4), pp.
645–651
Culaba,
A.B., Ubando, A.T., Ching, P.M.L., Chen, W.H., Chang, J.S., 2020. Biofuel from
Microalgae: Sustainable Pathways. Sustainability, Volume 12(19), pp. 1–19
El-Sheekh,
M.M., 2021. Biodiesel from Microalgae: Advantages and Future Prospective. Egyptian
Journal of Botany, Volume 61(3), pp. 669–671
Ghedini, E., Taghavi,
S., Menegazzo, F., Signoretto, M., 2021. A
Review on The Efficient Catalysts for Algae Transesterification to Biodiesel. Sustainability,
Volume 13(18), p. 10479
Kanagesan,
K., Palanisamy, K.M., Maniam, G.P., Hasbi, M., Rahim, A., Govindan, N., 2020.
Biodiesel Production by Microalgae Nannochloropsis sp. Grown in Palm Oil Mill
Effluent. Maejo International Journal of Energy and Enviromental
Communication, Volume 2(3), pp. 21–26
Kazemifard,
S., Nayebzadeh, H., Saghatoleslami, N., Safakish, E., 2019. Application of
Magnetic Alumina-Ferric Oxide Nanocatalyst Supported by KOH for in-situ
Transesterification of Microalgae Cultivated in Wastewater Medium. Biomass
and Bioenergy, Volume 129, p. 105338
Koech, A.K.,
Kumar, A., Siagi, Z.O., 2020. In Situ Transesterification of Spirulina
Microalgae to Produce Biodiesel Using Microwave Irradiation. Journal of
Energy, Volume 2020, pp. 1–10
Larida,
A.Q., Bañaga, J.B., 2021. Extraction and Characterization of Algal Oil from
Lake Sebu, South Cotabato: A Potential Source of Biodiesel. Journal of
Science and Science Education, Volume 5(1), pp. 12–25
Lomeu, A.A.,
Mendonça, H.V.de, Mendes, M.F., 2023. Microalgae As Raw Material for Biodiesel
Production: Perspectives and Challenges of The Third Generation Chain. Engenharia
Agrícola, Volume 2023, p. 43
Ma, X.N.,
Chen, T.P., Yang, B., Liu, J., Chen, F., 2016. Lipid production from
Nannochloropsis. Marine Drugs, Volume 14(4). p. 61
Makareviciene, V., Sendzikiene, E., 2022.
Application of Microalgae Biomass for Biodiesel Fuel Production. Energies,
Volume 15(11), p. 4178
Manurung,
R., Parinduri, S.Z.D.M., Hasibuan, R., Tarigan, B.H., Siregar, A.G.A., 2023.
Synthesis of Nano-CaO Catalyst with SiO2 Matrix Based on Palm Shell ash
as Catalyst Support for One Cycle Developed in The Palm Biodiesel Process. Case
Studies in Chemical and Environmental Engineering, Volume 7, p. 100345
Marlinda,
L., Prajitno, D.H., Roesyadi, A., Gunardi, I., Mirzayanti, Y.W., Al-Muttaqii,
M., Budianto, A., 2022. Biofuel From
Hydrocracking of Cerbera Mangas Oil Over Ni-Zn/HZSM-5 Catalyst. Ecletica
Quimica, Volume 47(1), pp. 17–39
Medipally,
S.R., Yusoff, F.M., Banerjee, S., Shariff, M., 2015. Microalgae as Sustainable
Renewable Energy Feedstock for Biofuel Production. BioMed Research
International, Volume 2015, p. 519513
Mirzayanti, Y.W., Alisa, A., Sari, D., 2020a. Biodiesel Production from Rice Bran using a
Two-Stage In-Situ Method Using Sulfuric Acid and CaO/Hydrotalcite Catalysts. Jurnal
Rekayasa Mesin, Volume 11(3), pp. 375–382
Mirzayanti,
Y.W., Kurniawansyah, F., Prajitno, D.H., Roesyadi, A., 2020b. Selectivity of
Zinc-Molybdenum Catalyst to Produce Gasoil Via Catalytic Hydrocracking of
Kapook Seed Oil (Ceiba Pentandra). Journal of Physics: Conference Series,
Volume 1442, p. 012055
Mohamad, M.,
Ngadi, N., Wong, S., Yahya, N.Y., Inuwa, I.M., Lani, N.S., 2018. Synthesis and
Characterization of CaO-TiO2 for Transesterification of Vegetable Palm Oil. International
Journal of Engineering, Transactions B: Applications, Volume 31(8), pp. 1326–1333
Navajas, A.,
Campo, I., Moral, A., Echave, J., Sanz, O., Montes, M., Odriozola, J.A.,
Arzamendi, G., Gandía, L.M., 2018. Outstanding Performance of Rehydrated Mg-Al
Hydrotalcites as Heterogeneous Methanolysis Catalysts for The Synthesis of
Biodiesel. Fuel, Volume 211, pp. 173–181
Nisar, S.,
Hanif, M. A., Rashid, U., Hanif, A., Akhtar, M.N., Ngamcharussrivichai, C., 2021).
Trends in Widely Used Catalysts for Fatty Acid Methyl Esters (Fame) Production:
A Review. Catalysts, Volume 11(9), p. 1085
Purkan, P.,
Safa, A., Abdulloh, A., Baktir, A., Arissirajudin, R., Hermansyah, H., Kim,
S.W., 2021. In-Situ Biodiesel Synthesis from Microalgae Nannochloropsis Oculata
Using Nio Nanocatalyst. Rasayan Journal of Chemistry, Volume 14(3), pp. 2097–2103
Rahma, F.N., Hidayat, A., 2023. Biodiesel
Production from Free Fatty Acid using ZrO2/Bagasse Fly Ash Catalyst. International
Journal of Technology, Volume 14(1), pp. 206–218
Salam, K.A., Velasquez-Orta, S.B., Harvey, A.P., 2016. A Sustainable Integrated in Situ
Transesterification of Microalgae for Biodiesel Production and Associated
Co-products- A Review. Renewable and Sustainable Energy Reviews, Volume
65, pp. 1179–1198
Salam, K.A., Velasquez-Orta, S.B., Harvey, A.P., 2016. Surfactant-Assisted Direct
Biodiesel Production from Wet Nannochloropsis Occulata by in Situ
Transesterification/Reactive Extraction. Biofuel Research Journal, Volume
3(1), pp. 366–371
Sardi, B., Ningrum, R.F., Ardiansyah, V.A.,
Qadariyah, L., Mahfud, M., 2022. Production of Liquid Biofuels from Microalgae
Chlorella sp. via Catalytic Slow Pyrolysis. International Journal of
Technology, Volume 13(1), pp. 147–156
Shirazi, Y., Viamajala, S., Varanasi, S.,
2020. In situ and Ex situ Catalytic Pyrolysis of Microalgae and Integration with
Pyrolytic Fractionation. Frontiers in Chemistry, Volume 8
Tabatabaei, M., Aghbashlo, M., Dehhaghi, M.,
Panahi, H.K.S., Mollahosseini, A., Hosseini, M., Soufiyan, M.M., 2019. Reactor
Technologies for Biodiesel Production and Processing: A Review. Progress in
Energy and Combustion Science, Volume 74, pp. 239–303
Wahyono, Y., Hadiyanto, Budihardjo, M.A.,
Hariyono, Y., Baihaqi, R.A., 2022. Multifeedstock Biodiesel Production from a
Blend of Five Oils through Transesterification with Variation of Moles Ratio of
Oil: Methanol. International Journal of Technology, Volume 13(3), pp. 606–618
Win, T.T.,
Khine, M.M., 2017. Synthesis and Characterization of CaO and KF Doped CaO
(KF/CaO) Derived from Chicken Eggshell Waste as Heterogeneous Catalyst in
Biodiesel Production. Technology, and Sciences (ASRJETS) American Scientific
Research Journal for Engineering, Volume 38(2), pp. 134–151
Wood, D.A., 2021.
Microalgae to Biodiesel - Review of Recent Progress. Bioresource Technology
Reports, Volume 14, p. 10065
Zhang, C.Y.,
Shao, W.L., Zhou, W.X., Liu, Y., Han, Y.Y., Zheng, Y., Liu, Y.J., 2019.
Biodiesel Production by Esterification Reaction on K+ Modified
MgAl-Hydrotalcite Catalysts. Catalysts, Volume 9(9), p. 742
Zul, N.A., Ganesan, S., Hamidon, T.S., Oh, W.-Da,
Hussin, M.H., 2021. A Review on The Utilization of Calcium Oxide as a Base
Catalyst in Biodiesel Production. Journal of Environmental Chemical
Engineering, Volume 9(4), p. 105741
