A Rapid Colorimetric Method to Investigate SARS-CoV-2 by Using Gold Nanoparticles Capped with O-hydroxybenzoic Acid
Corresponding email: agus147@brin.go.id
Published at : 25 Mar 2025
Volume : IJtech
Vol 16, No 2 (2025)
DOI : https://doi.org/10.14716/ijtech.v16i2.6476
Wulandari, M, Hasanah, N, Yuliarto, B, Angelina, M, Manurung, RV, Jenie, SNA & Andreani, AS 2025, ‘A rapid colorimetric method to investigate SARS-CoV-2 by using gold nanoparticles capped with O-hydroxybenzoic acid’, International Journal of Technology, vol. 16, no. 2, pp. 652-661
| Meyliana Wulandari | Chemistry Department, Syarif Hidayatullah State Islamic University, Jln. Ir. H. Djuanda No. 95, Ciputat, Tangerang Selatan, Banten, 15412, Indonesia |
| Nurachdiani Hasanah | 1. Chemistry Department, Syarif Hidayatullah State Islamic University, Jln. Ir. H. Djuanda No. 95, Ciputat, Tangerang Selatan, Banten, 15412, Indonesia 2. Research Centre for Chemistry, National Rese |
| Brian Yuliarto | 1. Department of Physics Engineering, Research Centre for Nanosciences and Nanotechnology, Institut Teknologi Bandung (ITB), Jln. Ganesha 10, Bandung, Jawa Barat 40312 Indonesia. 2. BRIN and ITB Coll |
| Marissa Angelina | 1. BRIN and ITB Collaboration Research Centre for Biosensor and Biodevices, Jln. Ganesha 10, Bandung, Jawa Barat 40132 Indonesia 2. Research Centre for Pharmaceutical Ingredients and Traditional Medi |
| Robeth Viktoria Manurung | 1. BRIN and ITB Collaboration Research Centre for Biosensor and Biodevices, Jln. Ganesha 10, Bandung, Jawa Barat 40132 Indonesia 2. Research Centre for Telecommunications, National Research and Innov |
| Siti Nurul Aisyiyah Jenie | 1. Research Centre for Chemistry, National Research and Innovation Agency (BRIN), Kawasan Puspiptek, Building 452, Serpong, Tangerang Selatan, Banten 15314 Indonesia 2. BRIN and ITB Collaboration Res |
| Agustina Sus Andreani | 1. Research Centre for Chemistry, National Research and Innovation Agency (BRIN), Kawasan Puspiptek, Building 452, Serpong, Tangerang Selatan, Banten 15314 Indonesia 2. BRIN and ITB Collaboration Res |
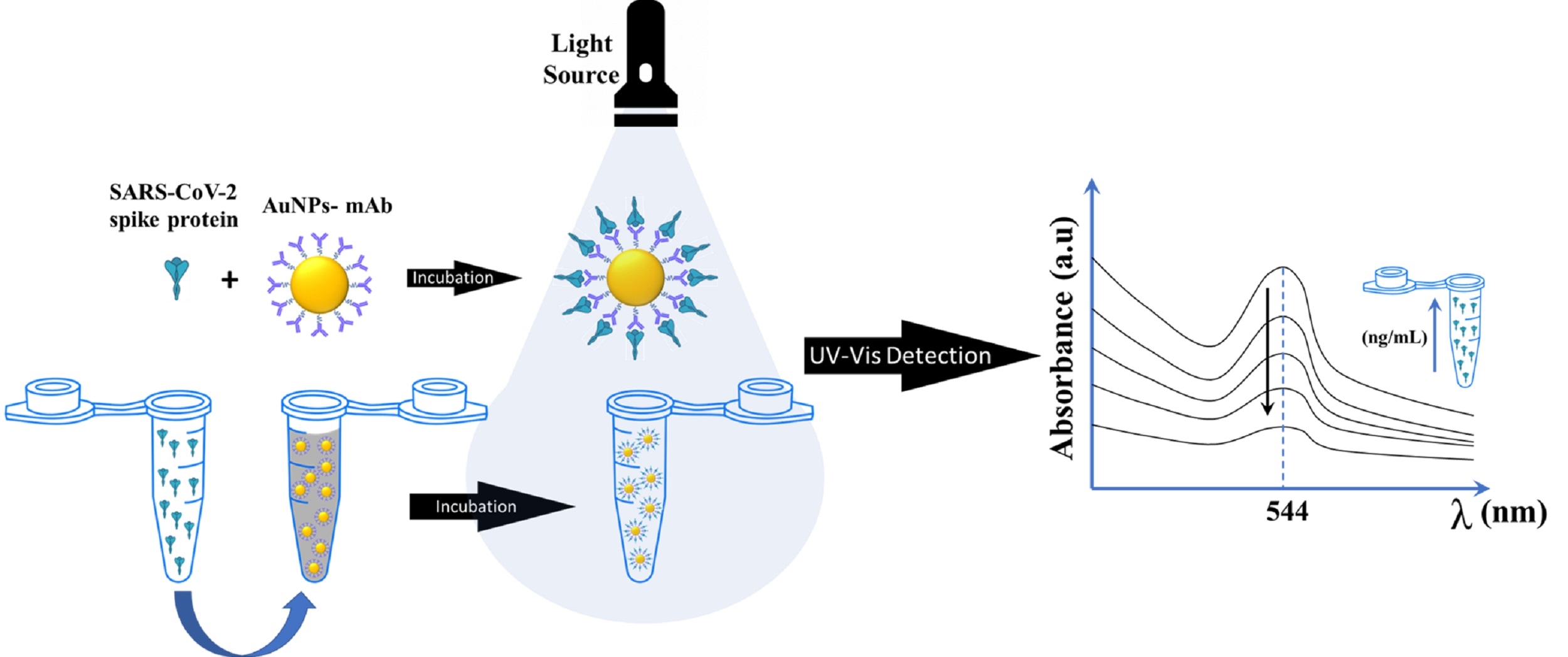
A rapid, sensitive, and stable colorimetric sensor applied to gold nanoparticles (AuNPs) has been established. AuNPs were synthesized by o-hydroxybenzoic acid; then, the surface was modified by N-ethyl-N’-(3-dimethyl aminopropyl) carbodiimide hydrochloride (EDC)/N-Hydroxysuccinimide (EDC/NHS) and bioconjugated with SARS-CoV-2 (SC-2) antibody (AuNPs-mAb). O-hydroxybenzoic acid consists of a carboxyl group and thus can reduce one step of the reaction in bioconjugation with SC-2 antibody. AuNPs-mAb were proven to detect the SC-2 antigen, as indicated by the decrease in UV-Vis absorbance and a visible color difference from purplish-red to colorless AuNPs. The detection limit (LoD) and quantification limit (LoQ) were 16.03 and 53.42 ng/mL, respectively, with a linear concentration range of 75-250 ng/mL. SC-2 can be detected with the naked eye using AuNPs-mAb in less than 5-minute incubation time (rapid), and AuNPs-SC-2 are stable for four weeks at 4 °C. The comparative study for AuNPs-mAb performance to detect the SC-2 antigen has been done with an enzyme-linked immunosorbent assay (ELISA). The data show that AuNPs have higher sensitivity than the conventional ELISA method in the same concentration range. Because the detection system based on AuNPs capped with o-hydroxybenzoic acid is rapid, stable, and practical, this method can be an alternative to early screening for COVID-19.
Colorimetric sensor; Gold nanoparticles (AuNPs); SARS-CoV-2
During the last three years, the epidemic of COVID-19 coming from SC-2 has attracted a big concern in the world (Ulyah et al., 2024; Sahlan et al., 2023; Ting et al., 2022; Yang et al., 2020; Zhu et al., 2020). Currently, molecular assays, i.e., real-time Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR), have been common to detect SC-2. Hence, RT-PCR analysis has crucial disadvantages, such as the need for costly equipment, skilled technicians, longer analysis time, complicated procedures, and susceptibility to false-negative results (Ayankojo et al., 2022; Lischer et al., 2021; Raziq et al., 2021; Tang et al., 2020). ELISA (enzyme-linked immunosorbent assays) offers a shorter analysis time but remains unsuitable for diagnosing an early infection stage. The human body requires two weeks for the level of antibody production to increase after being exposed to symptoms of this virus (Rump et al., 2021). This period of time can be used for health screening. Later, a CT imaging test was reported. This method was found not proper due to the long analysis time, expensive, and susceptibility to false positives and negatives (Srivastava et al., 2021). Given these limitations, an accurate, sensitive, specific, reliable, and much more straightforward yet rapid method is demanded to detect SC-2. Biosensors are practical, rapid, and sensitive, requiring only a few samples (Srivastava et al., 2021). Several biosensors, i.e., colorimetric (Basso et al., 2018; Lee et al., 2017), scanometric (Yi et al., 2015), electrochemical (Cesewski and Johnson, 2020), and fluorometric (Guirgis et al., 2012), were studied to detect various human viruses. The colorimetric method is the most straightforward visual detection method of all the reported methods. This method does not require complicated equipment as the electrometric method. The method of colorimetric analysis using metal nanoparticles has been used to analyze diseases in the human body (Yadavalli and Shukla, 2017). Biosensors allow colorimetric diagnostic analysis more rapidly and are suitable for home use.
Scientists have recently carried out the development of nanoparticle applications on a large scale (Adiwibowo et al., 2018). Among the most exciting applications of nanoparticles is the use of gold nanoparticles (AuNPs). Previous research has reported the success of AuNPs used in the medical world and biosensors (Funari et al., 2020). Various methods have also been reported to synthesize AuNPs, including chemical methods with the addition of stabilizers. Specifically, some advantages of colorimetric assays with gold nanoparticles (AuNPs) are that they are easy to synthesize, simple, practical, unique in terms of optical properties, and functional (Chang et al., 2019; Oliveira, et al., 2019; Jazayeri et al., 2018). AuNPs have surface plasmon resonance (SPR) characteristics that allow biosensor analysis by the colorimetric method. The SPR of AuNPs appears at ~520 nm in the absorption spectra of visible light. Absorption at these wavelengths can produce a red or blue color shift depending on the size, shape, and distance between nanoparticles (Krajczewski et al., 2017). The decrease in distance triggers aggregation, which changes the nanoparticle's optical properties. Therefore, this results in a red color shift in the AuNPs plasmon band and the color degradation from red to purple (Jazayeri et al., 2016).
There are several COVID-19 biosensing methods using AuNPs. The previous research (Ventura et al., 2020), a colorimetric method was employed to achieve sensitive detection of SC-2 using gold nanoparticles coupled with three different antibodies, i.e., spike, envelope, and membrane. The surface modification of AuNPs uses the photochemical immobilization technique (PIT) so that antibody activation occurs in just a few minutes. Those modified AuNPs develop excellent biosensing platforms because of their good biocompatibility, size-dependent optical property, and accessibility to modify the surface. A color shift of anti-spike antibody attached to AuNPs was detected with the bare eye. That method could detect antigen SC-2 even in the one ng/mL range at 1000 virus particles/mL. This method uses sampling from the nose and throat, which is not convenient for several age groups.
Similar research used polyethylene glycol (PEG) and EDC/NHS to modify the synthesized AuNPs with citrate (Pramanik et al., 2021). The direct detection was Surface-Enhanced Raman Spectroscopy (SERS). In the presence of SC-2 antigen, AuNPs aggregated and underwent a color degradation from pink to blue, which allowed very rapid detection at a concentration of 1 ng/mL for COVID-19 antigen at 1000 virus particles/mL. (Karakus et al., 2021) also modified AuNPs with antibodies to detect antigen (SC-2) by colorimetric in saliva and electrochemical methods. The difference is that AuNPs were synthesized with sodium citrate dihydrate, and EDC/NHS was used to alter the nanoparticle's surface to bind to antibodies. Before adding EDC/NHS, 11-mercaptoundecanoid acid (MUA) was used to obtain a carboxyl group. The results showed that the LoD for the colorimetric and electrochemical methods were 48 ng/ml and one pg/ml, respectively (Karakus et al., 2021). In that study, AuNPs were synthesized using sodium citrate, a weak reducing agent known to produce less-stable nanoparticles for a long time (Khalil et al., 2017; Zhao et al., 2013).
The weakness identified in the previous synthesis of AuNPs was the use of citrate, which led to low stability, lasting less than five months. However, research conducted by Andreani et al. (2019) offers a solution to overcome this problem. In their study, they successfully synthesized AuNPs using o-hydroxybenzoic acid, a benzoic derivative compound, as both a reducing and capping agent. The utilization of o-hydroxybenzoic acid resulted in AuNPs with significantly improved stability, lasting for more than five months. The hydroxyl group in hydroxybenzoic acid acted as a reducing agent, and the carboxyl group provided electrostatic repulsion to prevent the aggregation of AuNPs. The stable AuNPs made a colorimetric biosensor application easy (Andreani et al., 2019).
This research aims to synthesize AuNPs from [AuCl4]- produced by PT. Aneka Tambang Indonesia, which is cheaper than commercially available [AuCl4]-. Then, o-hydroxybenzoic acid was used to reduce and stabilize AuNPs because it had been shown to increase their stability. The use of o-hydroxybenzoic acid modified with N-ethyl-N’-(3-dimethyl aminopropyl) carbodiimide hydrochloride (EDC)/N-Hydroxysuccinimide (NHS) can reduce 1 step reaction, namely adding a compound to get a carboxyl group, because o-hydroxybenzoic acid already has a carboxyl group on its surface. AuNPs modified with EDC/NHS were used for conjugation with the SC-2 antibody and then applied selectively for the detection of the SC-2 antigen.
2.1. Synthesis of AuNPs
5 mL of the [AuCl4]- solution at pH 3 was added to 5 mL of o-hydroxy benzoic acid with a volume ratio of 1:1. The AuNPs were synthesized by adding 5 mL of [AuCl4]- 70 ppm and 0.01 M of 5 mL of o-hydroxybenzoic acid at pH 12 in a water bath at 98 °C for 20 minutes (Andreani et al., 2019).
2.2. AuNPs Surface Modification
The synthesized AuNPs were surface modified using EDC/NHS with an EDC/NHS concentration ratio of 2:1, 0.02 g of EDC was dissolved in 1 mL of PBS, and 0.05 g was dissolved in 1 mL of PBS. 10 µL of EDC, and 100 µL of NHS with a concentration ratio of 2:1 was added to 1 mL of synthesized AuNPs. The mixture was stirred at ambient temperature for about 30 minutes to produce the AuNPs-EDC/NHS samples.
2.3. Synthesis of AuNPs with SC-2 Antibodies (AuNPs-mAb)
The preparation of AuNPs-mAb samples was carried out by mixing SC-2 antibodies with the synthesized AuNPs. 50 µL of SC-2 200 ng/mL antibody was added to 1 mL of AuNPs-EDC/NHS, then incubated at 37oC for 1 hour and stirred at 200 rpm. The residue was washed twice with PBS (10 mM) at pH 7.4 (containing Tween-20 0.2 mg/mL), and the supernatant was discarded. Last, the AuNPs-mAb conjugate was resuspended in PBS (1 mL). For further experiments, the AuNPs-mAb was stored at 4oC.
2.4. Detection of SC-2 Antigen
The SC-2 antigen was prepared in various concentrations of 75, 100, 150, 200, and 250 ng/mL-1 in PBS. A total volume of 100 µL of each variation of SC-2 antigen was coated onto the well plate, and 100 µL of AuNPs-mAb was added, followed by incubation and shaking at ambient temperature. The incubation time varied from 0 to 60 minutes at ambient temperature. The absorbance analysis was carried out with a well-plate reader in a spectrophotometer with scanning at a wavelength of 300-800 nm. The calibration curve was created by plotting the absorbance values at 544 nm versus the concentration of SC-2 antigen with three repetitions. The equations below were used to calculate the limit of detection (LoD) (see equation 1) and limit of quantification (LoQ) (see equation 2):
where SD is standard deviation, and s is slope of calibration curve.
2.5. Comparison with The ELISA Method
The ELISA method was carried out according to the standard ELISA protocol (Cornuault and Knox, 2014). In this immunoassay protocol, the coating of the well plate (Corning Costar 96-well flat bottom) was performed using 100 µL of SC-2 antigen in PBS per well at 4 °C overnight. After coating, the plate was washed three times using PBS 0.1 % (v/v) and Tween-20, and the plate was blocked using 200 µL of BSA for 1 hour per well at ambient temperature. Subsequently, the well plate was incubated with 100 µL per well of 200 ng/mL antibody SC-2 for 1 hour at ambient temperature. Then, the plate was washed three times using PBS 0.1 % (v/v) and Tween-20, and 100 µL per well of secondary HRP-labeled antibody in PBS was added and allowed to incubate for 1 hour at ambient temperature. The well plates were washed three times using PBS 0.1 % (v/v) Tween-20. A total volume of 100 µL of TMB was added, and the enzymatic reaction was stopped by adding 50 µl of 2 N of sulphuric acid in each well. The absorbance analysis was carried out with a spectrophotometer scanning at 300-800 nm wavelengths.
3.1. Synthesis of AuNPs
In this research, AuNPs were formed due to the reduced of Au3+ into Au0 by o-hydroxy benzoic acid. This compound had lone pair electrons from the hydroxyl group, which were moved to Au3+ to form Au0. This reduction resulted in size-controlled AuNPs. The aromatic group of o-hydroxybenzoic acid located on the surface of AuNPs was used as a capping agent. The capping agent provided electrostatic repulsion, which repelled AuNPs and precluded them from aggregating. In previous studies, pH, concentration ratio, and reaction time were optimized to obtain AuNPs (Andreani, et al., 2019). The purplish-red AuNPs were observed at 540 nm on the UV-Vis spectrum due to the surface plasmon resonance (SPR) characteristic, with a narrow spectral bandwidth indicating the mono-dispersity of the AuNPs, as depicted in Figure 1. The TEM (inset in Figure 1) and FESEM (Figure S1a) images show unaggregated spherical particles ranging from 50-56 nm, corresponding to the PSA particle size of 51.2 nm. The surface composition of AuNPs based on the EDX results (Figure S1b) confirmed the presence of a strong signal of elemental gold of 45 wt%. AuNPs displayed an optical absorption band peak at about two keV. EDX revealed the presence of other compositions, such as carbon at 40.5 wt% and oxygen of 14.5 wt% corresponding to the capping agent.
Figure 1 Spectra of UV-Vis and TEM images (inset) of AuNPs.
3.2. AuNPs Surface Modification
AuNPs synthesized with o-hydroxybenzoic acid were covalently bound to the SC-2 antibody directly on the surface via a crosslinker, i.e., EDC/NHS. To activate the ester of the NHS group, adding a compound with a carboxyl group, such as 11-mercaptoundecanoic acid (MUA), was necessary (Kamra et al., 2016). However, a compound having a carboxyl group was not added to this work. Besides acting as a reducing agent and stabilizer for AuNPs, o-hydroxybenzoic acid also provided a carboxyl group for the surface modification of AuNPs. These steps reduced one step of the reaction in covalent immobilization. After modifying AuNPs with EDC/NHS, they changed the carboxyl group on the AuNPs surface into an ester of NHS, later bound to antibodies. Figure 2 shows an absorption peak at 1778 cm-1, which was shown as C=O NHS ester bound to the AuNPs surface. Absorption peaks at 1221 cm-1 and 1108 cm-1 were attributed to the C-O and N-O amine of EDC/NHS, respectively, indicating that the succinimidyl ester of NHS had covalent interaction with the group of carboxyl on the AuNPs surface (Widyasari et al., 2022). When the primary amine group in the SC-2 antibody reacted with the NHS ester, an amide bond was formed at an absorption peak of 1485 cm-1 (Figure S2 and Scheme 1-SI).
Figure 2 (a) Spectra UV-Vis of AuNPs, after modification with EDC/NHS, and AuNPs-mAb. TEM morphology of AuNPs-mAb at a scale bar of 100 nm as inset picture. (b) Spectra UV-Vis of AuNPs-mAb (black line) along with a purplish-red tube and AuNPs-mAb after being added with 250 ng/mL of SC-2 antigen (red line), colorless tube. The inset shows the TEM morphology of AuNPs-mAb after incubation for 10 minutes with the SC-2 antigen at the scale bar of 50 nm.
Figure 2a shows the spectrum of UV-Vis from modified AuNPs before and after immobilization with EDC/NHS and antibodies. The absorbance of AuNPs was observed at 0.55 with an SPR wavelength of 540 nm. When EDC/NHS (AuNPs-EDC/NHS) was added, the absorbance decreased to 0.51, and the wavelength shifted to 543 nm. When AuNPs bound to antibodies (AuNPs-mAb), the absorbance decreased to 0.48, and the wavelength shifted to 544 nm. The slight change and shift of the absorbance of AuNPs after functionalization with EDC/NHS mean that the addition of the EDC/NHS crosslinker can maintain the optical properties of the AuNPs (Widyasari et al., 2022). The red shift of AuNPs’ wavelength after immobilization with the antibody of ~4 nm indicates a size difference in the nanoparticles. The optical properties of nanoparticles will change when the distance declines, producing a red color in the band of AuNPs plasmon. The AuNPs-mAb, when stored in PBS-Tween 20 at four °C, remained stable for four weeks until being used for the detection of the SC-2 antigen.
The images of TEM (inset in Figure 2a) and FESEM (Figure S3a) show the AuNPs-mAb morphology. The distance among particles decreased while the AuNPs' size remained 52-58 nm. The decrease in the distance between nanoparticles analyzed by PSA was translated as nanoparticle aggregation. The particle size of 52.2 nm was in line with the TEM and FESEM results. The EDX results (Figure S3b) confirmed the presence of a strong signal of Au corresponding to AuNPs (53.3 wt%). There were other compositions, such as carbon (30.3 wt%), oxygen (9.3 wt%), and nitrogen (7.1 wt%), belonging to the capping agent and antibody. From the absorbance, micrographs, and PSA results, the AuNPs-mAb was then used to detect SC-2 antigen.
3.3. Detection of SC-2 Antigen Based on SPR
Figure 2b shows the AuNPs-mAb spectra before and after spiking with 250 ng/mL SC-2 antigen (AuNPs-SC-2) analyzed using a well-plate reader in a spectrophotometer. AuNPs-mAb was added to the SC-2 antigen coated on the well plate, and a spectrophotometer was used to read the absorbance of the AuNPs. AuNPs-mAb measurements were carried out under optimum conditions. In the presence of the 250 ng/mL of SC-2 antigen, AuNPs-mAb were bound with the antigen, thereby providing a decreased peak absorbance of AuNPs and a difference in color visible to the bare eye. There was no shift in the SPR wavelength between AuNPs-mAb and AuNPs-SC-2. Only a decrease in absorbance occurred. The decreased peak intensity due to SC-2 antigen bound to AuNPs-mAb reduced the distance between particles and aggregation (Scheme 2- SI). These results are comparable to those found in previous studies (Karakus et al., 2021).
The characteristics of AuNPs-SC-2 were analyzed in TEM (inset on Figure 2b) and FESEM (Figure S4a). Those pictures showed that AuNPs formed aggregates after being added to SC-2 antigen. The AuNPs-mAb were arranged specifically and led to networking on the surface of SC-2 antigen, which made the distance between each AuNPs decrease and aggregation occur. So, the AuNPs-mAb caused the particle size to become more prominent. The PSA analysis showed that the size of AuNPs-SC-2 was 72,590 nm. The particle size increased after incubation with the antigen SC-2, indicating that the method could detect the SC-2 antigen. EDX (Figure S4b) confirmed the presence of strong signals of carbon (48.5 wt%) and oxygen (45.6 wt%). The percentage of carbon and oxygen increased with the addition of the SC-2 antigen. Au showed a decrease of 47.4-fold in percentage due to the abundance of SC-2 antigen on the surface of AuNPs-mAb.
3.4. Sensitivity, Limit of Detection, and Limit of Quantification
Figure 3 describes the correlation of AuNPs-SC-2 antigen concentration versus absorbance. The concentration of the SC-2 antigen used in this study was in the linear region of 75 to 250 ng/mL at a wavelength of 544 nm and evaluated three times. The linear relationship had a correlation coefficient of 0.9946. It shows good linearity (Wulandari et al., 2024). The method's sensitivity refers to the calibration curve's slope (Forootan et al., 2017). The limit of detection (LoD) is the smallest amount of SC-2 antigen that can be detected with probability, although perhaps not precisely quantified. At the same time, the limit of quantification (LoQ) is the smallest amount of SC-2 antigen that could be determined with acceptable precision (Forootan et al., 2017). The calculation showed that LoD and LoQ exhibited 16.03 ng/mL and 53.42 ng/mL, respectively.
Figure 3 Calibration plot of absorbance of AuNPs-SC-2 antigen (544 nm) versus concentration of SC-2 antigen (ng/mL)
3.5. Stability of AuNPs-SC-2 Measurement
The absorbance of the AuNPs-SC-2 antigen versus incubation time during detection is shown in Figure S5a. Measurements were done with a UV-Vis spectrophotometer, and the absorbance was observed at a wavelength of 544 nm. The absorbance from the various incubation time from 0 to 60 minutes remained constant at around 0.08. Then, the absorbance of AuNPs-SC-2 decreased by 0.07 compared to the control. It shows that AuNPs can directly detect SC-2 in less than 5 minutes (rapid). Several studies reported that the detection time with a similar method (colorimetry) for SC-2 took 10 minutes (Moitra et al., 2020). Even conventional methods, such as RT-PCR, took 45 minutes to 4 hours to detect (Pokhrel et al., 2020; Santiago, 2020). In this research, AuNPs-mAb has the advantage of short detection time, which can be started in less than 5 minutes after adding SC-2.
Figure S5b describes the AuNPs-mAb stability probe in measuring 250 ng/mL of the SC-2 antigen from day 1 to week 4. Results described nearly the exact signal change after being stored at 4 °C for four weeks, suggesting that the AuNPs-mAb probe showed good, relatively long-term stability. Table 1 SI compares the analytical performances of various SC-2 biosensors in the linear range and LoD. The electrochemical method shows better LoD than colorimetric detection; however, it lacks practicality as it requires an additional instrument. Yet, this colorimetry method shows better LoD than voltammetry. For initial detection, the colorimetric method produces LoD, which has good quality because it can detect samples on the ng/mL scale. Compared to the previous colorimetric method (Karakus et al., 2021), this method has a better LoD and a short incubation time (less than 5 minutes) with the same probe stability of 4 weeks.
3.6. Comparison with Conventional Method (ELISA)
Figure S6a shows the absorbance of the SC-2 antigen in this work (AuNPs) in comparison with the conventional method, enzyme-linked immunosorbent assay (ELISA). In this work, the SC-2 antigen was coated onto a well plate, and AuNPs-mAb were added with a volume ratio of 1:1, then analyzed with an ELISA reader to read the absorbance produced at 544 nm. In conventional methods such as ELISA, several preparation steps are carried out as described, and then absorbance analysis is carried out with an ELISA reader at 454 nm. The AuNPs method requires a shorter preparation time when compared to the ELISA method.
A comparison of the SC-2 antigen detection methods between AuNPs and the ELISA method was conducted within the concentration range of the linearity curve, spanning from 75-250 ng/ml (Figure 3). he data clearly demonstrates that AuNPs exhibited higher sensitivity than ELISA within the same concentration range. This can be observed from the higher absorbance values obtained using the AuNPs method compared to the ELISA method. In addition, a comparison of the incubation time between AuNPs and SC-2 was also carried out. Figure Sb shows that AuNPs and ELISA had a relatively stable incubation time of 5-60 minutes. This means that AuNPs are superior in sensitivity and equal time in detection compared to the ELISA method.
This research aims to determine the ability of AuNPs as a colorimetric sensor to detect SC-2. SC-2 antibody immobilization on the AuNPs surface was carried out with the EDC/NHS crosslinker. Characterization using UV-Vis showed that after modification with EDC/NHS and SC-2 antibodies, the optical properties of AuNPs were still maintained. O-hydroxybenzoic acid consists of a carboxyl group and thus can reduce one-step reaction in bioconjugation with SC-2 antibody. FESEM and TEM characterization results showed that surface-modified AuNPs (AuNPs-mAb) reduced the distance between particles, but the size of AuNPs-mAb was still in the range of 52-58 nm. AuNPs-mAb was used to detect SC-2 antigen and reduce the absorbance of AuNPs, and the color degradation occurred from purplish red to colorless. There was no shift in the SPR wavelength between AuNPs-mAb and AuNPs-SC-2, and only a decrease in absorbance occurred. LoD (16.03 ng/mL) and LoQ (53.42 ng/mL) were calculated in the SC-2 antigen concentration range of 75-250 ng/mL at a wavelength of 544 nm. This colorimetric sensor has the advantage of a short detection time, namely an incubation time of less than 5 minutes and stability of up to 4 weeks. With the advantages of short detection time, good stability, and sensitivity, this method promises to be an alternative for early screening of COVID-19. However, this research still needs to be improved for selectivity and determination of actual samples.
Thank you to the RP-Research Organization for Health BRIN (6/III.9/HK/2024) and the BRIN-ITB Collaborative Research Center with program number 01/PKR/PPK-DFRI/2022; 01/PKR/PPK-DFRI/2022.
Author Contributions
MW: Writing – original draft, Investigation, Data curation. NH: Investigation, Formal analysis. BY: Validation, Supervision. MA: Validation, Supervision, Methodology. RVM: Validation, Supervision, Formal analysis. SNAJ: Validation, Supervision. ASA: Writing – review & editing, Validation, Supervision, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization.
Conflict of Interest
The authors declare no conflicts of interest.
| Filename | Description |
|---|---|
| R3-CE-6476-20230713133015.docx | --- |
Adiwibowo, MT, Ibadurrohman, M & Slamet 2018, ‘Synthesis of ZnO nanoparticles and their nanofluid stability in the presence of a palm oil-based primary alkyl sulphate surfactant for detergent application’, International Journal of Technology, vol. 9, no. 2, pp. 307-316, https://doi.org/10.14716/ijtech.v9i2.1065
Andreani, AS, Kunarti, ES & Santosa, SJ 2019, ‘Synthesis of gold nanoparticles capped-benzoic acid derivative compounds (o-, m-, and p-hydroxybenzoic acid)’, Indonesian Journal of Chemistry, vol. 19, no. 2, pp. 376-385, https://doi.org/10.22146/ijc.34440
Ayankojo, AG, Boroznjak, R, Reut, J, Öpik, A & Syritski, V 2022, ‘Molecularly imprinted polymer based electrochemical sensor for quantitative detection of SARS-CoV-2 spike protein’, Sensors and Actuators B: Chemical, vol. 353, p. 131160, https://doi.org/10.1016/j.snb.2021.131160
Basso, CR, Tozato, CC, Crulhas, BP, Castro, GR, Junior, JPA & Pedrosa, VA 2018, ‘An easy way to detect dengue virus using nanoparticle-antibody conjugates’, Virology, vol. 513, pp. 85-90, https://doi.org/10.1016/j.virol.2017.10.002
Cesewski, E & Johnson, BN 2020, ‘Electrochemical biosensors for pathogen detection’, Biosensors and Bioelectronics, vol. 159, p. 112214, https://doi.org/10.1016/j.bios.2020.112214
Chang, CC, Chen, CP, Wu, TH, Yang, CH, Lin, CW & Chen, CY 2019, ‘Gold nanoparticle-based colorimetric strategies for chemical and biological sensing applications’, Nanomaterials, vol. 9, no. 6, p. 861, https://doi.org/10.3390/nano9060861
Cornuault, V & Knox, JP 2014, ‘Sandwich enzyme-linked immunosorbent assay (ELISA) analysis of plant cell wall glycan connections’, Bio-protocol, vol. 8, no. 4, pp. 1-5
Forootan, A, Sjöback, R, Björkman, J, Sjögreen, B, Linz, L & Kubista, M 2017, ‘Methods to determine limit of detection and limit of quantification in quantitative real-time PCR (qPCR)’, Biomolecular Detection and Quantification, vol. 12, pp. 1-6, https://doi.org/10.1016/j.bdq.2017.04.001
Funari, R, Chu, KY & Shen, AQ 2020, ‘Detection of antibodies against SARS-CoV-2 spike protein by gold nanospikes in an opto-microfluidic chip’, Biosensors and Bioelectronics, vol. 169, article 112578, https://doi.org/10.1016/j.bios.2020.112578
Guirgis, BSS, Sá E Cunha, C, Gomes, I, Cavadas, M, Silva, I, Doria, G, Blatch, GL, Baptista, PV, Pereira, E, Azzazy, HME, Prudêncio, M & Franco, R 2012, ‘Gold nanoparticle-based fluorescence immunoassay for malaria antigen detection’, Analytical and Bioanalytical Chemistry, vol. 402, no. 3, pp. 1019-1027, https://doi.org/10.1007/s00216-011-5541-0
Guy, RC 2014,‘International conference on harmonisation’, Encyclopedia of Toxicology: Third Edition, vol. 2, no. November 1994, pp. 1070-1072, https://doi.org/10.1016/B978-0-12-386454-3.00861-7
Jazayeri, MH, Aghaie, T, Avan, A, Vatankhah, A & Ghaffari, MRS 2018, ‘Colorimetric detection based on gold nanoparticles (GNPs): an easy, fast, inexpensive, low-cost and short time method in detection of analytes (protein, DNA, and ion)’, Sensing and Bio-Sensing Research, vol. 20, pp. 1-8, https://doi.org/10.1016/j.sbsr.2018.05.004
Jazayeri, MH, Amani, H, Pourfatollah, AA, Pazoki-Toroudi, H & Sedighimoghaddam, B 2016, ‘Various methods of gold nanoparticles (GNPs) conjugation to antibodies’, Sensing and Bio-Sensing Research, vol. 9, pp. 17-22, https://doi.org/10.1016/j.sbsr.2016.04.002
Kamra, T, Chaudhary, S, Xu, C, Montelius, L, Schnadt, J & Ye, L 2016, ‘Covalent immobilization of molecularly imprinted polymer nanoparticles on a gold surface using carbodiimide coupling for chemical sensing’, Journal of Colloid and Interface Science, vol. 461, pp. 1-8, https://doi.org/10.1016/j.jcis.2015.09.009
Karakus, E, Erdemir, E, Demirbilek, N & Liv, L 2021, ‘Colorimetric and electrochemical detection of SARS-CoV-2 spike antigen with a gold nanoparticle-based biosensor’, Analytica Chimica Acta, vol. 1182, pp. 338939, https://doi.org/10.1016/j.aca.2021.338939
Khalil, M, Liu, N & Lee, RL 2017, ‘Synthesis and characterization of hematite nanoparticles using ultrasonic sonochemistry method’, International Journal of Technology, vol. 8, no. 4, pp. 582-590, https://doi.org/10.14716/ijtech.v8i4.9474
Krajczewski, J, Kolataj, K & Kudelski, A 2017, ‘Plasmonic nanoparticles in chemical analysis’, RSC Advances, vol. 7, no. 28, pp. 17559-17576, https://doi.org/10.1039/C7RA01034F
Lee, C, Wang, P, Gaston, MA, Weiss, AA & Zhang, P 2017, ‘Plasmonics-based detection of virus using sialic acid functionalized gold nanoparticles’, Methods in Molecular Biology, vol. 1571, pp. 109-116, https://doi.org/10.1007/978-1-4939-6840-4_7
Lischer, K, Avila, F, Sahlan, M & Whulanza, Y 2021, ‘Assessment of cost-efficient thermocycler prototype for polymerase chain reaction and loop-mediated isothermal amplification’, International Journal of Technology, vol. 12, no. 6, pp. 1207-1216, https://doi.org/10.14716/ijtech.v12i6.5207
Moitra, P, Alafeef, M, Dighe, K, Frieman, MB & Pan, D 2020, ‘Selective naked-eye detection of SARS-CoV-2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles’, ACS Nano, vol. 14, no. 6, pp. 7617-7627, https://doi.org/10.1021/acsnano.0c03822
Oliveira, JP, Prado, AR, Keijok, WJ, Antunes, PWP, Yapuchura, ER & Guimarães, MCC 2019, ‘Impact of conjugation strategies for targeting of antibodies in gold nanoparticles for ultrasensitive detection of -estradiol’, Scientific Reports, vol. 9, no. 1, pp. 1-8, https://doi.org/10.1038/s41598-019-50424-5
Pokhrel, P, Hu, C & Mao, H 2020, ‘Detecting the coronavirus (COVID-19)’, ACS Sensors, vol. 5, no. 8, pp. 2283-2296, https://doi.org/10.1021/acssensors.0c01153
Pramanik, A, Gao, Y, Patibandla, S, Mitra, D, McCandless, MG, Fassero, LA, Gates, K, Tandon, R & Chandra Ray, P 2021, ‘The rapid diagnosis and effective inhibition of coronavirus using spike antibody attached gold nanoparticles’, Nanoscale Advances, vol. 3, no. 6, pp. 1588-1596, https://doi.org/10.1038/s41598-019-50424-5
Raziq, A, Kidakova, A, Boroznjak, R, Reut, J, Öpik, A & Syritski, V 2021, ‘Development of a portable MIP-based electrochemical sensor for detection of SARS-CoV-2 antigen’, Biosensors and Bioelectronics, vol. 178, p. 113029, https://doi.org/10.1016/j.bios.2021.113029
Rump, A, Risti, R, Kristal, ML, Reut, J, Syritski, V, Lookene, A & Boudinot, SR 2021, ‘Dual ELISA using SARS-CoV-2 nucleocapsid protein produced in E. coli and CHO cells reveals epitope masking by N-glycosylation’, Biochemical and Biophysical Research Communications, vol. 534, pp. 457-460, https://doi.org/10.1016/j.bbrc.2020.11.060
Sahlan, M, Dewi, LK, Pratami, DK, Lischer, K & Hermansyah, H 2023, ‘In silico identification of propolis compounds potential as COVID-19 drug candidates against SARS-CoV-2 spike protein’, International Journal of Technology, vol. 14, no. 2, pp. 387-398, https://doi.org/10.14716/ijtech.v14i2.5052
Santiago, I 2020, ‘Trends and innovations in biosensors for COVID-19 mass testing’, ChemBioChem, vol. 21, no. 22, pp. 2880-2889, https://doi.org/10.1002/cbic.202000250
Srivastava, M, Srivastava, N, Mishra, PK & Malhotra, BD 2021, ‘Prospects of nanomaterials-enabled biosensors for COVID-19 detection’, Science of the Total Environment, vol. 754, pp. 142363, https://doi.org/10.1016/j.scitotenv.2020.142363
Tang, Y-W, Schmitz, JE, Persing, DH & Stratton, CW 2020, ‘Laboratory Diagnosis of COVID-19: Current Issues and Challenges’, Journal of Clinical Microbiology, vol. 58, no. 6, pp. 1–9, https://doi.org/10.1128/jcm.00512-20
Ting, C, Zakariah, H, Yusri, YZM, 2022 ‘Probabilistic risk assessment of COVID-19 patients at COVID-19 assessment centre, International Journal of Technology, vol. 13, no. 6, pp. 1193-1201, https://doi.org/10.14716/ijtech.v13i6.5882
Ulyah, SM, Susanti, R, Andreas, C, Rahmayanti, IA, Rifada, M, Fitriyani, NL, Ana, E, 2024 ‘A multivariate regression with time series error in forecasting jakarta composite index and stock prices of banking industry in indonesia by considering COVID-19 effect’, International Journal of Technology, vol. 15, no. 6, pp. 1839-1850, https://doi.org/10.14716/ijtech.v15i6.5469
Ventura, BD, Cennamo, M, Minopoli, A, Campanile, R, Censi, SB, Terracciano, D, Portella, G & Velotta, R 2020, ‘Colorimetric test for fast detection of SARS-CoV-2 in nasal and throat swabs’, ACS Sensors, vol. 5, no. 10, pp. 3043-3048, https://doi.org/10.1021/acssensors.0c01742
Widyasari, DA, Kristiani, A, Randy, A, Manurung, RV, Dewi, RT, Andreani, AS, Yuliarto, B & Jenie, SNA 2022, ‘Optimized antibody immobilization on natural silica-based nanostructures for the selective detection of E. coli’, RSC Advances, vol. 12, no. 33, pp. 21582-21590, https://doi.org/10.1039/D2RA03143D
Wulandari, M, Zahratussaadah, Z & Andreas, A 2024, ‘Separation of tannins and caffeine in black tea using modified microwave-assisted extraction and high-performance liquid chromatography’, International Journal of Technology, vol. 15, no. 6, pp. 2024–2037, https://doi.org/10.14716/ijtech.v15i6.7116
Yadavalli, T & Shukla, D 2017, ‘Role of metal and metal oxide nanoparticles as diagnostic and therapeutic tools for highly prevalent viral infections’, Nanomedicine: Nanotechnology, Biology, and Medicine, vol. 13, no. 1, pp. 219-230, https://doi.org/10.1016/j.nano.2016.08.016
Yang, Y, Xiao, Z, Ye, K, He, X, Sun, B, Qin, Z, Yu, J, Yao, J, Wu, Q, Bao, Z & Zhao, W 2020, ‘SARS-CoV-2: Characteristics and current advances in research’, Virology Journal, vol. 17, no. 1, pp. 1-17, https://doi.org/10.1186/s12985-020-01369-z
Yi, SY, Lee, U, Chung, BH & Jung, J 2015, ‘A scanometric antibody probe for facile and sensitive immunoassays’, Chemical Communications, vol. 51, no. 42, pp. 8865-8867, https://doi.org/10.1039/C5CC02838H
Zhao, P, Li, N & Astruc, D 2013, ‘State of the art in gold nanoparticle synthesis’, Coordination Chemistry Reviews, vol. 257, no. 3-4, pp. 638-665, https://doi.org/10.1016/j.ccr.2012.09.002
Zhu, N, Zhang, D, Wang, W, Li, X, Yang, B, Song, J, Zhao, X, Huang, B, Shi, W, Lu, R, Niu, P, Zhan, F, Ma, X, Wang, D, Xu, W, Wu, G, Gao, GF & Tan, W 2020, ‘A novel coronavirus from patients with pneumonia in China, 2019’, New England Journal of Medicine, vol. 382, no. 8, pp. 727-733, https://doi.org/10.1056/NEJMoa2001017
