Effect of Freeze-Thaw Cycles Method to Transfersome Characteristics for Growth Protein Encapsulation
Corresponding email: retno.wahyu01@ui.ac.id
Published at : 05 Feb 2024
Volume : IJtech
Vol 15, No 2 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i2.6670
Khayrani, A.C., Fahmi, M., Nurhayati, R.W., Manas, N.H.A., Suhaeri, M., 2024. Effect of Freeze-Thaw Cycles Method to Transfersome Characteristics for Growth Protein Encapsulation. International Journal of Technology. Volume 15(2), pp. 267-278
| Apriliana Cahya Khayrani | 1. Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Depok 16464, West Java, Indonesia. 2. Research Center for Biomedical Engineering, Faculty of Engineering, Universi |
| Muhammad Fahmi | Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Depok 16464, West Java, Indonesia |
| Retno Wahyu Nurhayati | 1. Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Depok 16464, West Java, Indonesia. 2. Research Center for Biomedical Engineering, Faculty of Engineering, Universi |
| Nor Hasmaliana Abdul Manas | 1. Faculty of Chemical and Energy Engineering, Universiti Teknologi Malaysia, Johor 81310, Malaysia. 2. Institute of Bioproduct Development, Universiti Teknologi Malaysia, Johor 81310, Malaysia |
| Muhammad Suhaeri | Universitas Indonesia Hospital, Universitas Indonesia, Depok 16424, West Java, Indonesia |
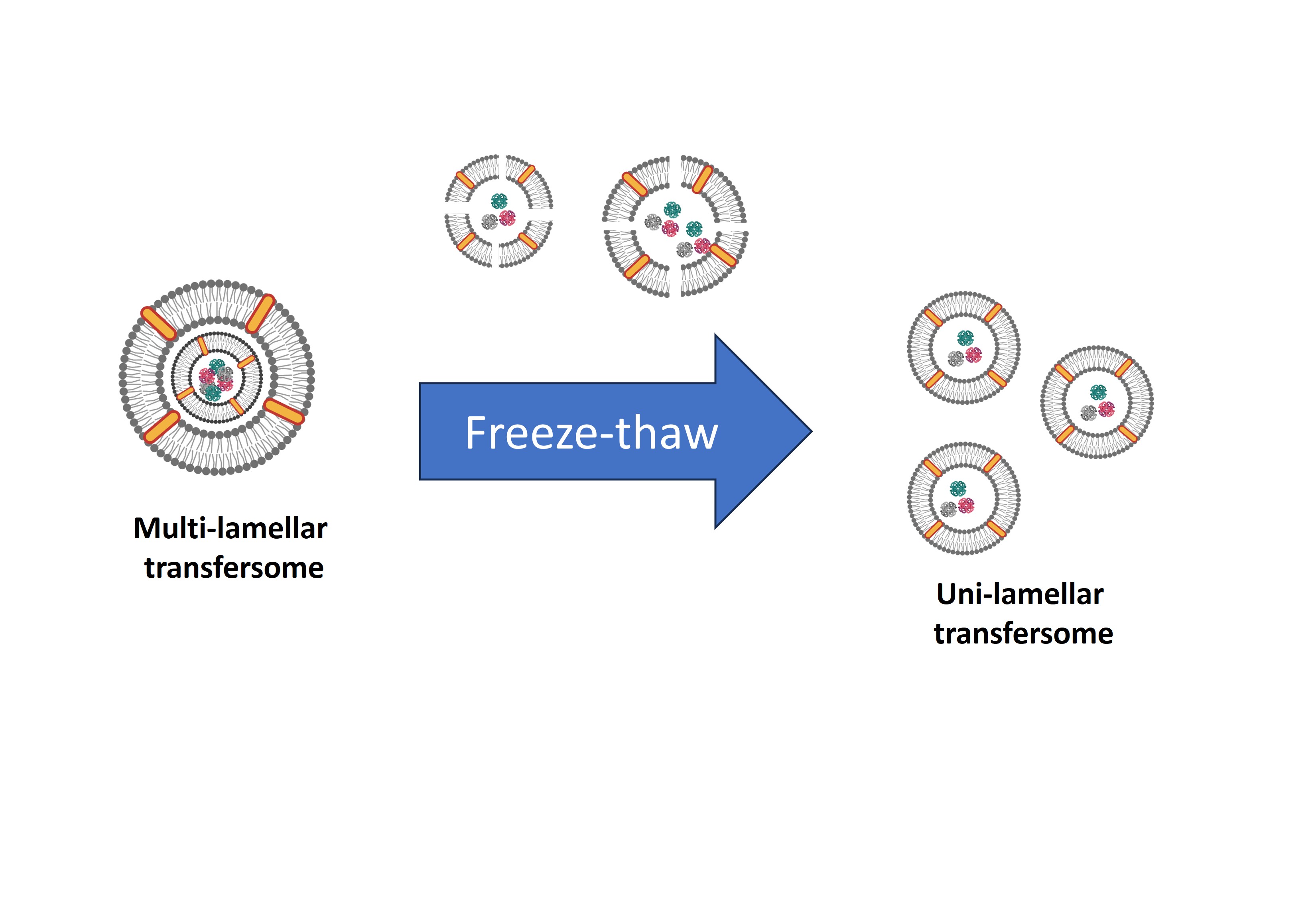
Transfersome, a lipid-based nanovesicle, can be a
suitable tool to improve the delivery of valuable growth factors. Through
transfersome technology, growth factors and active compounds can be transferred
transdermally without the need for invasive delivery procedures. In this study,
we evaluated the impact of freeze-thaw cycles on transfersome characteristics,
particularly particle size, polydispersity, and encapsulation efficiency.
Transfersome particles were prepared from dipalmitoylphosphatidylcholine (DPPC)
and Tween 80 with a 97.5:2.5 w/w% using thin film hydration at a temperature of
45° – 50°C. Then, the transfersome suspension was subjected to repeated
freeze-thaw for 1 minute of freezing and 3 minutes of thawing. The protein
release from all transfersome samples were evaluated using Bradford assay,
while the particle size and polydispersity were determined with a dynamic
scattering analyzer. It was found that freeze-thaw increased encapsulation
efficiency, particle size, and polydispersity of transfersomes up to
81.63±0.00%, 180.70±0.87 nm, and 0.369±0.02, respectively, from those without
freeze-thaw steps (73.35±0.03%, 144.93±0.21 nm and 0.202±0.02). Moreover, freeze-thawed
transfersomes exhibited a release of up to 52.80% of loaded protein within 78
hours, in contrast to 37.48% in non-freeze-thawed transfersomes. This study
shows that an additional freeze-thaw step is a promising method to improve the
properties of transfersome particles, especially encapsulation efficiency and
sustained protein release.
Dipalmitoylphosphatidylcholine; Encapsulation; Freeze-thaw; Growth protein; Transfersome
Degenerative disease, or disease caused by the functional and structural decline of tissues or organs, becomes a common disease among the elderly or even young people. The factors affecting degenerative disease are age, lifestyle, genetics, or the impact of an accident. In therapeutic approaches, there are various methods for treating degenerative diseases, including the utilization of stem cells in cell-based therapy. Stem cell therapy can be conducted by transplanting healthy cells to recipients (Mahla, 2016). However, cell-based therapy is a complicated process and may pose risks of immune rejection, tumorigenesis, and disease transmission (Rhatomy et al., 2020).
The
secretome is a stem cell culture medium containing protein, hormone, cytokine,
chemokine, an angiogenic factor, and growth factor that is secreted by stem
cells (Umar,
2023; Nurhayati et al., 2021). Secretomes can be an alternative to
stem cell therapy due to their ability to be immunomodulatory,
anti-inflammation, homeostasis, angiogenesis, and regenerative capacity.
However, some bioavailability challenges were caused by the first-pass effect
and secretome distribution into the unintended target tissue (Umar, 2023). To overcome
bioavailability problems, valuable molecules, including secretome, can be
encapsulated within nanovesicles composed of proteins,
peptides, phospholipids, or synthetic polymers (Aguilar-Toalá et al. 2022; Kusrini et al.,
2020; Sahlan et al., 2019).
Transfersomes, lipid-based nanovesicles, are also known
as elastic or deformable liposomes due to the presence of surfactants contained
in the bilayers, which make them more flexible and less stiff to pass through
holes and provide smaller vesicles than the conventional liposome (Apostolou et
al. 2021). The unique
characteristic of transfersome is ultra-flexibility, able to shrink its size 5
to 10 times smaller than its normal size (Opatha Titapiwatanakun,
and Chutoprapat, 2020), which is
needed for transdermal route administration of secretome. Transfersomes have
the ability to encapsulate molecules with a molecular weight ranging from 200
to 106 Da, making them intriguing candidates for utilization in
secretome-based delivery methods (Das, Nayak, and Mallick, 2022).
The encapsulation efficiency of
transfersome may vary depending on materials and fabrication methods, ranging
from 55 to 78% (Nojoki
et al., 2022; Vasileva et al., 2022; Luiz et al., 2021). In this study, we employed the
freeze-thaw method to promote the encapsulation efficiency of transfersome. The
lipid vesicle obtained from the thin film hydration method is an inhomogeneous
multilamellar vesicle (MLV), with the freeze-thaw method can be used to
homogenize the lamellarity and increase encapsulation at once (Costa, Xu, and Burgess, 2014; Sriwongsitanont and Ueno, 2011; Colletier et
al., 2002).
Freezing step expands the vesicle size due to ice crystal formation and dehydration of
lipid groups (Sriwongsitanont and
Ueno, 2011).
This process makes the membrane structure more brittle and susceptible to
disruption. Additionally, during the thawing process, membrane fusion occurs,
giving rise to new vesicles with a reduced lamellar number (Sriwongsitanont and Ueno, 2011).
Bovine
serum albumin (BSA), a common protein source for human cell culture, was used
in this study as a protein model loaded onto transfersomes. It consists of 583
amino acids (Topala et al.,
2014),
which can represent various proteins in the secretome. Furthermore, BSA has a
size of 66.4 kDa, which is close to secretome-derived protein, sizing 20 – 60
kDa (Weigent, 2011). In this study,
we evaluated the impact of freeze-thaw cycles on transfersome characteristics,
particularly particle size, polydispersity, and encapsulation efficiency, which
could be further utilized to produce transfersome particles with desirable
characteristics.
2.1. Materials
All
chemicals used in this study were analytical grade (purity >95%) and
purchased from Merck (Germany), otherwise specifically mentioned.
2.2. Protein Quantification
Protein
quantification was measured with a Bradford method, as described elsewhere.
Bradford reagent (1 mL) was mixed with 100 µL standard solution or protein
sample. The mixture was incubated for 5 minutes at room temperature and then
measured by UV-Vis spectrophotometer (Shimadzu, Japan) at 590 and 450 nm (Ernst and Zor, 2010).
2.3.
Transfersome
particles were prepared using a thin film hydration method (Nojoki et al., 2022; Luiz et
al., 2021; Opatha, Titapiwatanakun,
and Chutoprapat,
2020).
Briefly, Tween 80 (Vivantis, Malaysia) was dissolved in ethanol and then mixed
with DPPC (Avanti Polar Lipids, Inc., USA) with a ratio of 97.5:2.5 %w/w using
100 mL round bottom flasks. The mixture of DPPC and Tween 80 was dissolved with
chloroform and methanol mixture with a 2:1 v/v ratio. The organic solvent in
the mixture was removed by vacuum rotary evaporation at 50°C and 90 rpm for at
least 10 minutes until a thin film formed. The flask containing the lipid thin
film was stored in a vacuum desiccator overnight to remove the remaining
organic solvent. The amount of BSA used was a quarter of phospholipid mass. The
completely hydrated lipid film was rehydrated by BSA (ratio 1: 3) in PBS
solution and by rotation at 90 rpm and 45°C for 5 minutes. After rehydration,
transfersome suspension was vortexed for 1 minute. The heating in the 45°C
water bath process and vortex step were done with 3 times repetition for 1
minute in every procedure.
After the rehydration process,
transfersome suspension inside the egg flask was immersed in liquid nitrogen
(-196°C) for 1 minute, then thawed in a 45°C water bath while shaking the flask
by hand for 3 minutes (Castile and Taylor, 1999). The freeze-thaw steps were 5 cycles
(S1), 10 cycles (S2) or none (S0). The freeze-thawed suspension was subsequently
extruded 10 times by a mini extruder (Avanti Polar Lipid, Inc., Alabaster, AL,
USA) at 45°C. The extruded transfersome particles were purified by mini-column
centrifugation. The sample was transferred into the top part of an
ultra-centrifugal filter (Amicon tube, 100,000 MWCO) (Merck, Darmstadt,
Germany). Then, the tube was centrifuged for 15 minutes at 5000 rpm and 4°C
temperature. The
centrifugation step was repeated four times under the same operating
conditions, with the addition of 500 µL fresh PBS to the top part of the Amicon
tube at each repetition interval. The settled particle and filtrate were
separated for further analysis.
Figure 1 Schematic drawing of preparation technique for protein encapsulation with transfersome
2.4. Determination of Physicochemical
Properties
The
residue obtained from the purification process contains transfersome particles
used for physicochemical analysis, namely particle size (diameter),
polydispersity index (PDI), and zeta potential. A total of 500 µL residue
sample was dispersed into 5 mL bidistilled water. The sample was examined using
the dynamic light scattering (DLS) method with a particle size analyzer (SZ
100z, Horiba, Japan).
2.5. Determination of Encapsulation
Efficiency (%EE)
The
filtrate obtained from the purification process contains free BSA protein or
non-encapsulated BSA. The filtrate sample was tested for protein content using
the Bradford Assay procedure as described previously. Encapsulation efficiency
can be calculated using Equation 1:
where Mi is the total amount of
initial protein added, and Mf
is the total amount of free or unencapsulated protein.
2.6. In vitro Protein Release Assay
The
procedure of this assay was carried out using a modified method (Pisani et al., 2022; D’Souza,
2014; Li et al., 2001). The other residue sample obtained from the
purification process was transferred into the top part of an ultra-centrifugal
filter (Amicon tube, 100,000 MWCO) with a volume (ranging from 600 – 700 µL,
depending on sample encapsulation efficiency) that is equivalent to 1 mg of
BSA. After that, 500 µL fresh PBS (pH 7.4) solution was added to create a
gradient concentration of protein. The sample inside the tube was incubated in
a shaker incubator with 37°C set temperature and 100 rpm. The released BSA was
measured with Bradford assay. Hereinafter, the protein release profile data
were fitted into several drug release kinetic models, namely zero-order,
first-order, Higuchi, and Korsmeyer-Peppas. The data fitting model was analyzed
by Microsoft Excel with DDSolver add-ins (Mazhar et al., 2023; Khan et al., 2022; Zhang
et al., 2010), and the coefficient of determination (R2) was used as a
goodness-of-fit indicator (Costa and Lobo, 2001).
2.7. Statistical Analysis
Data were collected from triplicate
experiments and presented as mean ± standard deviation. Mean comparisons were
carried out using One-way ANOVA, followed by the Least Significant Difference
(LSD) multiple comparisons test. The p-value
less than 0.05 was considered statistically significant. For the BSA release
profile, the difference factor (f1)
was used to compare the profile release curve between samples (Zhang et al., 2010; Costa and
Lobo, 2001).
Furthermore, the difference factor (f1)
can be calculated using Equation 2 in Microsoft Excel with the DDSolver add-ins.
where Rt and Tt are cumulative amounts of released BSA from the
reference data group and test data group, respectively. t is a time-t point, and n
is the number of test repetitions. The BSA release profile between the two
samples was considered different if f1
> 15 and vice versa.
3.1. Encapsulation Efficiency (%EE)
In this study, all samples were prepared with a thin film hydration method; therefore, protein encapsulation or any active compound is affected by the thin film rehydration process. This is due to the thin film swelling and increased fluidity and permeability of the formed lipid bilayer (Lombardo and Kiselev, 2022). Two different freeze-thaw cycles (5 and 10 cycles) were evaluated in terms of encapsulation efficiency (Figure 2).
Figure 2 Encapsulation efficiencies of
transfersomes prepared with or without freeze-thaw (n=3). S0, S1, and S2
indicated transfersomes prepared without freeze-thaw, with 5 cycles of
freeze-thaw and 10 cycles of freeze-thaw, respectively. (**) p < 0.01; (***) p < 0.001; (ns) no significance
Thin film hydration creates
multilamellar vesicle (MLV) transfersome (Lombardo and Kiselev, 2022; Khayrani et al.,
2019).
Structural changes will increase the encapsulated protein amount within
transfersome particles due to disruption and membrane fusion during the
freeze-thaw process (Sriwongsitanont
and Ueno, 2011). The formed multilamellar becomes fragile and disrupted
into bilayer fragments during the freezing process because of ice crystal
formation on the polar part membrane of the vesicle. Furthermore, during the
thawing process (45°C) above the transition lipid temperature (DPPC = 41.3°C),
the bilayer fragments will be re-assembled by membrane fusion to form new
vesicles with less lamellar number, which subsequently makes them able to
encapsulate free proteins. Besides that, a decrease in the lamellar number on
the membrane obtains a bigger internal volume within the particle, so it will
increase the encapsulation efficiency (Sriwongsitanont and Ueno, 2011).
The encapsulation efficiency of
transfersome with 5 cycles of freeze-thaw had no significant differences (p > 0.05) as compared to those
without freeze-thaw. The possible explanation for this result is a shorter
freezing process compared to 10 cycles of freeze-thaw, leading to suboptimal
vesicle disruption. In other words, each bilayer layer in the formed
multilamellar vesicle was not completely disrupted with 5 cycles of
freeze-thaw, resulting in suboptimal encapsulation of free BSA by the new
vesicle.
3.2. Physicochemical Properties
The physicochemical characteristics such
as size (diameter), polydispersity index (PDI), and zeta potential can give
information about particle ability in drug delivery, particle homogeneity, and
particle stabilization. Based on Figure 3, transfersome particles with a
diameter lower than 300 nm were obtained. That particle size indicates that
transfersome particles can penetrate the skin epidermis (Das, Nayak,
and Mallick, 2022; Wang et al.,
2020). In
other words, the obtained transfersome particle is able to deliver protein
transdermally. Moreover, based on the polydispersity index value, the obtained
particle has a uniform size or homogenous size distribution because the PDI
value is lower than 0.5 (Danaei et al., 2018). Because of that PDI value, the
obtained transfersome particles could efficiently deliver valuable molecules to
a precise target (Danaei
et al., 2018). Nevertheless, the three samples have low particle
stability because the zeta potential value is between -10 mV and 10 mV (Wang et al., 2020). The low zeta
potential value indicates that the obtained transfersome particle is prone to
agglomeration, coagulation, and flocculation during storage in the colloidal
system (Manaia et al.,
2017).
The low transfersome particle stability in the colloidal system can lead to
inadequate transdermal delivery and suboptimal release of protein or drugs.
Figure
3
Physicochemical characteristics of transfersome (n = 3). (a) Particle
diameter (b) PDI, (c) Zeta potential. S0, S1, and S2 indicated transfersomes
prepared without freeze-thaw, with 5 cycles of freeze-thaw and 10 cycles of
freeze-thaw, respectively. (**) p
< 0.01; (***) p < 0.001; (ns)
no significance
The results of particle diameter and
polydispersity index measurement showed that the diameter and polydispersity
increased (p < 0.0001) due to the
freeze-thaw cycle process. The increase in diameter and PDI can be caused by
the formation of new particles and aggregate during the thawing process, which
can increase both the particle size and size distribution. This result is
consistent with Castile
and Taylor (1999) study, reporting an increase in diameter and size
distribution caused by freeze-thaw. In addition, the freezing step can decrease
the repulsive force and distance between membranes because of particle
dehydration caused by ice crystal formation (Bernal-Chávez et al., 2023; Boafo et al.,
2022).
Based on the diameter and polydispersity
measurement, transfersome particles produced in this study were quite suitable
for transdermal protein delivery. However, the obtained particle diameter and
polydispersity index can still be reduced by cryoprotectant addition. The
presence of cryoprotectants, such as cellobiose, sucrose, lactose, and glycerol
on transfersome formulation can inhibit ice crystal formation, which can
prevent nanovesicle damage that impacts particle size distribution (Bernal-Chávez et al., 2023;
Boafo et al., 2022). Based on that statement, inhibition of ice
crystal formation is slightly contradicted by the freeze-thaw cycle, which is
intended to reduce lamella numbers and increase encapsulation efficiency.
However, the report by Susa et al. (2021) showed that particle sizes could be
reduced with cryoprotectant due to the freezing process, which further disrupts
membranes into bilayer fragments. Then, the fragments are re-assembled to form
new vesicles with smaller sizes in the thawing process. Therefore, the addition
of cryoprotectant into transfersome that contain surfactant can prevent
particle aggregation during the freezing process. Further research is needed to
study the influence of cryoprotectants in lamellarity and encapsulation
efficiency of freeze-thawed transfersome.
The zeta potential measurement showed
zeta potential changes on S1 transfersome particles, which indicated more
neutral potential than the control sample (S0) and sample S2. These changes can
be affected by constituent material and/or hydration medium (Khan et al., 2021; Heurtault
et al., 2003). If the constituent material affects the particle zeta potential,
particle structure changes due to freeze-thaw can lead to a zeta potential
shift (Bernal-Chávez et
al., 2023; Sungpud et al., 2020; Costa, Xu, and Burgess, 2014). Nevertheless,
in this study, zeta potential changes on sample S1 were not affected by
freeze-thaw cycles. It happens because the DPPC used is neutral in a PBS medium
with pH 7.4, and Tween 80 is categorized as a non-ionic surfactant. Then, the
structural changes due to freeze-thaw cannot alter the zeta potential of
samples S1 and S2. It indicates that the zeta potential shift is due to pH
changes in the medium. In the S1 sample preparation, there may be impurities
that lower the pH of the medium, thereby reducing the negative charge on the S1
sample transfersome (Heurtault
et al., 2003).
The zeta potential is one of the
important parameters to measure particle stability. Generally, transfersome is
used for topical administration of protein or drugs through the skin in the
colloidal form like ointment, so it must have high particle stability to
prevent particle aggregation during storage. The transfersome particles are
considered stable if the zeta potential is more than 30 mV and less than -30 mV
(Manaia et al.,
2017).
However, every transfersome particle obtained in this study, mainly with the
freeze-thaw cycles, has low stability in the colloidal system. Consequently,
these particles are prone to aggregation and an increase in diameter. To
enhance transfersome particle stability in the colloidal system, cholesterol
addition to the transfersome formulation can be employed (Maritim, Boulas, and Lin, 2021). The increased particle stability is
obtained from the increased rigidity of the particle membrane due to
cholesterol presence, which can maintain particle fluidity by lowering the tilt
angle of the phospholipid component (Yeo, Yoon, and Lee, 2022; Khan et al., 2021). On the other
hand, cholesterol addition also reduces leakage of the encapsulated compound so
it can maintain or even increase the encapsulation efficiency (Hsieh et al., 2021; Khan et
al., 2021; Maritim, Boulas, and
Lin, 2021; Lu et al., 2014). Despite this, cholesterol addition into
transfersome formulation also contradicts the ultra-flexibility properties of
transfersome because the addition of cholesterol can reduce deformability and
transfersome permeability into the skin (Duangjit et al., 2013). The cholesterol addition into
transfersome formulation needs further research to obtain optimal cholesterol
amounts so it can form a stable transfersome but still maintain its
flexibility.
3.3. In vitro Protein Release Studies
In vitro BSA release from transfersome
was evaluated for 78 hours using an incubation temperature of 37°C and
phosphate-buffered saline (PBS) with pH 7.4 as the release medium in order to
mimic human physiology conditions (Yilmaz et al., 2020; D’Souza, 2014). Figure 4 shows
protein released from various transfersomes. In 78 hours, the BSA release for
samples S0, S1, and S2 were 37.48%, 40.39%, and 52.80%, respectively. The BSA
release profile showed a biphasic release pattern, which was indicated by
initial burst release and sustained release.
Figure 4 Protein release profile of
encapsulated BSA
Based on different factors (f1), results show that
between S0 and S1 have similarities (f1
< 15), whereas between S2 with two other samples have differences (f1 > 15). Furthermore, the
BSA release profile graph shows that for 78 hours, a transfersome sample with
10 cycles of freeze-thaw tends to release BSA more than the two other samples.
The difference in the amount of BSA released by each transfersome sample is
closely related to the number of lamellar on the transfersome membrane.
Transfersome sample S2 is suspected of having a lower lamellar number or even
an unilamellar membrane that makes BSA diffuse out of particles easily. On the
other hand, S0 and S1 transfersome samples have less released BSA compared to
the S2 sample.
The
slower release observed in S0 and S1 samples can be attributed to a higher
number of lamellar structures compared to S2 samples. Due to the presence of
multilamellar on particle membranes, the particle has thicker boundaries, so
BSA takes more time to diffuse out (Khan et al., 2021; Sungpud et al., 2020). It is also
consistent with the research results of Matsuura-Sawada et al. (2023), which show
that paclitaxel (PTX) release is slower in multilamellar liposomes.
In the sustained release phase in vitro,
there will be a slow decline in vivo due to drug distribution, metabolism, or
drug excretion in drug pharmacokinetics (Adepu and Ramakrishna, 2021; Yoo and Won, 2020; Rahul et
al., 2015). On
the other hand, the BSA release profile in this study shows the sustained
release of the amount of BSA released less than 60%, which suggests the
transfersome particle can increase protein bioavailability. The biphasic
release pattern can reduce dosage frequency and improve patient compliance (Rahul et al., 2015). However, the
drawbacks of this pattern are the possibility of drug excess to the toxicity
threshold in blood circulation due to initial burst release. Thus, it would
give drug side effects (Yoo and Won, 2020).
The
mathematical modeling of release kinetic was performed using Microsoft Excel
add-ins, namely DDSolver (Mazhar et al., 2023; Khan et al., 2022; Zhang et
al., 2010).
The model fitting results for BSA release kinetic were summarized in Table 1.
Table
1 Release Kinetic Model-fitting Results
|
Kinetic Model |
Parameters |
Sample Code | ||
|
S0 |
S1 |
S2 | ||
|
Zero Order |
k0 |
0.703 |
0.761 |
0.974 |
|
R2 |
-2.79 |
-3.26 |
-2.97 | |
|
First Order |
k1 |
0.013 |
0.015 |
0.020 |
|
R2 |
-2.41 |
-2.76 |
-2.19 | |
|
Higuchi |
kH |
6.510 |
7.082 |
8.925 |
|
R2 |
-0.72 |
-0.98 |
-0.76 | |
|
Korsmeyer-Peppas |
K |
20.42 |
24.26 |
29.50 |
|
n |
0.191 |
0.160 |
0.169 | |
|
R2 |
0.77 |
0.82 |
0.87 | |
The
BSA release kinetic model fitting shows a negative coefficient of determination
(R2) for all samples in
zero order, first order, and Higuchi models. A negative coefficient of
determination indicates that release profile data has a worse fitting with
those three models. When compared with all release kinetic models,
Korsmeyer-Peppas has the highest R2
value in all samples. This indicates that S0, S1, and S2 have the
Korsmeyer-Peppas release model. Based on the Korsmeyer-Peppas model,
transfersome particles in every sample have a Fickian diffusion mechanism with n value less than 0.43 (Bayer, 2023). That parameter
shows that the BSA release mechanism from a transfersome particle is diffusion
caused by the concentration gradient between the vesicle core and the outer
side of the particle. Such diffusion mechanisms can be triggered by the hydrophilic
properties of BSA and the presence of pores in the transfersome membrane due to
the surfactant content as an edge activator, so the BSA can diffuse easily (Opatha, Titapiwatanakun, and Chutoprapat, 2020). In addition, Korsmeyer-Peppas release
kinetic model in every transfersome sample also indicates that protein release
occurs over a long period of time due to slow release on the release profile (Mazhar et al., 2023), which may
benefit in less frequency for repeated protein injection for a therapy (Khan et al., 2022).
This study evaluated the influence of repeated
freeze-thaw process for transfersome characteristics in terms of encapsulation
efficiency, particle size, and polydispersity. It was found that 10 cycles
freeze-thaw had more significant effects than 5 cycles freeze-thaw. The
encapsulation efficiency of transfersome was improved up to 81.63±0.00% (10
cycles freeze-thaw) from 73.35±0.025% (without freeze-thaw). However, the
particle sizes of transfersome fabricated with freeze-thaw were enlarged from
144.93±0.21 to 180.70±0.87 nm, and polydispersity was increased from 0.202±0.02
to 0.369±0.02 in the freeze-thawed samples. The proteins released from the
transfersome were then evaluated in the in vitro system. Out of the four
kinetic models, it was observed that the protein release from transfersomes
exhibited the best fit with the Korsmeyer-Peppas model. After 78 h incubation,
transfersomes prepared from freeze-thaw had better protein release (52.80%)
than those without freeze-thaw (37.48%). In conclusion, freeze-thaw is a
suitable method to improve the characteristics of DPPC-based transfersome, and
further study is needed to understand the impact of cryoprotectant addition to
freeze-thawed transfersome.
This research was partially funded by Korea
Institute of Science and Technology (KIST) School Partnership Project 2022
awarded to Dr. Muhammad Suhaeri and Indonesian National Research Priority Research
Funding 2021 (Program Flagship Pendanaan Prioritas Riset Nasional (PRN) untuk
Perguruan Tinggi 2021; No. PKS-193/UN2.INV/HKP.05/2021) from Indonesian
Ministry of Education, Culture, Research, and Technology awarded to Dr. Retno
Wahyu Nurhayati. This research was also partially funded by the Directorate
Research and Development Universitas Indonesia through the Q2 PUTI Grant (Hibah
Publikasi Terindeks Internasional (PUTI) Q2 No. NKB-800/UN2.RST/HKP.05.00/2023)
awarded to Apriliana Cahya Khayrani, Ph.D.
Adepu, S., Ramakrishna, S., 2021. Controlled Drug
Delivery Systems: Current Status and Future Directions. Molecules, Volume 26(19), p. 5905
Aguilar-Toalá,
J.E., Quintanar-Guerrero, D., Liceaga, A.M., Zambrano-Zaragoza, M.L. 2022. Encapsulation of Bioactive Peptides: A Strategy to
Improve the Stability, Protect the Nutraceutical Bioactivity, and Support Their
Food Applications. RSC advances, Volume 12(11), pp. 6449–6458
Apostolou, M., Assi, S., Fatokun, A.A., Khan, I., 2021. The Effects of Solid and
Liquid Lipids on the Physicochemical Properties of Nanostructured Lipid
Carriers. Journal of Pharmaceutical Sciences, Volume 110(8), pp.
2859–2872
Bayer, I.S., 2023. Controlled Drug Release from
Nanoengineered Polysaccharides. Pharmaceutics,
Volume 15(5), p. 1364.
Bernal-Chávez, S.A., Romero-Montero, A., Hernández-Parra,
H., Peña-Corona, S.I., Del Prado-Audelo, M.L., Alcalá-Alcalá, S., Cortés, H.,
Kiyekbayeva, L., Sharifi-Rad, J., Leyva-Gómez, G., 2023. Enhancing Chemical and
Physical Stability of Pharmaceuticals Using Freeze-Thaw Method: Challenges and
Opportunities for Process Optimization Through Quality By Design Approach. Journal of Biological Engineering,
Volume 17(1), p. 35
Boafo, G.F., Magar, K.T., Ekpo, M.D.,
Qian, W., Tan, S., Chen, C., 2022. The Role of Cryoprotective Agents in
Liposome Stabilization and Preservation. International Journal of Molecular
Sciences, Volume 23(20), p. 12487
Castile, J.D., Taylor, K.M.G., 1999. Factors Affecting
the Size Distribution of Liposomes Produced by Freeze-Thaw Extrusion. International Journal of Pharmaceutics,
Volume 188(1), pp. 87–95
Colletier, J.P., Chaize, B., Winterhalter, M., Fournier,
D., 2002. Protein Encapsulation in Liposomes: Efficiency Depends on
Interactions Between Protein and Phospholipid Bilayer, BMC Biotechnology, Volume 2(1), pp. 1–8
Costa, A.P., Xu, X., Burgess, D.J.,
2014. Freeze-Anneal-Thaw Cycling of Unilamellar Liposomes: Effect on
Encapsulation Efficiency. Pharmaceutical
Research, Volume 31(1), pp. 97–103
Costa, P., Lobo, J.M.S., 2001. Modeling and Comparison of Dissolution Profiles. European Journal of Pharmaceutical Sciences,
Volume 13(2), pp. 123–133
D’Souza, S., 2014. A Review of In Vitro Drug Release Test
Methods for Nano-Sized Dosage Forms. Advances
in Pharmaceutics, Volume 2014, pp. 1–12.
Danaei, M., Dehghankhold, M., Ataei, S., Hasanzadeh
Davarani, F., Javanmard, R., Dokhani, A., Khorasani, S., Mozafari, M., 2018.
Impact of Particle Size and Polydispersity Index on the Clinical Applications
of Lipidic Nanocarrier Systems. Pharmaceutics,
Volume 10(2), p. 57
Das, B., Nayak, A.K., Mallick, S., 2022. Transferosomes:
A Novel Nanovesicular Approach for Drug Delivery. In: Systems of Nanovesicular Drug Delivery, Nayak, A.K., Hasnain, M.S.,
Aminabhavi, T., Torchilin V.P., (ed.), Elsevier, pp. 103–114
Duangjit, S., Opanasopit, P.,
Rojanarata, T., Ngawhirunpat, T., 2013. Evaluation of Meloxicam-Loaded Cationic Transfersomes as Transdermal
Drug Delivery Carriers. AAPS PharmSciTech,
Volume 14(1), pp. 133–140
Ernst, O., Zor, T., 2010. Linearization of the Bradford
Protein Assay. Journal of Visualized Experiments: JoVE, Volume 38,
p. 1918
Heurtault, B., Saulnier, P., Pech, B., Proust, J.E.,
Benoit, J.P., 2003. Physico-Chemical Stability of Colloidal Lipid Particles, Biomaterials, Volume 24(23), pp.
4283–4300
Hsieh, W.-C., Fang, C.-W., Suhail, M., Lam Vu, Q.,
Chuang, C.-H., Wu, P.-C., 2021. Improved Skin Permeability and Whitening Effect
of Catechin-Loaded Transfersomes Through Topical Delivery. International Journal of Pharmaceutics, Volume 607, p. 121030
Khan, I.,
Needham, R., Yousaf, S., Houacine, C., Islam, Y., Bnyan, R., Sadozai, S.K.,
Elrayess, M.A., Elhissi, A., 2021. Impact Of Phospholipids, Surfactants and
Cholesterol Selection on The Performance of Transfersomes Vesicles Using
Medical Nebulizers for Pulmonary Drug Delivery, Journal of Drug Delivery Science and Technology, Volume 66, p.
102822
Khan, M.I., Yaqoob, S., Madni, A., Akhtar, M.F., Sohail,
M.F., Saleem, A., Tahir, N., Khan, K.-R., Qureshi, O.S., 2022. Development and
In Vitro/Ex Vivo Evaluation of Lecithin-Based Deformable Transfersomes and
Transfersome-Based Gels for Combined Dermal Delivery of Meloxicam and
Dexamethasone. BioMed Research
International, Volume 2022, pp. 1–16
Khayrani, A.C., Mahmud, H., Oo, A.K.K., Zahra, M.H., Oze,
M., Du, J., Alam, M.J., Afify, S.M., Quora, H.A.A., Shigehiro, T., Calle, A.S.,
Okada, N., Seno, A., Fujita, K., Hamada, H., Seno, Y., Mandai, T., Seno, M.,
2019. Targeting Ovarian Cancer Cells Overexpressing CD44 with Immunoliposomes
Encapsulating Glycosylated Paclitaxel. International
Journal of Molecular Sciences, Volume 20(5), p. 1042
Kusrini, E., Asvial,
M., Budiyanto, M.A., Kartohardjono, S., Wulanza, Y., 2020. The Future of
Nanotechnology and Quantum Dots for the Treatment of COVID-19. International
Journal of Technology. Volume 11(5), pp. 873–877
Li, Y.-P., Pei, Y.-Y., Zhang, X.-Y., Gu, Z.-H., Zhou,
Z.-H., Yuan, W.-F., Zhou, J.-J., Zhu, J.-H. and Gao, X.-J., 2001. PEGylated
PLGA Nanoparticles as Protein Carriers: Synthesis, Preparation and
Biodistribution in Rats. Journal of
Controlled Release, Volume 71(2), pp. 203–211
Lombardo, D., Kiselev, M.A.,
2022. Methods of Liposomes Preparation: Formation
and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine
Application. Pharmaceutics, Volume
14(3), p. 543
Lu, K., Xie, S., Han, S., Zhang, J., Chang, X., Chao, J.,
Huang, Q., Yuan, Q., Lin, H., Xu, L., Shen, C., Tan, M., Qu, S., Wang, C.,
Song, X., 2014. Preparation of a Nano Emodin Transfersome and Study on its
Anti-Obesity Mechanism in Adipose Tissue of Diet-Induced Obese Rats. Journal of Translational Medicine,
Volume 12(1), p. 72
Luiz, M.T., Viegas, J.S.R., Abriata, J.P., Tofani, L.B.,
Vaidergorn, M. de M., Emery, F. da S., Chorilli, M., Marchetti, J.M., 2021.
Docetaxel-Loaded Folate-Modified TPGS-Transfersomes for Glioblastoma Multiforme
Treatment, Materials Science and
Engineering: C, Volume 124, p. 112033
Mahla, R.S., 2016. Stem Cells Applications in
Regenerative Medicine and Disease Therapeutics. International Journal of Cell Biology, Volume 2016, p. 6940283
Manaia, E.B., Abuçafy, M.P., Chiari-Andréo, B.G., Silva,
B.L., Oshiro-Júnior, J.A., Chiavacci, L., 2017. Physicochemical
Characterization of Drug Nanocarriers. International
Journal of Nanomedicine, Volume 12, pp. 4991–5011
Maritim, S., Boulas, P., Lin, Y.,
2021. Comprehensive Analysis of Liposome Formulation Parameters and Their
Influence on Encapsulation, Stability and Drug Release In Glibenclamide
Liposomes. International Journal of
Pharmaceutics, Volume 592, p. 120051
Matsuura-Sawada, Y., Maeki, M.,
Uno, S., Wada, K., Tokeshi, M., 2023. Controlling
Lamellarity and Physicochemical Properties of Liposomes Prepared Using a
Microfluidic Device. Biomaterials Science,
Volume 11(7), pp. 2419–2426
Mazhar, D., Haq, N.U., Zeeshan, M., Ain, Q.U., Ali, H.,
Khan, S., Khan, S.A., 2023. Preparation, Characterization, and Pharmacokinetic
Assessment of Metformin HCl Loaded Transfersomes Co-Equipped with Permeation
Enhancer to Improve Drug Bioavailability via Transdermal Route. Journal of Drug Delivery Science and
Technology, Volume 84, p. 104448
Nojoki, F., Ebrahimi-Hosseinzadeh, B., Hatamian-Zarmi,
A., Khodagholi, F., Khezri, K., 2022. Design and Development of
Chitosan-Insulin-Transfersomes (Transfersulin) as Effective Intranasal
Nanovesicles for The Treatment of Alzheimer’s Disease: in Vitro, in Vivo, and
Ex Vivo Evaluations, Biomedicine &
Pharmacotherapy, Volume 153, p. 113450
Nurhayati, R.W., Lubis, D.S.H., Pratama, G., Agustina,
E., Khoiriyah, Z., Alawiyah, K., Pawitan, J.A., 2021. The Effects of Static and
Dynamic Culture Systems on Cell Proliferation and Conditioned Media of
Umbilical Cord-derived Mesenchymal Stem Cells. International Journal of Technology. Volume 12(6), pp. 1187-1197
Opatha, S.A.T., Titapiwatanakun, V., Chutoprapat, R.,
2020. Transfersomes: A Promising Nanoencapsulation Technique for Transdermal
Drug Delivery, Pharmaceutics, Volume
12(9), p. 855
Pisani, S., Chiesa, E., Genta, I., Dorati, R., Gregorini,
M., Grignano, M.A., Ramus, M., Ceccarelli, G., Croce, S., Valsecchi, C., Monti,
M., Rampino, T., Conti, B., 2022. Liposome Formulation and in Vitro Testing in
Non-Physiological Conditions Addressed to Ex Vivo Kidney Perfusion. International Journal of Molecular Sciences,
Volume 23(14), p. 7999
Rahul, K.S., Kadam, V.S., Shendarkar, G.R., Jadhav, S.B.,
Bharkad, V.B., 2015. Sustained Release Drug Delivery System: Review. Indian Journal of Research in Pharmacy and
Biotechnology, Volume 2015, pp. 246–251
Rhatomy, S., Prasetyo, T.E., Setyawan, R., Soekarno,
N.R., Romaniyanto, F.N.U., Sedjati, A.P., Sumarwoto, T., Utomo, D.N., Suroto,
H., Mahyudin, F., Prakoeswa, C.R.S., 2020. Prospect of Stem Cells Conditioned
Medium (Secretome) In Ligament and Tendon Healing: A Systematic Review. Stem Cells Translational Medicine,
Volume 9(8), pp. 895–902
Sahlan, M., Fadhan, A.M., Pratami, D.K.,
Wijanarko, A., Lischer, K., Hermansyah, H., Mahira, K.F., 2019. Encapsulation
of Agarwood Essential Oil with Maltodextrin and Gum Arabic. International
Journal of Technology, Volume 10(8), pp. 1541–1547
Sriwongsitanont, S., Ueno, M., 2011. Effect of
Freeze-Thawing Process on the Size and Lamellarity of PEG-Lipid Liposomes. The Open Colloid Science Journal, Volume
4(1), pp. 1–8
Sungpud, C.,
Panpipat, W., Chaijan, M., Sae Yoon, A., 2020. Techno-Biofunctionality of
Mangostin Extract-Loaded Virgin Coconut Oil Nanoemulsion and Nanoemulgel. PLOS ONE, Volume 15(1), p. e0227979
Susa, F., Bucca, G., Limongi, T., Cauda, V., Pisano, R.,
2021. Enhancing The Preservation of Liposomes: The Role of Cryoprotectants,
Lipid Formulations and Freezing Approaches. Cryobiology,
Volume 98, pp. 46–56
Topala, T., Bodoki, A., Oprean,
L., Oprean, R., 2014. Bovine Serum
Albumin Interactions with Metal Complexes. Medicine
and Pharmacy Reports, Volume 87(4), pp. 215–219
Umar, A.K., 2023. Stem Cell's Secretome Delivery Systems. Advanced Pharmaceutical Bulletin, Volume 13(2), 244–258
Vasileva,
L., Gaynanova, G., Zueva, I., Lyubina, A., Amerhanova, S., Buzyurova, D.,
Babaev, V., Voloshina, A., Petrov, K. and Zakharova, L., 2022. Transdermal
Delivery of 2-PAM as a Tool to Increase the Effectiveness of Traditional
Treatment of Organophosphate Poisoning. International Journal of Molecular
Sciences, Volume 23(23), p. 14992
Wang, D.Y., van der Mei, H.C., Ren, Y., Busscher, H.J.,
Shi, L., 2020. Lipid-Based Antimicrobial Delivery-Systems for the Treatment of
Bacterial Infections. Frontiers in
Chemistry, Volume 7, p. 872
Weigent, D.A., 2011. High Molecular Weight Isoforms of
Growth Hormone in Cells of The Immune System. Cellular Immunology, Volume 271(1), pp. 44–52
Yeo, S., Yoon, I., Lee, W.K.,
2022. Design and Characterisation of pH-Responsive Photosensitiser-Loaded
Nano-Transfersomes for Enhanced Photodynamic Therapy, Pharmaceutics, Volume 14(1), p. 210
Yilmaz, B., Pazarceviren, A.E., Tezcaner, A., Evis, Z.,
2020. Historical Development of Simulated Body Fluids Used in Biomedical
Applications: A Review. Microchemical
Journal, 155, p. 104713
Yoo, J., Won, Y.-Y., 2020. Phenomenology of the Initial
Burst Release of Drugs from PLGA Microparticles. ACS Biomaterials Science & Engineering, Volume 6(11), pp.
6053–6062
Zhang, Y., Huo, M., Zhou, J., Zou, A., Li, W., Yao, C.,
Xie, S., 2010. DDsolver: an Add-In Program for Modeling and Comparison of Drug
Dissolution Profiles. AAPS Journal,
Volume 12(3), pp. 263–271
