Effect of Coagulation Bath Composition on Cellulose-Based Polymer Electrolyte Fabricated via Non-Solvent-Induced Phase Separation Method
Corresponding email: adam.febriyanto04@ui.ac.id
Published at : 07 Dec 2023
Volume : IJtech
Vol 14, No 7 (2023)
DOI : https://doi.org/10.14716/ijtech.v14i7.6677
Ratri, C.R., Aguta, T.B., Arundati, A.H., Rohib., Chalid, M., Astutiningsih, S., Nugraha, A.F., 2023. Effect of Coagulation Bath Composition on Cellulose-Based Polymer Electrolyte Fabricated via Non-Solvent-Induced Phase Separation Method. International Journal of Technology. Volume 14(7), pp. 1605-1614
| Christin Rina Ratri | 1. Green Polymer Technology Laboratory, Department of Metallurgical and Materials Engineering, Faculty of Engineering, Universitas Indonesia, Depok, Jawa Barat, 16424, Indonesia, 2. Research Center fo |
| Tegar Budi Aguta | Green Polymer Technology Laboratory, Department of Metallurgical and Materials Engineering, Faculty of Engineering, Universitas Indonesia, Depok, Jawa Barat, 16424, Indonesia |
| Annisaa Hayya Arundati | Graduate Institute of Ferrous and Energy Materials Technology, Pohang University of Science and Technology, Pohang 37673, Republic of Korea |
| Rohib Rohib | Institut De Recherches Sur La Catalyse Et L'environnement De Lyon, Umr 5256, Cnrs Avenue Albert Einstein, 69626, Lyon, France |
| Mochamad Chalid | Green Polymer Technology Laboratory, Department of Metallurgical and Materials Engineering, Faculty of Engineering, Universitas Indonesia, Depok, Jawa Barat, 16424, Indonesia |
| Sotya Astutiningsih | Green Polymer Technology Laboratory, Department of Metallurgical and Materials Engineering, Faculty of Engineering, Universitas Indonesia, Depok, Jawa Barat, 16424, Indonesia |
| Adam Febriyanto Nugraha | Green Polymer Technology Laboratory, Department of Metallurgical and Materials Engineering, Faculty of Engineering, Universitas Indonesia, Depok, Jawa Barat, 16424, Indonesia |
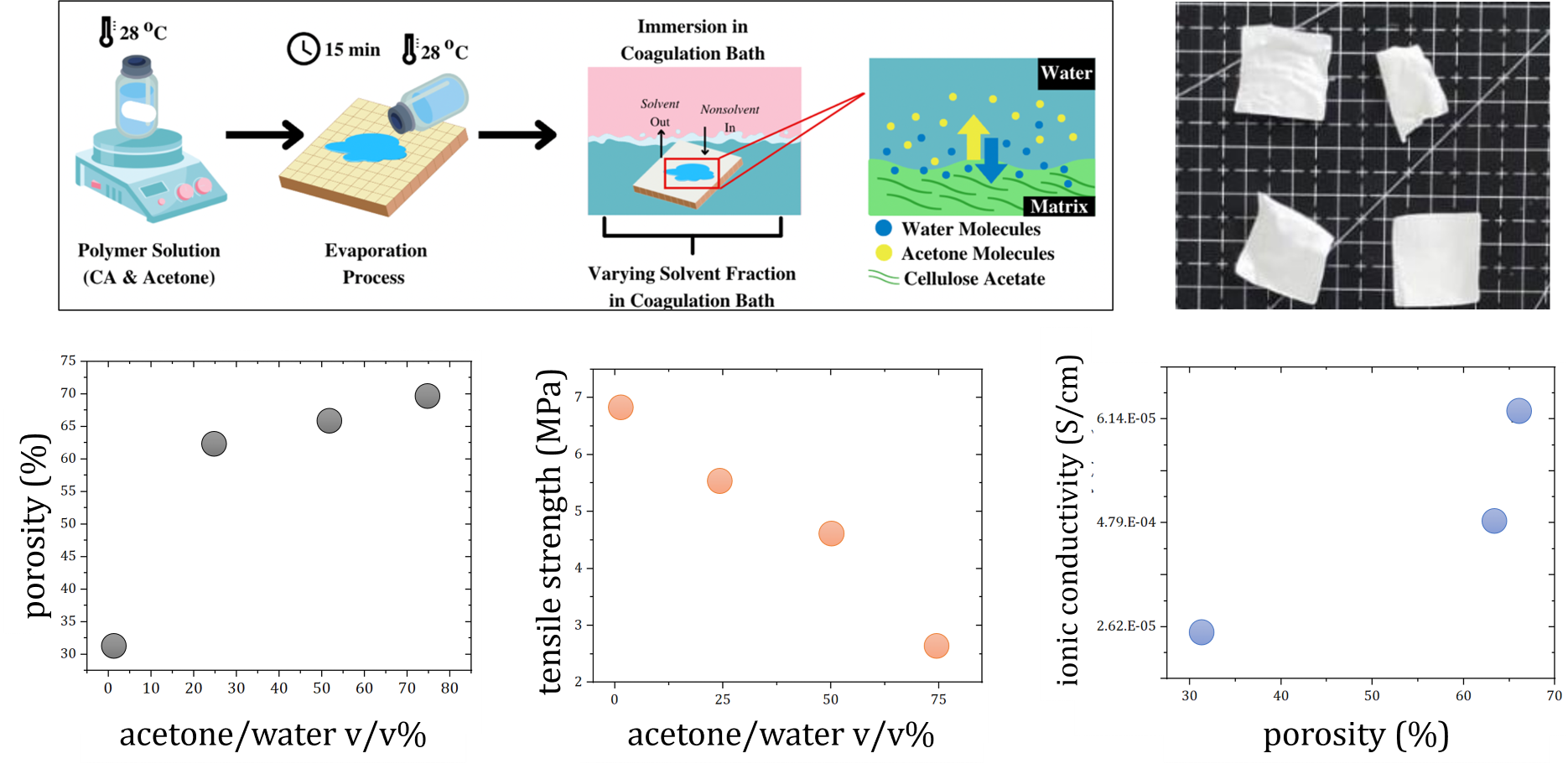
Cellulose acetate (CA) membrane was developed through
a non-solvent-induced phase separation (NIPS) technique to replace the
commercial petroleum-based Celgard separator membrane in Li-ion battery (LIB).
The morphology of a membrane can have a substantial impact on both its
mechanical and electrochemical properties, which are influenced by the
solvent-nonsolvent interaction. Therefore, this study examined the effect of
solvent fraction in an acetone-water system on the membrane morphology. CA
dissolved in acetone was cast on a glass plate and immersed in the coagulation
bath with varying acetone-water ratios. The resulting free-standing membrane
was analyzed subsequently and showed increased porosity, hydrophilicity, and
electrolyte uptake with higher acetone ratios in the coagulation bath. It was
also found that a more porous membrane contributes to a lower tensile strength,
including, 6.8 MPa, 5.5 MPa, 4.6 MPa, and 2.6 MPa for the coagulation baths
containing 0%, 25%, 50%, and 75% acetone, respectively. These results showed
that the mechanical properties of CA membranes are higher than those of
commercial Celgard membranes (1.42 MPa). LIB separator performance was measured
using electrochemical impedance spectroscopy (EIS). CA membrane fabricated with
50% acetone content in the coagulation bath possessed the highest ionic
conductivity, 4.79×10-4 S/cm, which is higher than the ionic
conductivity of the Celgard membrane (9.41×10-7 S/cm). Considering
their superior mechanical properties and electrical performance, CA membranes
could potentially substitute Celgard as a more sustainable alternative for LIB
separators.
Cellulose acetate; Coagulation bath; LIB separator; NIPS
The increasing demand for mobile devices and electric or hybrid vehicles has led to the widespread use of lithium-ion batteries (LIBs). Their popularity is attributed to outstanding energy density, substantial specific capacity, relatively low self-discharge rate, durability, and lightweight design (Tabani, Maghsoudi, and Fathollahi Zonouz, 2021). The important role of LIBs separators includes preventing short circuits between the electrodes and second, to absorb and optimize lithium-ion conductivity by absorbing and retaining electrolytes (Yang et al., 2022).
Polyolefin
separators, such as polyethylene (PE) and polypropylene (PP) membranes, are widely used due to their suitably sized pores, excellent mechanical
strength, and chemical stability (Setiaji et al., 2022; Razalli et al., 2015). However,
their primary disadvantage is insufficient liquid electrolyte capacity and the
inability to absorb high-dielectric-constant electrolytes on their hydrophobic
surfaces (Xu et al.,
2017).
To address these challenges, biodegradable
battery separators, made from renewable natural-resource-based polymers, are
being developed as a sustainable, non-toxic, and biodegradable alternative to
petroleum-based membranes. Among these
solutions, cellulose acetate (CA) is a promising host
polymer for LIBs separators (Febriasari et al., 2021). Subsequently,
CA polymers, characterized by carbonyl and hydroxyl groups, exhibit a strong
affinity for electrolytes and superior compatibility with electrodes. CA's
versatility extends to film formation and efficient electrical insulation,
rendering it well-suited for use as a matrix in LIBs (Li et al., 2017).
Polymeric
membranes can be produced in various ways including
nonsolvent-induced phase separation (NIPS), thermally induced phase separation
(TIPS), melt extrusion, electrospinning, and track etching (Rochardjo et al., 2021). The easy
processing and high reliability of NIPS membranes make them attractive choices.
As a widely used method for fabricating membranes made from polymers, NIPS has
several advantages over other methods. Subsequently,
with
NIPS, this approach facilitates fine control and generates small, evenly
distributed micropores (Choi, Ingole, and Park, 2022). Furthermore, this method
is superior to alternative methods, such as dry spinning and hot drawing, in
terms of achieving appropriate tensile strength, biaxial strength, and puncture
resistance (Kahrs and
Schwellenbach, 2020; Li et al., 2017). In
the NIPS process, the polymer and solvent are homogeneously dissolved, poured
onto a glass plate, and submerged in a nonsolvent bath (Li et al., 2008). After
phase separation and removal of residual
solvent, the nonsolvent must have a high affinity for the solvent but not for
the polymer (Wang et al.,
2019).
Previous NIPS studies used
various approaches to fabricate porous membranes, such as changing the
composition of the coagulation bath (Thankamony et al., 2018). The
properties and morphology of membranes are significantly influenced by the bath
composition during immersion in the coagulation bath (Pagliero et al., 2020). The porous
membrane structure was affected by the solvent fraction in the coagulation bath
(Asghar et al. 2018). The results
showed that increasing the solvent concentration in the coagulation bath
increased membrane crystallinity (Jung et al., 2016).
This
study specifically focuses on the preparation of CA membranes
using the NIPS method. There is a gap in study
addressing the contribution of solvent fractions in acetone-water systems to
the effectiveness of CA-based membranes as LIB separators. In the NIPS
method, the solvent amount in the coagulation bath plays a crucial
role in the demixing process, influencing pore formation and potentially
affecting the electrochemical performance of the membrane. Using a
facile approach, a porous CA membrane with superior ionic conductivity was successfully produced for use as a lithium-ion battery separator.
To prepare the separators through NIPS, CA (Mn =
30,000) was dissolved in acetone until homogeneous. The
dissolution process occurred at room temperature, using a vial and a magnetic
stirrer
(IKA C-MAG HS 7) to maintain constant stirring. After achieving homogeneity, the solution was set aside for a period to
eliminate any gas bubbles and was subsequently placed on a glass plate
at room temperature.
A 15-minute evaporation process was used
to partially evaporate the polymer solution. Subsequently, the glass plate was
placed in a room-temperature water coagulation bath for 15 min and the membrane
was peeled off slowly. Three variations of porous membranes were prepared, each
based on the solvent fraction used in the coagulation bath, as shown in Table 1. The membrane was dried on
sandwich-like filter paper using a vacuum desiccator for further analysis. An overview
of the NIPS procedure is shown in Figure 1.
Table 1 Sample
specification for the membrane fabrication
|
Sample code |
Water/acetone (v/v) |
|
A0W100 |
100/0 |
|
A25W75 |
75/25 |
|
A50W50 |
50/50 |
|
A75W25 |
25/75 |
Figure 1 Overview
process of the NIPS method
2.2. Cellulose Acetate
Membrane Characterization
Fourier
transform infrared (FTIR, Thermo Scientific iS-10 Spectra) spectroscopy was
used to evaluate the functional groups of CA membrane at
4000–400 cm-1 absorbance under ambient conditions.
To evaluate thermal stability, changes in membrane dimensions were
calculated.
Approximately 2 cm diameter pieces were cut from the samples. After a 2-hour
storage at 90°C, the membrane was examined for heat-induced shrinkage caused by
heat. Based on Equation (1), the shrinkage was calculated (Liang et al., 2018):
where So is the membrane surface
area before heating and S is the membrane surface area after heating.
The contact
angle was determined using the sessile
drop method, using wet agents such as water, ethylene glycol, and
an electrolyte (0.67 M LiClO4). Using a desiccator vacuum overnight,
residual humidity was removed from the membrane before the wettability test.
After dropping the electrolyte solution for 3 and 30 s, the contact angles were
measured.
To measure separator porosity (P), a gravimetric method based on n-butanol absorption was used. Each sample measured approximately 2 cm in diameter. Equation (2) was used to calculate the porosity of the separator (Cui et al., 2017).
where P is the separator porosity, the mass
of the separator at the start is Mi; the mass after an hour of
n-butanol soaking is Mn, the density of n-butanol is (0.81 g/cm3), the separator surface area is s, and its thickness is
d.
The surface and
cross-section of the separator were observed using a
scanning electron microscope (SEM, Hitachi SU3500, Tokyo, Japan) with an
acceleration voltage of 10 kV. After analyzing the surface images, the ImageJ
software was used to determine the pore sizes.
The tensile
properties of the membranes were determined using a universal testing machine
(UTM, Shimadzu AGS-X Series 5 kN) in accordance with ASTM D1708.
The test was conducted under ambient conditions at a
speed of 0.25 mm/min.
A gravimetric measurement of membrane electrolyte uptake was conducted by weighing the membranes before and after soaking in 0.67 M LiClO4 for 2 h. Electrolyte uptake was measured following Equation (3).
where W1 shows the separator
weight before and W2 shows the weight after electrolyte soaking. The
mean and standard deviation were calculated from three measurements.
Electrochemical
impedance spectroscopy (EIS, Metrohm Autolab, Potentiostat Mode) was performed
at frequencies ranging from 1 Hz to 1 MHz to quantify the membrane ionic
conductivity between the stainless-steel electrodes. Cell assembly started with
the membranes being soaked in 0.67 M LiClO4 electrolyte solution for
two hours before assembly. Coin cells (CR2032) were assembled using a separator
and blocking electrodes. Equation (4) was used to calculate the ionic
conductivity, (Luiso
et al., 2021).
where t is the membrane thickness, Rb is the bulk resistance, and A is the contact area between the separator and electrodes.
The
coagulation process plays a crucial role in the formation of hollow membranes,
influenced by both thermodynamics and kinetics. Membrane
morphology is impacted by factors such as polymer-solvent interactions,
solution viscosity, and solvent diffusivity. Typically, water serves as the most common nonsolvent. The addition
of a solvent can delay instant demixing by lowering polymer concentration and
decreasing nonsolvent activity. This delay in liquid-liquid demixing is the
predominant effect, as this method is used to produce dense membranes (Cui et al., 2017).
The
identification of functional groups in CA membrane was based on FTIR spectra,
analyzing the location and intensity of spectral peaks. In Figure 2, the FTIR spectrum of CA
membrane shows two characteristic peaks at wavelengths of 1730 and 1220
cm-1, corresponding to the C=O and C–O–C functional groups,
respectively (Ramesh, Shanti, and Morris, 2013).
The stretching vibration of hydroxyl group O-H was denoted by the peak at 3497
cm-1, and methyl group C-H stretching at 2945 cm-1 (Sudiarti et al., 2017), confirming the formation of true CA. Figure 2
shows the FT-IR spectra of CA in the powder and membrane forms.
Figure 2 FT-IR spectra of
cellulose acetate powder and membrane form
Porosity is a crucial factor
for separators because it impedes the penetration of active components and
prevents dendritic Li penetration (Ngamaroonchote
and Chotsuwan 2016). The membrane obtained using the NIPS process
exhibited a more porous structure compared to the solution casting method. This
is showed by the SEM results shown in Figure 3.
Figure 3 SEM micrograph of (top row) surface and (bottom row) cross-sectional
morphology of (a) Celgard and cellulose acetate membrane obtained with (b) NIPS
and (c) solution casting
The observation in Figure 4 shows that an increase in acetone content in the coagulation bath leads to higher porosity in CA separator. This effect is attributed to the higher acetone concentration reducing the solubility difference between the solvent and nonsolvent. This reduction promotes demixing during the coagulation process, ultimately yielding a membrane with increased porosity.
Figure 4 Influence of solvent fraction on porosity of the membrane
In LIB, specifically those
with high power and energy, dimensional stability is crucial for separators.
The separator should not shrink or wrinkle significantly when the temperature increases
and it should minimize the thermal shrinkage during drying. As shown in Figure
5, the Thermal Shrinkage Ratio (TSR) for each composition of the coagulation
bath was less than 5%. This satisfies the dimensional stability requirements of
battery separator applications.
Figure
5 Influence of (a) solvent fraction and (b)
porosity to thermal shrinkage ratio
Membrane hydrophilicity plays
a significant role in interfacial interactions and can be modified by adjusting
the phase-inversion process conditions. Valuable information is obtained by
measuring the contact angle between the wetting agent and the membrane surface (Song, Birbach, and Hinestroza, 2012). The results
are shown in Figure 6. Increasing hydrophilicity was also observed with higher
acetone composition in the coagulation bath, and this is attributed to the
increasing miscibility, which accelerates the precipitation process and
promotes pore formation (Silva, Belmonte-Reche and
Amorim, 2021).
Figure 6 Influence of (a) solvent
fraction and (b) porosity to the membrane wettability
Figure 7 shows the influence of the solvent
fraction and porosity on the membrane tensile strength. These results show that
the tensile strength of CA membrane decreased with increasing acetone content
in the coagulation bath. This is in accordance with the literature, as an
increase in the composition of acetone causes an increase in porosity, and
negatively affects the tensile strength of the membrane (Kartha and Mallik, 2020; Zhao et al., 2008). The tensile strength of the
Celgard membrane was measured at 1.42 MPa. All the fabricated CA separator membranes had a tensile strength that
exceeded that of a conventional Celgard separator membrane.
Figure 7 Influence of (a) solvent
fraction and (b) porosity to the tensile strength
Figure
8
Influence of (a) solvent fraction and (b) porosity to the electrolyte uptake
As shown in
Figure 8, a higher acetone ratio in the coagulation bath resulted in a higher
electrolyte uptake. This is caused by higher absorption of the electrolyte in
the membrane. It is important to note that electrolyte uptake increases
linearly, while the porosity increases logarithmically (Shi et al., 2015). An excellent ionic conductivity is required for
the separator because it controls ions passing between the positive and
negative terminals (Arrieta, Barrera, and Mendoza,
2022). Subsequently, EIS measurements were performed by applying a
frequency within a certain range, resulting in real and imaginary impedances
presented in the Nyquist plot (Figure 9). The calculated ionic conductivities
are listed in Table 2.
Figure
9
(a) Nyquist plot of cellulose acetate membranes and (b) influence of porosity
on ionic conductivity
Table
2
Ionic conductivities () of
cellulose acetate and conventional Celgard membrane
The ionic conductivity
calculation shows that a higher acetone composition in CA-based membrane leads
to higher ionic conductivity compared to using water only as a non-solvent.
Additionally, the results of all CA-based solid polymer electrolyte membranes show
better performance than that of Celgard.
In conclusion, CA separator membrane was
successfully fabricated using the NIPS method with acetone as the solvent and
water as the nonsolvent. Higher acetone composition
in the coagulation bath facilitated faster phase
separation, potentially leading
to larger
pores, higher electrolyte absorption, lower tensile
strength, and lower thermal shrinkage. Increased
acetone content also correlated with higher ionic conductivity. The contact
angle between CA membrane and water and ethylene glycol decreased with
increasing acetone content in the coagulation bath. The wetting ability of the
membrane electrolyte increased because of increased membrane porosity. CA-based
membranes have shown better mechanical and
electrical properties than commercial Celgard membranes. The tensile strength
of the pure CA membrane was 6.8 MPa while that of Celgard was 1.42 MPa. The
ionic conductivity of the CA membrane with 50% acetone content was 4.79×10-4
S/cm while that of Celgard was 9.41×10-7 S/cm. These results showed
that CA has potential as a viable alternative to replace polyolefin separators.
The
authors express
profound gratitude to
the Integrated Laboratory of Advanced Materials Characterization, National
Research and Innovation Agency of Indonesia (BRIN), and the Material Research
Center, Universitas Indonesia, for the laboratory and materials
characterization facility. Special appreciation to the Indonesia Endowment Fund for Education (LPDP) for providing
financial support in the PhD program (awardee number 202112210108100).
Arrieta,
A., Barrera, I., Mendoza, J., 2022. Impedantiometric Behavior of Solid
Biopolymer Electrolyte Elaborated from Cassava Starch Synthesized in Different
pH. International Journal of Technology, Volume 13(4), pp. 912–920
Asghar,
M.R., Zhang, Y., Wu, A., Yan, X., Shen, S., Ke, C., Zhang, J., 2018.
Preparation of Microporous Cellulose/Poly (Vinylidene
Fluoride-Hexafluoropropylene) Membrane for Lithium Ion Batteries by Phase
Inversion Method. Journal of Power Sources, Volume 379, pp. 197–205
Choi,
O., Ingole, P.G., Park, C.H., 2022. Precision-Aiming Tuning of Membranes
Prepared by NIPS And Its Performance Enhancement. Journal of Cleaner
Production, Volume 365, p. 132858
Cui, J.,
Liu, J., He, C., Li, J., Wu, X., 2017. Composite of Polyvinylidene
Fluoride–Cellulose Acetate with Al(OH)3 as a Separator For
High-Performance Lithium Ion Battery. Journal of Membrane Science,
Volume 541, pp. 661–667
Febriasari,
A., Suhartini, M., Yunus, A.L., Rahmawati, R., Sudirman, S., Hotimah, B., Hermana,
R.F., Kartohardjono, S., Fahira, A. and Permatasari, I.P., 2021. Gamma
Irradiation of Cellulose Acetate-Polyethylene Glycol 400 Composite Membrane and
Its Performance Test for Gas Separation. International Journal of Technology,
Volume 12(6), pp. 1198–1206
Jung,
J.T., Kim, J.F., Wang, H.H., Di-Nicolo, E., Drioli, E., Lee, Y.M., 2016.
Understanding The Non-Solvent Induced Phase Separation (NIPS) Effect During the
Fabrication of Microporous PVDF Membranes via Thermally Induced Phase
Separation (TIPS). Journal of Membrane Science, Volume 514, pp. 250–263
Kahrs,
C., Schwellenbach, J., 2020. Membrane Formation via Non-Solvent Induced Phase
Separation using Sustainable Solvents: A Comparative Study. Polymer,
Volume 186, p. 122071
Kartha, T.R., Mallik, B.S., 2020. Revisiting LiClO4 as an Electrolyte for Li-ion
Battery: Effect of Aggregation Behavior on Ion-Pairing Dynamics and
Conductance. Journal of Molecular Liquids, Volume 302, p. 112536
Li, L.,
Yu, M., Jia, C., Liu, J., Lv, Y., Liu, Y., Zhou, Y., Liu, C., Shao, Z., 2017.
Cellulosic Biomass-Reinforced Polyvinylidene Fluoride Separators with Enhanced
Dielectric Properties and Thermal Tolerance. ACS Applied Materials and
Interfaces, Volume 9(24), pp. 20885–20894
Li,
Z.H., Zhang, H.P., Zhang, P., Li, G.C., Wu, Y.P., Zhou, X.D., 2008. Effects of
the Porous Structure on Conductivity of Nanocomposite Polymer Electrolyte for
Lithium Ion Batteries. Journal of Membrane Science, Volume 322(2), pp.
416–422
Liang,
S., Yan, W., Wu, X., Zhang, Y., Zhu, Y., Wang, H., Wu, Y., 2018. Gel Polymer
Electrolytes for Lithium Ion Batteries: Fabrication, Characterization and
Performance. Solid State Ionics, Volume 318, pp. 2–18
Luiso,
S., Williams, A.H., Petrecca, M.J., Roh, S., Velev, O.D. Fedkiw, P.S., 2021.
Poly(Vinylidene Difluoride) Soft Dendritic Colloids as Li-Ion Battery
Separators. Journal of The Electrochemical Society, Volume 168(2), p.
020517
Ngamaroonchote,
A., Chotsuwan, C., 2016. Performance and Reliability of Cellulose Acetate-Based
Gel Electrolyte for Electrochromic Devices. Journal of Applied
Electrochemistry, Volume 46(5), pp. 575–582
Pagliero,
M., Bottino, A., Comite, A., Costa, C., 2020. Novel Hydrophobic PVDF Membranes
Prepared by Nonsolvent Induced Phase Separation for Membrane Distillation. Journal
of Membrane Science, Volume 596, p. 117575
Ramesh,
S., Shanti, R., Morris, E., 2013. Characterization of Conducting Cellulose
Acetate Based Polymer Electrolytes Doped With “Green” Ionic Mixture. Carbohydrate
Polymers, Volume 91(1), pp. 14–21
Razalli,
S.M.M., Saaid, S.I.Y.S.M., Ali, A.M.M., Hassan, O.H., Yahya, M.Z.A., 2015.
Cellulose Acetate-Lithium Bis (Trifluoromethanesulfonyl) Imide Solid Polymer
Electrolyte: ATR-FTIR And Ionic Conductivity Behavior. Functional Materials
Letters, Volume 8(3), p. 1540017
Rochardjo,
H.S.B., Fatkhurrohman, Kusumaatmaja, A., Yudhanto, F., 2021. Fabrication of
Nanofiltration Membrane based on Polyvinyl Alcohol Nanofibers Reinforced with
Cellulose Nanocrystal using Electrospinning Techniques. International
Journal of Technology, Volume 12(2), pp. 329–338
Setiaji,
D.A., Chalid, M., Abuzairi, T., Efroza, M., Nugraha, A.F., 2022. Effect of Cold
Plasma Treatment on Physical Properties of Multilayer Plastics for Polymer
Asphalt Applications. Piston: Journal of Technical Engineering, Volume
6(1), pp. 1–14
Shi, J.,
Xia, Y., Yuan, Z., Hu, H., Li, X., Zhang, H., Liu, Z., 2015. Porous Membrane
with High Curvature, Three-Dimensional Heat-Resistance Skeleton: A New and
Practical Separator Candidate for High Safety Lithium Ion Battery. Scientific
Reports, Volume 5(1), pp. 1–9
Silva,
M.A., Belmonte-Reche, E., Amorim, M.T.P.De., 2021. Morphology and Water Flux of
Produced Cellulose Acetate Membranes Reinforced by The Design of Experiments
(DOE). Carbohydrate Polymers, Volume 254, p. 117407
Song,
J., Birbach, N.L., Hinestroza, J.P., 2012. Deposition of Silver Nanoparticles
on Cellulosic Fibers via Stabilization of Carboxymethyl Groups. Cellulose,
Volume 19(2), pp. 411–424
Sudiarti,
T., Wahyuningrum, D., Bundjali, B., Arcana, I.M., 2017. Mechanical Strength and
Ionic Conductivity of Polymer Electrolyte Membranes Prepared from Cellulose
Acetate-Lithium Perchlorate. In: IOP Conference Series: Materials
Science and Engineering, Volume 223(1), p. 012052
Tabani,
Z., Maghsoudi, H., Fathollahi-Zonouz, A., 2021. High Electrochemical Stability
of Polyvinylidene Fluoride (PVDF) Porous Membranes Using Phase Inversion
Methods For Lithium-Ion Batteries. Journal of Solid State Electrochemistry,
Volume 25(2), pp. 651–657
Thankamony,
R.L., Li, X., Fan, X., Sheng, G., Wang, X., Sun, S., Zhang, X., Lai, Z., 2018.
Preparation of Highly Porous Polymer Membranes with Hierarchical Porous
Structures via Spinodal Decomposition of Mixed Solvents with UCST Phase
Behavior. ACS Applied Materials and Interfaces, Volume 10(50), pp.
44041–44049
Wang,
H.H., Jung, J.T., Kim, J.F., Kim, S., Drioli, E., Lee, Y.M., 2019. A Novel
Green Solvent Alternative for Polymeric Membrane Preparation via
Nonsolvent-Induced Phase Separation (NIPS). Journal of Membrane Science,
Volume 574, pp. 44–54
Xu, R.,
Huang, X., Lin, X., Cao, J., Yang, J., Lei, C., 2017. The Functional Aqueous
Slurry Coated Separator Using Polyvinylidene Fluoride Powder Particles for
Lithium-Ion Batteries. Journal of Electroanalytical Chemistry, Volume
786, pp. 77–85
Yang, B., Yang, Y., Xu, X., Ke, Y., Pan, Y., Su, L., Wang, Y., Wang, S., Qian, J., Xia, R., Fu, E., 2022. Hierarchical Microstructure and Performance Of PVDF/PMMA/SiO2lithium Battery Separator Fabricated by Thermally-Induced Phase Separation (TIPS). Journal of Materials Science, Volume 57(24), pp. 11274–11288
Zhao, H., Jiang, C., He, X., Ren, J., Wan, C., 2008. Preparation of Micro-Porous Membrane Electrodes and Their Application in Preparing Anodes of Rechargeable Lithium Batteries. Journal of Membrane Science, Volume 310(1–2), pp. 1–6
