Synthesis of Chemical Adsorbent for Purification of Heavy Oil Residue
Corresponding email: galimjan_87@mail.ru
Published at : 17 May 2024
Volume : IJtech
Vol 15, No 3 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i3.6507
Maldybayev, G., Shayakhmetova, R., Nurzhanova, S., Sharipov, R., Negim, E.-S., Alimzhanova, A., Osipov, P., Mukhametzhanova, A., Usman, A., 2024. Synthesis of Chemical Adsorbents for Purification of Heavy Oil Residues. International Journal of Technology. Volume 15(3), pp. 792-802
| Galymzhan Maldybayev | 1. National Center on complex processing of mineral raw materials of the Republic of Kazakhstan, 67 Jandossov Str., Almaty 050036, Republic of Kazakhstan, 2. School of Materials Science and Green Tech |
| Roza Shayakhmetova | National Center on complex processing of mineral raw materials of the Republic of Kazakhstan, 67 Jandossov Str., Almaty 050036, Republic of Kazakhstan |
| Saule Nurzhanova | Sokolsky Institute of Organic Catalysis and Electrochemistry, 142 Kunaev St., Almaty 050010, Republic of Kazakhstan |
| Rustam Sharipov | School of Materials Science and Green Technologies, Kazakh-British Technical University, St. Tole Bi, 59, Almaty 050000, Kazakhstan |
| El-Sayed Negim | 1. School of Materials Science and Green Technologies, Kazakh-British Technical University, St. Tole Bi, 59, Almaty 050000, Kazakhstan, 2. School of Petroleum Engineering, Satbayev University, 22 Satp |
| Aliya Alimzhanova | National Center on complex processing of mineral raw materials of the Republic of Kazakhstan, 67 Jandossov Str., Almaty 050036, Republic of Kazakhstan |
| Petr Osipov | National Center on complex processing of mineral raw materials of the Republic of Kazakhstan, 67 Jandossov Str., Almaty 050036, Republic of Kazakhstan |
| Anar Mukhametzhanova | National Center on complex processing of mineral raw materials of the Republic of Kazakhstan, 67 Jandossov Str., Almaty 050036, Republic of Kazakhstan |
| Anwar Usman | Department of Chemistry, Faculty of Science, University Brunei Darussalam, Jalan Tungku Link, Gadong BE1410, Brunei Darussalam |
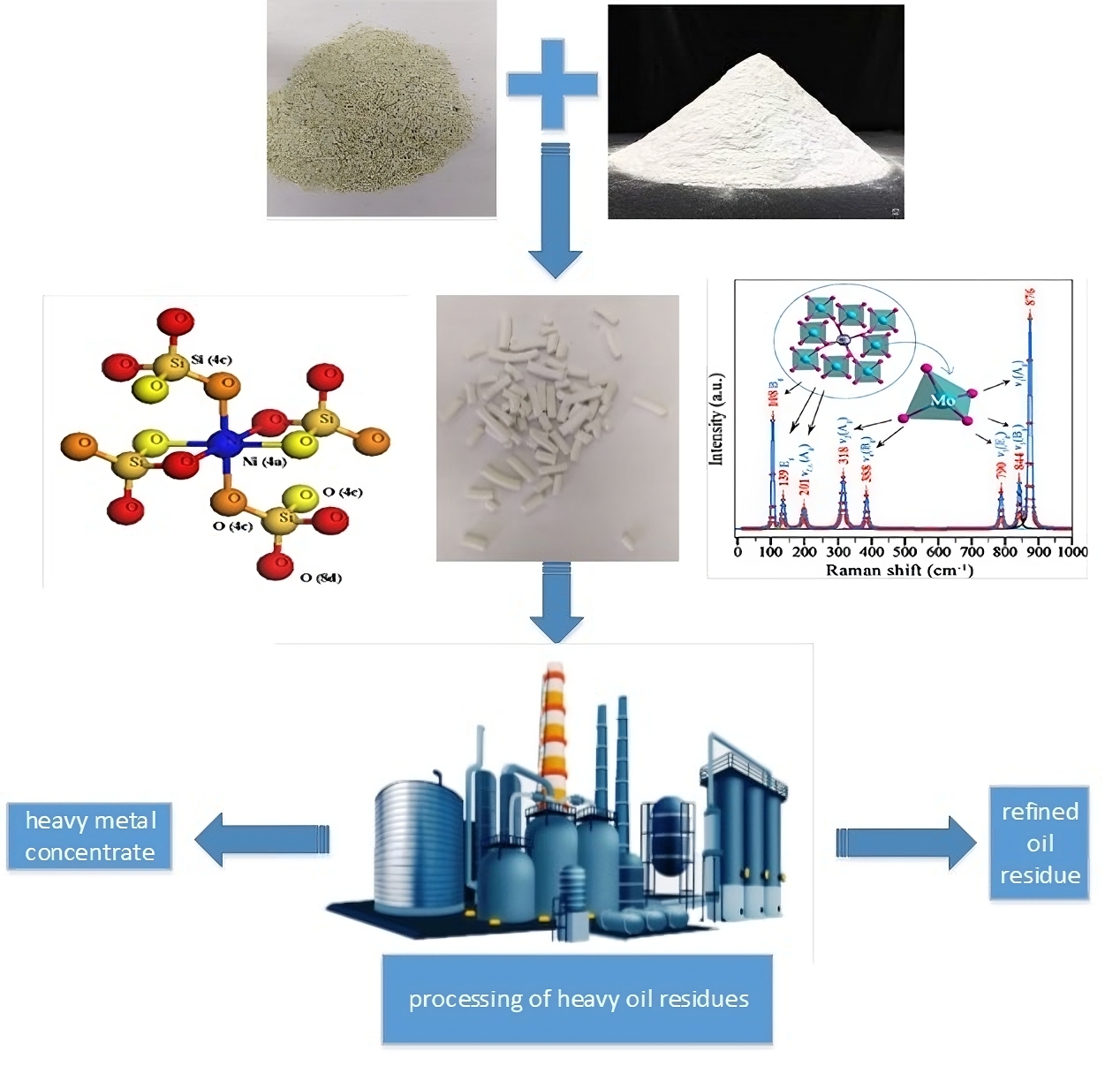
This study
aimed to investigate synthesized wollastonite based on amorphous silicon
dioxide and calcium carbonate as adsorbent for cleaning hydrocarbon feedstock
from heavy organometallic compounds and sulfur. During the analysis,
physicochemical parameters including the specific surface area and intensity of
synthesized wollastonite were determined. The results showed that the optimal
temperature zone for demetallization of hydrocarbon raw materials was 653 - 683
K, with a contact time of 60 minutes, and pressure of 8 - 10 atm. Furthermore,
the content of vanadium, nickel, iron, and sulfur was found to decrease from
540 to 52 µg/kg, 50 to 26 µg/kg, 50 to 12 µg/kg, and 3.50% to 2.39%,
respectively.
Adsorbent; Asbestos waste; Demetallization; Heavy oil; Sulfur
Cracking of oil in zeolites is one of the most significant
industrial applications in oil refining. In this field, attention is focused on
developing catalytic systems using nanosized zeolites and micro-mesoporous
materials. The wide use of zeolites as a valuable tool in nanotechnology is
attributed to their unique properties, such as nanoscale of cavities, capacity
for precise arrangement of active centers, selectivity, and directional effect
on chemical groups of molecules (Johansson et
al., 2023; Ulfiati et al., 2022; Ongarbayev et
al., 2020; Li et al., 2020; Attia et al., 2020; Shang et al., 2016; Jo et
al., 2014).
Recently,
various inorganic substances such as hydroxides, silicates, phosphates, and
aluminosilicates, have gained significant attention. This is due to the
widespread availability of inorganic substances, along with high thermal and
chemical resistance, serving as effective and accessible sorbents. Although the
activity of sorbents containing zeolites depends on the concentration of
hydrogen ions and mobility, regulation can be
Sorbents are generally obtained
from natural minerals and secondary raw materials, such as waste from
extraction plants, which mainly contain silicate and aluminosilicate non-metallic minerals. Moreover, obtaining effective sorbents
based on anthropogenic raw materials can solve both technological issues by expanding the range of products and creating innovative technologies, as well as ecological problems through
purification and disposal of waste (Suryanti et
al., 2023; Solouki, Jaffer, and Chaouki, 2022; Li et al.,
2019; Zhang et al., 2016; Galarneau et al., 2014; Milinchuk and
Shilina, 2010). Due to the
huge amount of waste produced in various industries, there is a need for
asbestos mineral extraction plants to explore innovative technological
solutions focusing on the construction and diversification of materials for
other purposes. This strategic shift can expand the range of manufactured
products and solve environmental problems for the processing of deposited waste
(Baigenzhenov et al., 2024; 2022; Kraus et
al., 2018; Jenifer, 2015). Recent study has described the methods of
obtaining molecular sieves and zeolites from rice husks (Zainal et al., 2024; Zharmenov et al., 2018; Yefremova et
al., 2016; Jo et al., 2015), showing properties comparable
with commercial types. Therefore, the development of technologies for deep
processing of heavy oil with high metal and sulfur content is essential,
requiring the search for new efficient conversion and integrated technological
solutions.
The natural fibrous mineral serpentinite and waste generated can be considered as a potential source of
producing sorption-filtering materials.
Specifically, serpentinite consists mainly
of serpentine group minerals with
admixture of carbonates, amphiboles, talc, ore
minerals magnetite, and chromite, with composition described by the general formula Mg3Si2O5(OH)4.
The fibrous variety of serpentine, known as chrysotile asbestos, is widely used
in chemical industry for obtaining high-purity magnesium and silicon
oxides, heat-resistant ceramics,
refractories, and adsorbent (Lv et al., 2020; Yerdos et al.,
2019; Zakaria et
al., 2019; Lee, Jo, and Ryoo, 2017; Zhang et al., 2017;
Baigenzhenov et
al., 2015; Jo et al., 2013).
Based on the background above, this study aimed
to develop and create new zeolite chemical adsorbent using technogenic waste of
asbestos production for purification of heavy hydrocarbon feedstock. The
novelty of this study lies in the high-temperature synthesis of zeolite
chemical adsorbent using technogenic waste of asbestos production containing
SiO2, as the main mass, nickel sulfate (NiSO4·7H2O),
and molybdenum oxychloride (MoOCl2) for demetallization and
desulfurization of heavy oil feedstock.
In previous studies by Ongarbayev et al. (2019), demetallization and desulfurization of heavy vacuum fraction were carried out in the presence of zeolites adsorbent containing vanadium oxide xerogel at a temperature of 613 K, pressure 1 atm, and volumetric feed rate 1 h. The results showed a significant decrease in vanadium, nickel, and iron content by 90, 70, and 60%, respectively, along with a reduction in sulfur content from 1.97 to 1.36%.
2.1. Materials
The activity of chemical adsorbent used in this study depends on the
rate of formation and transformation of surface intermediates. This process is
determined by the nature of the interaction, including bond breaking through
spatial configuration. The basic principle of activation is that the material
is subjected to thermal treatment under appropriate conditions, inducing the
formation of numerous pores, gaps, and cracks to increase the surface area of
pores per unit mass. In engineering applications, both chemical and vapor-gas
methods of material activation are widely used. Therefore, the initial
materials used in this study for the charge preparation and production of the active chemical
adsorbent include asbestos waste (AW) obtained after hydrochloric acid leaching
provided by Kostanay Minerals (Republic
of Kazakhstan). Chemical composition of the asbestos waste is presented in Table 1, with particle size of the material being -2.0 + 1.0 mm.
Table 1 Chemical composition of AW after
leaching
|
Component |
Chemical formula |
Content, % wt. |
|
Silicon oxide |
SiO2·nH2O |
90.0 |
|
Magnesium oxide |
MgO |
6.5 |
|
Iron oxide |
Fe2O3 |
2.5 |
|
Remaining impurities |
- |
1.0 |
- calcium
carbonate CaCO3,
99-99.9%,). – Sigmatec;
- sodium
carbonate ("chemically pure" 99.8%)
– Sigmatec;
- liquid
glass, diluted to 10 % – Sigmatec.
2.2. Mixing
Asbestos waste from hydrochloric acid leaching was obtained using the
method of (Shayakhmetova et al.,
2019). The procedure for the preparation of mixed adsorbent is similar to ceramic production applications. This
includes initial components preparation. grinding,
followed by mixing and wetting, pellet molding, hardening, and calcination.
Grinding of the initial components is essential, ensuring that the material
dispersion is suitable for the process adopted and meets the requirements for
the properties of adsorbent. The active adsorbent was prepared
by adding soluble salts of nickel and molybdenum
as semi-hydrous nickel sulfate and ammonium paramolybdate to the base
material, namely serpentine after leaching
and calcium carbonate. The weight ratio of the materials to be added and the
active ingredient in mixed catalysts are given below:
Table 2 Composition of synthetic chemical adsorbent
|
Name of a
compound |
The formula |
Content, % |
|
Serpentine after
leaching |
SiO2 |
38.0 |
|
Calcium carbonate |
CaCO3 |
44.0 |
|
Baking soda |
Na2CO3 |
5.0 |
|
Nickel sulphate |
NiSO4·7H2O |
3.0 |
|
Ammonium
paramolybdate |
(NH4)6Mo7O24·7H2O |
10.0 |
|
Total |
|
100.0 |
As shown in
Table 2, the mixing of charge components and grinding to achieve a homogeneous
mass composition was carried out for 10 minutes in the dry state using a
grinder to reduce the particle size to less than 100 microns. Subsequently, plasticizers in the form of sugar molasses
(0.5% of the total mass), and
sodium lignosulphonate (2.0%) were added to the mixture. Plastic molding
with the addition
of water was carried out and the molded materials obtained were
subjected to a two-stage firing at 923 K for 60 minutes to form wollastonitic structure and 1323 K for 30
minutes. Consequently, this processing led to the formation of the synthetic
adsorbent with wollastonite structure.
2.3. Test
The following equipment was used to determine
the mineralogical composition, chemical composition,
and formation structure of the synthetic chemical adsorbent obtained with various activator additives. Specifically, X-ray data were
obtained using a BRUKER D8 ADVANCE machine. X-ray fluorescence analysis was performed
on a Venus 200 PANalyical
B.V. (Holland) spectrometer with wave
dispersion. Chemical analysis of samples was
performed on an optical emission spectrometer with inductively coupled
plasma Optima 2000 DV (USA, PerkinElmer). Mapping
of elemental and phase composition of samples was carried out on
electron-probe analyzer JXA-8230 by
JEOL (Japan). Thermal analysis was performed using Jupiter STA 449 F3 synchronous thermal analysis unit and the
results were processed with NETZSCH Proteus software.
To determine the value of the total specific surface
of meso- and macroporous substances and materials, the SORBTOMETR-M device was used by the method
of thermal desorption of gas-adsorbate - BET method
based on the Brunauer-Emmett-Teller equation. The specific surface area measurement range is 0.3-2000
m2/g, with a temperature of 323-573
K.
3.1.
Study of temperature regimes (thermal analysis) to produce synthetic chemical adsorbent
The prepared mixture was thermally heated to 1273 K with an exposure time of 1 hour to determine the decomposition products of calcium carbonate and secondary calcium silicates. The analysis was carried out using differential thermal analysis (DTA) and thermogravimetric analysis (TGA) on a Q-1000D derivatograph. When the sample was dynamically heated from 293 to 1273 K, a series of thermal peaks were observed on the DTA curve. Among these peaks, 413, 873, and 1093 K were identified as endothermic, formed by the release of hydroxyl and carbon dioxide water molecules into the atmosphere. The formation of the fourth peak at 998 K was due to the exothermic destruction of the hydro-silicate, which caused an influx of thermal energy into the system, as shown in Figure 1.
Figure 1 TGA of the sample
The morphology of the DTA-curve in the
interval of 623 - 1003 K was similar to the differential
curve of serpentine decomposition. Based on the configurations of TG and DTG
lines, the low intensity of dehydration of sample components and the weak similarity of trajectories
of thermal peaks concerning the peaks of classical serpentine can be traced. This discrepancy showed
that the structure of the supposed mineral was found to be very defective, and unable to fully meet the crystalloid-chemical requirements of the reference serpentine. Therefore, the
composition of the sample was determined using the amount of constitutional
water (OH), corresponding to the sum of weight loss ?m3/2+?m4
= 1.05%+2.5%, which is the addition of second part ?m3 and the whole
part ?m4, as shown in Table 3. Considering the stoichiometric formula
of serpentine, the amount of this mineral
in the composition of the sample was found to be 24.6%.
Table 3 Thermogravimetric readings
of the test sample between
293-1273 K
The other component encountered in the sample tested is calcite,
detected by the strong emission of carbon dioxide into the atmosphere. The
removal of calcite from the system occurs at the high temperature, forming a
plumb line of weight loss on the TG curve. In contrast, on the DTG-curve, the release of carbon dioxide from the system
formed a very deep peak at 1073 K,
showing a high dissociation rate. Consequently, the DTA
curve showed an intensive peak from
1093 K, indicating a substantial outflow of thermal energy from the sample as presented in Figure 1. The
thermal degradation pattern observed
for CaCO3 corresponded
with well-oxidized calcite.
According to the amount of
CO2 emission (1.05% + 22.5%) shown in Table 3 and the stoichiometric formula, the content in
the sample was found to be 53.5%.
Meanwhile, the 3.3% of water ejected from the sample at 293 – 473 K was not part of the serpentine and
calcite, leading to the classification as mechanically bound
water of impurity status. This
showed that di-calcium silicate Ca2SiO4
was formed at 773 K, while tri-calcium silicate Ca3Si2O7
and wollastonite CaSiO3 began at 1123 K. Based on these results, it
can be concluded that Ca2SiO4 and Ca3Si2O7 are unstable intermediate compounds. According to these thermograms, the formation of wollastonite commenced when Ca3Si2O7 formed at temperatures above
1173 K. The formation of the mineral wollastonite occurred at high
temperatures with the release of
carbon dioxide, as expressed by chemical equation 1, indicated by the thermodynamic
data of chemical reaction
presented in Table 4:
CaCO3 +SiO2 ? CaSiO3
+CO2 (1)
Table 4 Thermodynamics of the wollastonite formation reaction
|
T (K) |
deltaH |
deltaS |
deltaG |
Log (K) |
|
(kJ) |
(J/K) |
(kJ) |
| |
|
273 |
89.135 |
162.947 |
44.627 |
-8.535 |
|
473.0 |
87.550 |
158.744 |
12.441 |
-1.374 |
|
673.0 |
84.903 |
154.135 |
-18.853 |
1.463 |
|
873.0 |
80.404 |
148.380 |
-49.154 |
2.941 |
|
1073.0 |
77.377 |
145.263 |
-78.513 |
3.822 |
|
1173.0 |
73.652 |
141.875 |
-92.788 |
4.132 |
|
1273.0 |
71.827 |
140.382 |
-106.901 |
4.386 |
|
1373.0 |
69.907 |
138.931 |
-120.866 |
4.598 |
Based on the thermal analysis and thermodynamic reactions, the formation
of synthetic chemical adsorbent in
the form of mineral wollastonite
occurred at 1173-1373 K.
3.2. X-ray phase and analyses of
samples
X-ray diffraction analysis of synthetic chemical adsorbent in Figure 2 and Table 5 showed the formation of new mineral. Specifically, pavellite (CaMoO4) crystallized in tetragonal syngony was identified, forming dipyramidal and tabular crystals, which showed a density of 4.25 - 4.52 g/cm3, with 72% MoO2 and 10% Mo composition. Libenbergite (Ni2SiO4) was detected, characterized by fine pyramidal crystals, grains crystallized in rhombic syngony, and a density of 4.50 - 4.60 g/cm3, containing 56% nickel.
Figure 2 Diffractogram of the
obtained synthetic chemical adsorbent
Table 5 Results of semi-quantitative analysis of crystalline
phases
|
Phase name |
Formula |
SemiQuant [%] |
|
Wollastonite |
CaSiO3 |
85.0 |
|
Powellite, syn |
CaMoO4 |
6.0 |
|
liebenbergite, high,
syn |
Ni2SiO4 |
2.0 |
|
Silicon Oxide |
SiO2 |
7.0 |
The XRD data
showed that the high-temperature synthesis produced a well-formulated synthetic
material, with wollastonite CaSiO3 constituting 80 - 85% of the main mineral
composition. The integration of activators in the form of molybdenum oxychloride
(MoOCl2) and nickel sulfate
(NiSO4·7H2O) promoted the formation of new minerals in
the form of powellite (CaMoO4) and libenbergite (Ni2SiO4),
which are fully synthesized based on free calcium and silicon compounds. The presence of these compounds did not
impair the physicochemical properties of the synthesized material but promoted
the formation of a porous structure
due to its small density and specific surface
area. The resulting product also contained free amorphous silica in the form of cristoballite, constituting 7% of the
composition.
3.3. BET
method
In selecting adsorbent for a particular practical application,
information about porous structure is
essential. For appropriate selection, there is a need to know whether the inherent
pore radius distribution corresponds to the molecular
sizes of given sorbates and ensures rapid penetration of
substances deep into sorbent granules.
Adsorption potential in micropores is significant due to the
superposition from neighboring walls. Therefore, molecules capable of
penetrating micropores show significantly higher adsorption affinity compared
to those fixed in meso- or macropores. To determine the specific
surface area and pore radius distribution of porous bodies, the BET method was applied using SORBTOMETR-M device, as shown in Table 6.
As shown in Table 6, the values of the specific surface area and pore volume of the obtained active component are small.
Moreover, micropores are important
for the activity of material and demetallization process due to their diameters of approximately 2 nm, which corresponds
to the size of the adsorbing molecules. The presence of micropores also facilitates the development of the main part of
the inner surface of the active
component, positively influencing the properties of the synthetic
chemical adsorbent.
Table 6 Specific surface area, specific
volume, and average pore size of materials
|
Sample |
Specific surface
area, m2/g |
Specific pore
volume, cm3 |
Average pore
size, Å |
Weight, g |
|
Obtained chemical
adsorbent |
4.0-6.0 |
0.137 |
1.714 |
0.1345 |
3.4. Cleaning of heavy crude oil from metal
impurities
The destructive-adsorptive thermal contact
process of demetallization consists of short-term contact of oil feedstock with adsorbent
in the two-section reactor heated
at temperatures of 573 - 723 K. Due to the contact between the feedstock and the hot adsorbent,
hydrocarbon vapors are formed, which are mixed with water vapor and transported
to the gas venting line, as shown in Figure 3.
Figure 3 Process flow diagram for demetallization and desulfurization of heavy
oil residue
Testing was carried out using tar obtained from
the delayed coking unit of "Pavlodar petrochemical plant" LLP, a viscous slow-moving liquid with a mass
fraction of water of approximately 0.1 wt.%.
This tar contains metals, such
as vanadium 400-600.0 mkg/kg, nickel 50
– 70.0 mkg/kg, iron 30 – 70.0 mkg/kg,
the mass fraction of sulfur 2.5 – 3.5%, ash content – 0.02 wt. %, coking - 18 wt. %, density at 273K - 1000.0
kg/m3, the boiling point – 653 K. Furthermore, testing of demetallization of heavy
oil residue was carried out at temperature zone of 593 - 693 K,
contact time of 60 minutes, and the pressure was kept within 8 - 10 atm.
The results obtained were
presented in Table 7, where the optimum temperature zone of heavy oil demetallization was found to be 673 - 683
K. However, the tar commenced to boil and coke due to an increase in
temperature above 683 K, which adversely affected demetallization process. This
phenomenon also affected the content of vanadium, nickel, iron, and sulfur in
vacuum residue, which decreased from
540 to 52 mkg/kg, 50 to 26 mkg/kg, 50 to 12 mkg/kg, and 3.50 % to 2.39 %,
respectively. The maximum degree of extraction
at these metals was found to be 90.37%, 48.0%, 76.0%, and 31.7%,
respectively.
Table 7 Metal and
sulfur content of tar before and after testing as a function of process temperature
|
Raw material and type of adsorbent |
T, K |
Element content | |||
|
V, µg/kg |
Ni, µg/kg |
Fe, µg/kg |
S, % | ||
|
Initial tar |
- |
540.0 |
50.0 |
50.0 |
3.50 |
|
Tar after
contact with modified chemical adsorbent |
593 |
500.0 |
50.0 |
50.0 |
2.65 |
|
613 |
488.0 |
50.0 |
50.0 |
2.61 | |
|
633 |
450.0 |
43.0 |
40.0 |
2.58 | |
|
653 |
330.0 |
35.0 |
33.0 |
2.53 | |
|
673 |
70.0 |
31.0 |
21.0 |
2.45 | |
|
683 |
52.0 |
26.0 |
12.0 |
2.39 | |
|
693 |
23.0 |
22.0 |
7.0 |
1.50 | |
In conclusion, this study showed the developmental processes for
dehydrating and obtaining active components to create a matrix base of synthetic chemical adsorbent from composite materials using chrysotile asbestos production waste. The adsorption properties of
obtained composite catalysts were practically investigated. The results showed
that the optimal temperature zone for demetallization of heavy oil residue was 653-683 K, with a contact time
of 60 minutes and a pressure of 8-10 atm. Under these conditions, a significant
decrease was observed in the content of vanadium, nickel, iron, and sulfur from
540 to 52 mkg/kg, 50 to 26 mkg/kg, 50 to 12 mkg/kg, and 3.50% to 2.39%, respectively.
The authors are grateful for the financial support provided
by project NTP
No. O.003 (2021-2023) titled "Creation of new composite
materials with high-performance properties based on rare and rare earth
elements" funded by the Committee for Industrial Development of the Ministry of Industry
and Infrastructure
Development
of the Republic of Kazakhstan.
Attia, M., Farag, S., Shaffiq, A.J., Chaouki, J., 2020. Metal And Sulfur
Removal from Petroleum Oil Using a Novel Demetallization-Desulfurization Agent
and Process. Journal of Cleaner Production, Volume 275, pp. 1–14
Baigenzhenov, O., Khabiyev, A.,
Mishra, B., Aimbetova, I., Yulusov, S., Temirgali, I., Kuldeyev, Y.,
Korganbayeva, Z., 2022. Asbestos Waste Treatment—An Effective Process to
Selectively Recover Gold and Other Nonferrous Metals. Recycling, Volume 7(6), p. 85
Baigenzhenov,
O.S., Chepushtanova, T.A., Altmyshbayeva, A. Zh., Temirgali, I.A., Maldybayev,
G., Sharipov, R.H., Altaibayev, B.T., Dagubayeva, A.T., 2024. Investigation of
Thermodynamic and Kinetic Regularities of Asbestos Waste Leaching
Processes. Results in Engineering,
Volume 21, p. 102000
Baigenzhenov, O.S., Kozlov, V.A., Luganov, V.A., Mishra, B.,
Shayahmetova, R.A., Aimbetova, I.O., 2015. Complex Processing of Tailings Generated in Chrysotile
Asbestos Production. Mineral
Processing and Extractive Metallurgy Review, Volume 36(4), pp. 242–248
Baikonurova, A.O.,
Ussoltseva, G.A., Markametova, ?.S., Nurzhanova, S.B., 2021. Zeolite Modification by
Synthesized Vanadium Xerogel. Journal of the Balkan Tribological Association,
Volume 27(3), pp.
445–456
Farghadani,
M.H., Mahdavi, V., 2022. Novel Synthesis of Highly Dispersed
Molybdenum Oxide Over Nanorods Cryptomelane Octahedral Manganese
Oxide Molecular Sieve (MoOx/nanorod-OMS-2) As a High-Performance Catalyst
For Oxidative Desulfurization Process. Fuel Processing Technology, Volume 236, p. 107415
Galarneau, A., Villemot, F., Rodriguez, J., Fajula, F., Coasne, B.,
2014. Validity of the Tplot Method To Assess Microporosity In Hierarchical
Micro/Mesoporous Materials. Langmuir, Volume 30, pp. 13266–13274
Jenifer, A.C., Sharon, P., Prakash, A., Sande, P.C., 2015. A Review of
The Unconventional Methods Used for The Demetallization Of Petroleum Fractions
Over The Past Decade. Energy and Fuels, Volume 29(12), pp. 7743–7752
Jo, C., Cho, K., Kim, J., Ryoo, R., 2014. MFI Zeolite Nanosponges Possessing Uniform Mesopores Generated by Bulk
Crystal Seeding In The Hierarchical Surfactant-Directed Synthesis. Chemical
Communications, Volume 50, pp. 4175–4177
Jo, C., Lee, S., Cho, S.J., Ryoo, R., 2015. Synthesis Of Silicate
Zeolite Analogues Using Organic Sulfonium Compounds as Structure-Directing
Agents. Angewandte Chemie International Edition, Volume 54, pp.
12805–12808
Jo, C., Ryoo, R., ?Zilkov´a, N.,
Vitvarov´a, D., ?Cejka, J., 2013. The Effect of MFI Zeolite Lamellar and
Related Mesostructures on Toluene Disproportionation and Alkylation. Catalysis
Science & Technology, Volume 3, pp. 2119–2129
Johansson, A.C., Bergvall, N., Molinder, R., Wikberg,
E., Niinipuu, M., Sandström, L., 2023. Comparison of Co-Refining of Fast
Pyrolysis Oil from Salix Via Catalytic Cracking and Hydroprocessing. Biomass and Bioenergy. Volume
172, p. 106753
Kamal, M.S., Razzak, S.A., Hossain, M.M., 2016. Catalytic Oxidation of
Volatile Organic Compounds (VOCs)–A Review. Atmospheric Environment, Volume
140, pp. 117–134
Kraus, M., Trommler, U., Holzer, F., Kopinke, F.D., Roland, U., 2018.
Competing Adsorption Of Toluene And Water On Various Zeolites. Chemical
Engineering Journal, Volume 351, pp. 356–363
Lee, S., Jo, C., Ryoo, R., 2017. Tomographic
Imaging Of Pore Networks And Connectivity Of Surfactant-Directed Mesoporous
Zeolites. Journal of Materials Chemistry A, Volume 5(12), pp.
11086–11093
Li, R., Chong, S., Altaf, N., Gao, Y., Louis, B., Wang, Q., 2019.
Synthesis of ZSM-5/ Siliceous Zeolite Composites For Improvement Of Hydrophobic
Adsorption Of Volatile Organic Compounds. Frontiers in Chemistry, Volume
7, p. 505
Li, X., Zhang, L., Yang, Z., Wang, P., Yan, Y., Ran, J., 2020.
Adsorption Materials for Volatile Organic Compounds (VOCs) and the Key Factors
for VOCs Adsorption Process: A Review. Separation and Purification
Technology, Volume 235, p. 116213
Lv, Y., Sun, J., Yu, G., Wang, W., Song, Z., Zhao, X., Mao, Y., 2020.
Hydrophobic Design of Adsorbent for VOC Removal in Humid Environment and Quick
Regeneration by Microwave. Microporous and Mesoporous Materials, Volume
294, p. 109869
Markametova,
M.S., Baikonurova, A.O., Kozlov, V.A.,
Nurzhanova, S.B., Ermolaev, V., 2014.
Obtaining Vanadium Nanostructures by Sol-Gel Process. Industry in Kazakhstan,
Volume 3(66), pp. 73–75
Milinchuk, V.K., Shilina,
A.S., 2010. Sorption Purification of Natural and Industrial
Waters from Heavy Metal Cations and Radionuclides by A New Type of High-Temperature Aluminosilicate Adsorbent. Sorption and Chromatographic Processes, Volume 2. pp. 237–245
Ongarbayev, Y.,
Oteuli, S., Tileuberdi, Y., Maldybaev, G., Nurzhanova, S., 2020.
Demetallization Of Heavy Vacuum Residuum by Titanium –Vanadium Zeolite
Adsorbents. Studia Universitatis Babes-Bolyai, Volume 65(1), pp. 219-231
Ongarbayev, Y., Oteuli,
S., Tileuberdi, Y., Maldybayev, G., Nurzhanova, S., 2019. Demetallization and
Desulfurization of Heavy Oil Residues by Adsorbents. Petroleum Science and
Technology, Volume 37(9), pp. 1045–1052
Prihadiyono, F.I.,
Lestari, W.W., Putra, R., Aqna, A.N.L., Cahyani, I.S., Kadja, G.T.M., 2022.
Heterogeneous Catalyst based on Nickel Modified into Indonesian Natural Zeolite
in Green Diesel Production from Crude Palm Oil. International Journal
of Technology, Volume 13(4), pp. 931–943
Shang,
H., Liu, Y., Shi, J.-C., Shi, Q., Zhang, W.-H., 2016. Microwave-Assisted Nickel
and Vanadium Removal From Crude Oil. Fuel Processing Technology, Volume
142, pp. 250–257
Shayakhmetova, R.A. Mukhametzhanova A.A., Samatov I.B., Akbayeva D.N., 2019. Technogenic Raw Materials for The Production
of Magnesium and Silicon- Containing Compounds.
Machines. Technologies. Materials.
Volume 13(2), pp. 90–92
Solouki, A., Jaffer, S.A., Chaouki, J., 2022. Process Development and
Techno-Economic Analysis of Microwave-Assisted Demetallization and
Desulfurization of Crude Petroleum Oil. Energy Reports, Volume 8, pp.
4373–4385
Sudibandriyo, M., Putri, F.A.,
2020. The Effect of Various Zeolites as an Adsorbent for Bioethanol
Purification using a Fixed Bed Adsorption Column. International Journal of
Technology, Volume 11(7), pp. 1300–1308
Suryanti, V., Kusumaningsih, T., Safriyani, D., Cahyani, I.S., 2023. Synthesis
and Characterization of Cellulose Ethers from Screw Pine (Pandanus tectorius)
Leaves Cellulose as Food Additives. International
Journal of Technology. Volume 14(3), pp. 659–668
Ulfiati, R., Dhaneswara, D.,
Harjanto, S., Fatriansyah, J.F., 2022. Synthesis and Characterization ZSM-5
Based on Kaolin as a Catalyst for Catalytic Cracking of Heavy Distillate. International
Journal of Technology. Volume 13(4), pp. 860–869
Yefremova, S.V., Korolev, Y.M., Sukharnikov, Y.I., Kablanbekov, A.A.,
Anarbekov, K.K., 2016. Structural Transformations of Carbon Materials in The
Processes of Preparation from Plant Raw Materials. Solid Fuel Chemistry,
Volume 50, pp.
152–157
Yerdos, O., Shynar, O., Yerbol, T., Galymzhan, M., Saule, N., 2019.
Demetallization And Desulfurization of Heavy Oil Residues by Adsorbents. Petroleum
Science and Technology, Volume 37(9), pp. 1045–1052
Zainal,
Z.S., Hoo, P., Ahmad, A.L., Abdullah, A.Z., Qihwa N., Shuit, S., Rahim, S.K.E.,
Andas, J., 2024. Plant-Based Calcium Silicate from Rice Husk Ash: A Green
Adsorbent For Free Fatty Acid Recovery From Waste Frying Oil. Heliyon,
Volume 10(4), p. e26591
Zakaria, M.Y., Sulong, A.B., Muhamad, N., Raza, M.R., Ramli, M.I., 2019.
Incorporation Of Wollastonite Bioactive Ceramic with Titanium for Medical
Applications: An Overview. Materials Science and Engineering: Volume 97,
pp. 884–895
Zarraoa, L., Gonz´alez, M.U., Paulo, ´A.S., 2019. Imaging
Low-Dimensional Nanostructures by Very Low Voltage Scanning Electron
Microscopy: Ultra-Shallow Topography And Depth-Tunable Material Contrast. Scientific
Reports, Volume 9, p. 16263
Zhang, L., Peng, Y., Zhang, J., Chen, L., Meng, X., Xiao, F.S., 2016.
Adsorptive And Catalytic Properties in The Removal Of Volatile Organic
Compounds Over Zeolite-Based Materials. Chinese Journal of Catalysis, Volume
37, pp. 800–809
Zhang, X., Gao, B., Creamer, A.E., Cao, C., Li, Y., 2017. Adsorption of VoCs onto Engineered Carbon Materials: A Review. Journal of Hazardous Materials, Volume 338, pp. 102–123
Zharmenov, A., Yefremova, S., Sukharnikov, Y., Bunchuk, L., Kablanbekov, A., Anarbekov, K., Murtazayeva, D., Yessengarayev, Y., 2018. Carbonaceous Materials from Rice Husk: Production and Application in Industry and Agriculture. In?ynieria Mineralna, Volume 19(1), pp. 263–274
