Catalytic Synthesis of Diethyl Carbonate from Carbon Dioxide using Catalyst KI/EtONa with Propylene Oxide as Dehydration Agent and Process Optimization Based on Box-Behnken Design
Corresponding email: gwibawa@chem-eng.its.ac.id
Published at : 31 Jan 2025
Volume : IJtech
Vol 16, No 1 (2025)
DOI : https://doi.org/10.14716/ijtech.v16i1.6417
Anggerta, LA, Kurniawansyah, F, Tetrisyanda, R & Wibawa, G 2025, 'Catalytic synthesis of diethyl carbonate from carbon dioxide using catalyst KI/EtONa with propylene oxide as dehydration agent and process optimization based on box-behnken design', International Journal of Technology, vol. 16, no. 1, pp. 243-254
| Lintang Alivia Anggerta | Department of Chemical Engineering, Faculty of Industrial Technology and System Engineering, Institut Teknologi Sepuluh Nopember, Surabaya, 60111, Indonesia |
| Firman Kurniawansyah | Department of Chemical Engineering, Faculty of Industrial Technology and System Engineering, Institut Teknologi Sepuluh Nopember, Surabaya, 60111, Indonesia |
| Rizky Tetrisyanda | Department of Chemical Engineering, Faculty of Industrial Technology and System Engineering, Institut Teknologi Sepuluh Nopember, Surabaya, 60111, Indonesia |
| Gede Wibawa | Department of Chemical Engineering, Faculty of Industrial Technology and System Engineering, Institut Teknologi Sepuluh Nopember, Surabaya, 60111, Indonesia |
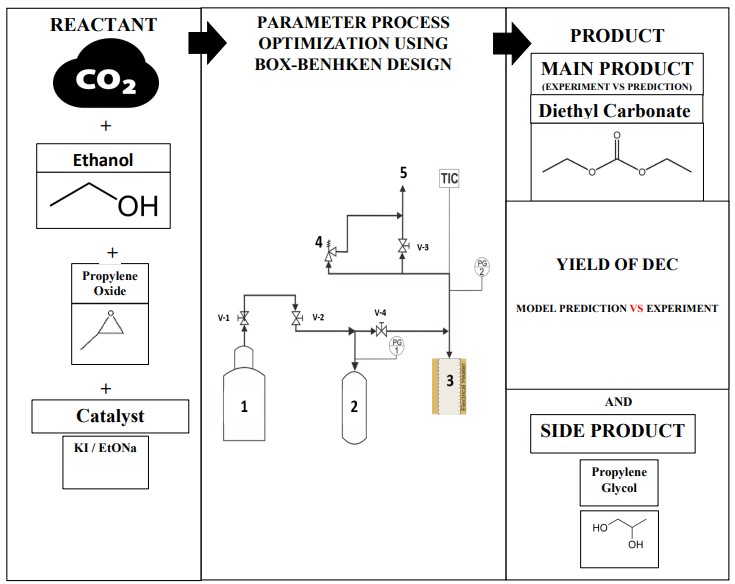
Diethyl carbonate (DEC) was synthesized through catalytic conversion from carbon dioxide (CO2) and ethanol. However, common challenges in synthesizing DEC from CO2 have been high energy consumption, catalysts-dehydrating agent selection, and relatively complex reaction. In this study, propylene oxide (PO) was used as a dehydrating agent, and KI/Sodium ethoxide was employed as a catalyst, resulting in the highest yield of DEC. The synthesis was conducted in a stainless steel reactor under batch conditions, with an initial CO2 pressure ranging from 20 to 40 bar, a reaction temperature between 130-190°C, and a reaction time of 1-5 hours. Product identification was conducted with gas chromatography analysis with FID detector. Besides kinetic study, optimizing the parameter process in DEC synthesis is necessary to find the highest yield of DEC because it is difficult to achieve optimum conditions using trial and error. So, this parameter process synthesis was also optimized with the Box-Bhenken Design (BBD) method to get optimal conditions and an equation to predict the yield of DEC. As confirmed with the BBD method, an initial pressure of CO2 40 bar, 190oC, and a 3-hour reaction were expected to perform optimized processing. By applying these optimized process parameters in experimental work, a DEC yield of up to 24.07% was obtained. This experimental result was relatively consistent with the findings of the simulation study, which achieved a yield of 24.3%.
Carbon dioxide; Catalytic;; Diethyl carbonate; Optimization; Synthesis
As a greenhouse gas, CO2 is a significant contributor to global warming. The CO2 release mainly originated from anthropogenic sources (Creamer et al., 2016). About 10 billion tons of CO2 are released into the atmosphere every year (Qi et al., 2013). CO2 emission causes climate change, followed by effects such as global warming, sea-level rise, hurricane, aggravation, and species extinction (Zhao et al., 2016).
Several technologies have been developed to capture or utilize CO2 to reduce greenhouse gas emissions. CO2 has been converted into other valuable chemicals by cataly- tic or non-catalytic reactions (Wang et al., 2016; Zhao et al., 2016; Leino et al., 2011; Bistline and Rai, 2010). CO2 has been used to produce chemical products such as urea and ammonia for the non-catalytic reaction (Zhao et al., 2016). Additionally, some innovation have been attempted to convert CO2 through a catalytic processing strategy (Leino et al., 2017; Wang et al., 2016; Zhao et al., 2016; Leino et al., 2011).
Diethyl Carbonate (DEC) is a valuable organic chemical that can be synthesized from CO2 through catalytic processes. DEC has gained recognition as a promising gasoline additive due to its advantageous properties, including a high octane number, low vapor pressure, and improved miscibility in water compared to ethanol. When used as a co-additive with ethanol, DEC enables an increase in ethanol content in gasoline by more than 15% by volume. Besides, using DEC as an additive in a gasoline mixture has been found to reduce the vapor pressure of the mixture (Anugraha et al., 2018).
According to several studies, DEC may be synthesized from various raw materials, for example, from the reaction of urea and ethanol (Shukla and Srivastava, 2017; Xin et al., 2014; Wang et al., 2007), carbon monoxide (CO) and ethanol (Zhang et al., 2015b; Zhu et al., 2011; Briggs et al., 2010; Zhang and Ma, 2010; Zhang et al. 2008), DMC (Murugan and Bajaj, 2011), ethyl carbamate, and ethanol (Zhao et al., 2014; An et al., 2012; Lian et al., 2012), ethylene carbonate and ethanol (Iida, Kawaguchi and Okumura, 2018), from CO2 with ethanol (Leino et al., 2017; Wang et al. 2016; Zhang et al., 2014; Leino et al., 2013, 2011; Gasc et al., 2009).
CO2 is one of the raw materials used for synthesizing DEC. CO2 has some characteristics that have many advantages over the other material used for DEC synthesis above, such as being renewable, not flammable, non-toxic, and widely available in the environment (Zhang et al., 2011).
Synthesis of DEC from CO2 as raw material can be affected by several factors, such as dehydrating agents, catalysts, and operating conditions used as reported by previous researchers (Leino et al., 2017; Wang et al. 2017; Zhang et al., 2014; Leino et al., 2013, 2011; Gasc et al., 2009). The reaction from only CO2 and ethanol to produce DEC is difficult to occur spontaneously, even in harsh conditions. The production of water as a by-product affects the equilibrium of the reaction process and imposes thermodynamic limitations. Additionally, the catalyst plays a crucial role in the formation of DEC. Leino et al. noted that the amount of catalyst affects the cycloaddition reaction, which can lead to the formation of DEC (Leino et al., 2017). So, the dehydration agent will shift the reaction to the carbonated side (Wang et al., 2016).
Ethylene oxide (EO) and propylene oxide (PO) have been reported as dehydration agents used in the synthesis of DMC from CO2. The synthesis route of DMC was similar to DEC synthesis. The difference is the use of alcohol in their synthesis. DEC synthesis uses ethanol, while DMC synthesis uses methanol as a reactant (Yan et al., 2011; Cui et al., 2003). At the same time, other researchers have also reported the effect of a liquid dehydration agent such as butylene oxide (BO) or propylene oxide (PO) used with other catalyst additions. Leino et al. (2011) reported that DEC could be synthesized from CO2 using BO as a dehydration agent and CeO2 as a catalyst. The experiment achieved about 15.6% yield of DEC (Leino et al., 2011). Wang et al. reported a DEC synthesis through a one-pot reaction using PO as a dehydrating agent with KBr-KNO3-CeO2 as a catalyst. They reported that a 13% yield of DEC was achieved at a temperature of 150oC and initial pressures of 50 bar for 3 hours of reaction time (Wang et al., 2016). DEC has also been synthesized through a coupling reaction in the study of Wang et al. PO was used as dehydrating agent, while PVEImBr/MgO was used as a catalyst in their experiment. The reaction was carried out at 170oC and initial pressures of 30 bar for 4.5 hours reaction time, yielding DEC of approximately 38 % (Wang et al., 2017). This report indicates that the yield of DEC and DMC with PO was higher than BO because the PO chain is shorter than BO. Not only it, but Wang et al. also said that in DEC synthesis, PO would react with CO2 to produce Propylene Carbonate (PC), and the PC will react with ethanol to produce DEC as the main product and Propylene Glycol (PG) as a side product. So, PO is chosen in this experiment to produce the optimum yield of DEC.
On the other hand, the catalyst used was also affected in DEC synthesis. Some experiment reports that using a single catalyst only produces a lower yield of DEC, such as Wang et al. report that using only a CeO2 catalyst in DEC synthesis with PO as a dehydration agent only produces a 1.3% yield of DEC. In contrast, the other combined catalyst in their experiment produced a higher DEC yield (Wang et al., 2016). So it is recommended to use a combination of catalysts in DEC synthesis. Catalysts from potassium halides such as KI, KBr, and KCl were also reported by Wang et al. in their DEC synthesis experiment The addition of KI combined with CeO2 and KNO3 has shown higher performance in terms of ethanol conversion during DEC synthesis compared to KBr and KCl. However, the result of DEC yield in their experiment shows a non-significance value to using only CeO2 catalyst in their works (Wang et al., 2016). Moreover, previous studies proved that basic catalysts were also effective for DEC synthesis (Branco, Brito and Ferreira, 2020; Zhang et al., 2015a; Leino et al., 2011). So, In this study, the combination of catalysts from potassium halides (KI) and a basic catalyst Sodium Ethoxide (EtONa) was also tried in this DEC synthesis to get a higher yield of DEC.
Thus, common challenges in synthesizing DEC from CO2 have been high energy consumption, catalysts-dehydrating agent selection, and relatively complex reaction ( Wang et al., 2017, 2016; Xin et al., 2014). So, PO with KI/EtONa as a catalyst was chosen for this synthesis.
Not only dehydration agent and catalyst used, but operating conditions or process parameters are also essential to consider in achieving successful conversion. Since the synthesis process is complex, there will be many experiments to find optimal synthesis conditions. Using trial and error to find the optimal condition is also rigid and requires more time for an investigation. So the design of the experiment using the Response Surface Methodology (RSM) analysis can fit the experimental domain studied in the theoretical design through a response function. The RSM analysis method can evaluate and optimize an experiment's parameter process using combined arithmetical and statistical methods (Esraa et al., 2022; Petrus et al., 2021; Hawashi et al., 2019). At the same time, Box-Behnken Design (BBD) is modified of Central Composite Design (CCD) and is widely used in the RSM analysis method. This design can shorten the time and cost of an experiment to get the optimal condition (Lubis et al., 2019). Many researchers have also reported that using BBD will reduce the number of experiments (Wang et al., 2021; Variyana et al., 2019; Belhaj et al., 2017; Bayraktar, 2001). Not only can it be optimized, but it also can make an equation to predict the best product of the synthesis.
So, in this work, optimization of process parameters in the catalytic synthesis of DEC from CO2, ethanol, and PO was attempted. The optimization was based on the Box – Behnken method, using response surface methodology (RSM), while catalyst KI/EtONa was used throughout the study.
2.1. Materials
Materials used in this experiment were CO2 (99.8% purity) purchased from PT. Aneka Gas Industri – Samator - Indonesia, ethanol (99.5% purity), propylene oxide (99.5%), and butylene oxide (99%) were purchased from Sigma-Aldrich – Singapore, while KI and sodium ethoxide (EtONa) was purchased from Merck - Germany.
2.2. Methods
Experimental Apparatus and Procedure for DEC Synthesis
The schematic diagram of the experimental apparatus is presented in Figure 1. The synthesis of the DEC reactor was made of 340 cm3 cylindrical stainless steel 316 equipped with a stirrer, heating jacket, electric heater, and a temperature controller to keep the temperature reaction constant.
The reactor was operated in batch operation mode where initially, 0.2 g of total catalyst KI and EtONa and also the mixture of PO and ethanol was prepared to obtain the mole ratio of PO and ethanol of 1:10. The catalyst KI and EtONa were prepared with a mass ratio of 1:3.
Figure 1 System for DEC synthesis.
The mixture was loaded into the reactor, and all valves were closed. Impurities and air were evacuated from the reactor by opening V1 to allow CO2 to flow to the buffer, reactor, and line package for at least 5 minutes. Then, the buffer tank was loaded with CO2 by opening valve V-1 until the pressure reached 60 bar. Then, the CO2 started to be introduced into the reactor by opening valve V-2, and the valve would be closed when the pressure in the reactor reached the desired initial pressure of CO2. The stirrer was then turned on and stirred at 500 rpm speed while the reactor was heated until the desired temperature was achieved. The initial synthesis reaction was varied at the initial pressure of CO2, reaction temperature, and time reaction as shown in Table 1. After the reaction time, the heater and stirrer were turned off, the reactor was cooled to room temperature, the remaining gas was released, and the reactor was slowly depressurized by opening valve V-3 until it reached atmospheric pressure. Then, the reaction products were identified qualitatively using GC-MS (Agilent 6980-433 HP-5MS), and the product compositions were analyzed using gas chromatography (Shimadzu) with a capillary column (Stabilwax 30m x 0.25mm x 0.25µm) equipped with a flame ionization detector (FID).
Box-Behnken Design
The relationship between the parameter process and experimental output (yield of DEC synthesis) could be assessed with empirical modeling from response surface methodology. This study used the RSM of BBD to understand the combination of process parameters that influence the yield of DEC.
The independent variable responses and code levels derived after preliminary runs are shown in Table 1. To obtain a high yield of DEC synthesis, three factors from BBD were applied to optimize the process parameter conditions in this work.
Table 1 presents the independent variables and their corresponding levels designed by the BBD for DEC synthesis.
A total of 17 experiments were designed in Table 3, where each synthesis experiment was evaluated based on the yield (Yield, %):
Y=0+iXi+iiXi2+ijXiXj
where0, i, ii and ijare regression coefficients in the intercept, linear, quadratic, and interaction packages. The BBD was performed using (Design Expert 13th trial version; State-Ease Inc., Minneapolis, USA). This software was used to get the coefficients of the polynomial quadratic model.
Catalytic Synthesis of DEC
This study used a catalyst to conduct DEC synthesis via cycloaddition and transesterification. As reported by Wang et al., the amount of catalyst affects the cycloaddition reaction, which can lead to the formation of DEC (Wang et al., 2014). The effect of the catalyst in this work has been similar to Wang et al. experiment, which employs a different catalyst. Wang et al. also used propylene oxide as a dehydration agent and potassium halides as the catalyst. However, the difference in the catalyst used between Wang et al. works and this experiment is also shown in Table 2.
Table 2 Effects of Different catalyst between Wang et al. (Wang et al., 2016) and this works.
(a)Wang et al works (Parameter Process: T=150oC; Initial Pressure= 50 bar; time= 2 hours)(Wang et al., 2016)
(b)This works(Highest Yield of DEC)(Parameter Process: T= 190oC; Initial Pressure = 40 bar; time= 3 hours)
Table 2 shows that combined catalysts have a DEC yield higher than individual catalysts in using KCl, KNO3, and KBr. The yield of DEC in that experiment increases 10-fold from using only the CeO2 catalyst. In contrast, using KI as a combination catalyst in their works has a non-significant result other than using CeO2 as an individual catalyst. On the other hand, KI and CeO2 showed better performance as a catalyst than KBr and KCl in diesel soot oxidation. Zhang et al. work also proved that both potassium halides and basic catalysts behaved a poor catalytic activity when used alone (Zhang et al., 2006). So, in this study, KI was combined with EtONa as a catalyst in DEC synthesis.
The result of DEC synthesis using KI/EtONa as a catalyst in this synthesis is higher than KI/CeO2 or the other combined catalyst used by Wang et al. (Wang et al., 2016). The highest yield of DEC in this work was achieved at 24.07%. It indicates that KI combined with EtONa performs better than KI combined with CeO2 or the other combination of potassium halides and CeO2 in DEC synthesis. The use of basic catalysts in the synthesis causes this performance. CeO2 was a moderate basic catalyst, while EtONa was a strong base catalyst (Wang et al., 2016). So, the combined catalyst KI/EtONa will have higher catalytic activity in DEC synthesis.
3.2. Process Optimization Based on BBD
The effect of individual and combined parameter processes in DEC synthesis, such as initial pressure of CO2, temperature, and Time Reaction, was statistically assessed using the BBD optimization method. The number of experiments and the responses of BBD, both analyses predicted and experiment, were also shown in Table 3.
3.2.1. Model Summary Statistic for Fitting a Quadratic Model
Design-Expert was used to fit a series of models, including linear, 2FI (two-factor interaction), quadratic, and cubic response surface models, to the data (Variyana et al., 2019). The quadratic model has a standard deviation of less than 0.05 in the model summary, as shown in Table 4. In addition, adjusted R2 and predicted R2 were close to 1. Therefore, the summary statistic recommended in Design-Expert suggests the quadratic model for optimization.
Table 3 Box-Behnken design matrix and response for the yield of DEC synthesis.
Catalyst ratio: KI: EtONa = 1:3
Dehydration agent ratio: PO: Ethanol = 1:10
Stirred in 500 rpm
Table 4. Model summary of statistics for the yield of DEC synthesis.
3.2.2. Analysis of Synthesis Variants and Parameters
The ANOVA results are in Table 5. show the parameter response to the yield of DEC synthesis significantly. The significance of the models is determined by the p-value and F-value of ANOVA, which are less than 0.0001 and 185.21. Results of a p-value less than 0.0500 indicate that model terms are significant. The Model F-value of 185.21 implies that the model is significant. There is only a 0.01% chance that an F-value this large could occur due to noise. Bayraktar also reported that the significance value of the model, which has a high F-test value with a small p-value, will significantly affect the test results (Bayraktar, 2001). A, B, C, AC, BC, B², and C² are significant model terms in this case.
Furthermore, the coefficient of determination (R2) is 0,9958, close to 1.00. It means the degree of relationship between the experimental data and the optimization model is very strong (Belhaj et al., 2017). As same as the previous study of some researchers who used BBD method analysis in their experiment, the other terms in the BBD model with p-values greater than 0.05 were not neglected in a quadratic polynomial expression to obtain the output of appropriate fitness (Saud et al., 2021; Jawad et al., 2021, 2020a, 2020b). So from the optimization results obtained, a quadratic equation is as follows:
Yield of DEC Synthesis = 2.01+ 0.1487 A + 1.31 B + 0.5340 C - 0.0655 AB + 0.2213 AC + 0.2796 BC + 0.0757 A² - 0.2824 B² - 1.61 C²
Table 5 Analysis of ANOVA variants against response results.
Comparison of residual distribution and correlation between experimental and model predicted yield of DEC were also used to confirm the BBD model using a graphical method, as shown in Figure 2.
Figure 2 Comparison between model predictions and DEC synthesis experiment data
Figure 2 states the relationship between experimental data and optimization models that intersect through linear regression. This figure proves mathematically that the results of optimization models and experiments have an R2 close to 100%, so the line tends to be straight. The straight line pattern means that the result confirmed the excellent agreement of data between experimental and model-predicted values (Arifan et al., 2022; Saud et al., 2021). So, the trend of the string confirms that the optimization model can reasonably predict the yield of DEC synthesis in experiments.
3.2.1. Effect of Process Parameter Interactions on DEC Synthesis
The effect of parameter interaction between the initial pressure of CO2 and temperature reaction can be seen in Figure 3 (a). The contour shows a response when the initial pressure of CO2 is at the minor variable with low-temperature variation resulting in the smallest yield. As the temperature increases, the yield of DEC synthesis gets bigger. This result indicates that the increase in temperature reaction has a linear influence on the yield of DEC synthesis. While Figure 3 (b). describes the interaction between the initial pressure of CO2 and the time reaction. It can be seen that the brightest and most intense dome colors are at a reaction time of 3 hours, so it can be concluded that the highest yield is obtained when the reaction time reaches 3 hours. Furthermore, Figure 3 (c) illustrates the interaction between reaction temperature and reaction time. It shows that as the temperature increases within the reaction range of 3 hours, the highest yield of DEC is obtained.
(a) (b)
(c)
Figure 3. Interaction between process parameters; (a): A-Initial Pressure and B-Temperature, (b): A-Initial Pressure and C-Time Reaction, (c): B-Temperature and C-Time Reaction
3.2.2. Validation for Parameter Process Optimization of DEC Synthesis
In the optimized study of the parameter process DEC synthesis with BBD response surface methodology, the optimum process parameters to produce the highest DEC yield were obtained with an initial pressure of CO2 of about 39.56 bar, temperature reaction of 189.93oC, and reaction time of 2.99 hours achieving 24.34% yield of DEC and value of desirability about one which means have reached ideal conditions. Raissi and Farsani reported in 2009 that the yield response by experimental and model parameters could reach ideal conditions when the desirability value is 1 (Raissi and Farsani, 2009).
Meanwhile, the highest DEC synthesis yield in the experiment was obtained with the parameters of the initial pressure of the CO2 process of about 40 bar, temperature reaction of 190oC, and reaction time of 3 hours, achieving a 24.07% yield of DEC.
Based on these results, it is evident that the error rates between the two models have a value of <0.05%. Therefore, it can be concluded that the quadratic model design is suitable for determining the influence of parameters and identifying the optimal conditions for the synthesis of DEC.
Based on existing challenges in synthesizing DEC from CO2 such as high energy consumption, catalysts-dehydrating agent selection, and relatively complex reaction wich can affects the high number of experiment, so the BBD analysis will help the experiments of DEC synthesis to find optimal synthesis conditions. Finally, Propylene Oxide as a dehydration agent and KI/EtONa as catalyst as conducted in this study produced the highest yield of DEC, about 24.07% with an initial pressure of CO2 of 40 bar, an operating temperature of 190oC, and 3 hours of reaction time are appropriate reaction parameters that can be applied in the synthesis. This result was also in good agreement with the Box-Behnken Design analysis, which obtained 24.34% highest yield of DEC with an initial pressure of CO2 of 39.56 bar, temperature reaction of 189.93oC, and reaction time of 2.99 hours. This comparison between experimental and predicted yield responses has an error percentage of <0.05%. So, this DEC synthesis route from CO2, ethanol and Propylene oxide as reactant with KI/EtONa as catalyst with the parameter process optimized by BBD can also be used as a reference for process parameter design of DEC synthesis if used on a larger scale.
Financial support for this work was provided by “PENELITIAN DISERTASI DOKTOR” scheme under Indonesian Ministry of Research,Technology, and Higher Education SK No. 112/E5/PG.02.00.PL/2023 dated June 19, 2023 and Derivative Agreement / Contract No. 1900/PKS/ITS/2023 dated June 20, 2023 for "Studi Utilisasi Karbon Dioksida Menjadi Dialkyl Carbonate: Sintesis Katalitik dan Kesetimbangan Uap Cair" research.
Author Contributions
Lintang Alivia Anggerta is responsible for providing research ideas, making experimental designs, methodology, theoretical approach, data treatment, and manuscript writing Firman Kurniawansyah is responsible for the catalytic reaction and correcting the draft manuscript. Rizky Tetrisyanda is responsible for designing experimental equipment and conducting data analyses. Gede Wibawa provides research ideas, experimental equipment design, methods, and manuscripts.
Conflict of Interest
The authors declare no conflicts of Interest
An, H, Zhao, X, Guo, L, Jia, C, Yuan, B & Wang, Y 2012, 'Synthesis of diethyl carbonate from ethyl carbamate and ethanol over ZnO-PbO catalyst', Applied Catalysis A: General, vol. 433, pp. 229-235, https://doi.org/10.1016/j.apcata.2012.05.023
Anugraha, RP, Wiguno, A, Altway, A & Wibawa, G 2018, 'Vapor pressures of diethyl carbonate + ethanol binary mixture and diethyl carbonate + ethanol + isooctane / toluene ternary mixtures at temperatures range of 303 , 15 – 323 , 15 K', Journal of Molecular Liquids, vol. 264, pp. 32-37, https://doi.org/10.1016/j.molliq.2018.05.049
Arifan, F, Teguh, R, Wisnu, D & Sumardiono, S 2022, 'Effect of thermal pretreatment of pineapple peel waste in biogas production using response surface methodology', International Journal of Technology, vol. 13, no. 3, pp. 619-632, https://doi.org/10.14716/ijtech.v13i3.4747
Bayraktar, E 2001, Response surface optimization of the separation of DL- typtophan using an emulsion liquid membrane Response surface optimization of the separation of using an emulsion liquid membrane, Process Biochemistry, vol. 37, pp. 169-175, https://doi.org/10.1016/S0032-9592(01)00192-3
Belhaj, D, Frikha, D, Athmouni, K, Jerbi, B, Ahmed, MB, Bouallagui, Z, Kallel, M, Maalej, S, Zhou, J & Ayadi, H 2017, 'Box-Behnken design for extraction optimization of crude polysaccharides from Tunisian Phormidium versicolor cyanobacteria (NCC 466): partial characterization, in vitro antioxidant and antimicrobial activities', International Journal of Biological Macromolecules, vol. 105, no. 2, pp.1501-1510, https://doi.org/10.1016/j.ijbiomac.2017.06.046
Bistline, JE & Rai, V 2010, 'The role of carbon capture technologies in greenhouse gas emissions-reduction models?: A parametric study for the U , S , power sector', Energy Policy, vol. 38, no. 2, pp.1177-1191, https://doi.org/10.1016/j.enpol.2009.11.008
Branco, JB, Brito, PE & Ferreira, AC, 2020, 'Methanation of CO2 over nickel-lanthanide bimetallic oxides supported on silica', Chemical Engineering Journal, vol. 380, p. 122465, https://doi.org/10.1016/j.cej.2019.122465
Briggs, DN, Bong, G, Leong, E, Oei, K, Lestari, G & Bell, AT 2010, 'Effects of support composition and pretreatment on the activity and selectivity of carbon-supported PdCu n Cl x catalysts for the synthesis of diethyl carbonate', Journal of Catalysis, Volume 276(2), pp. 215-228, https://doi.org/10.1016/j.jcat.2010.08.004
Creamer, AE, Gao, B & Wang, S 2016, 'Carbon dioxide capture using various metal oxyhydroxide – biochar composites', Chemical Engineering Journal, vol. 283, pp. 826-832, https://doi.org/10.1016/j.cej.2015.08.037
Cui, H, Wang, T, Wang, F, Gu, C, Wang, P & Dai, Y 2003, One-pot synthesis of dimethyl carbonate using ethylene oxide, methanol, and carbon dioxide under supercritical conditions, Industrial and Engineering Chemistry Research, vol. 42, no. 17, pp. 3865-3870, https://doi.org/10.1021/ie021014b
Esraa, A, Putra, A, Mosa, AI, Dan, RM & Attia, OH 2022, 'An empirical model for optimizing the sound absorption of single layer MPP based on response surface methodology', International Journal of Technology, vol. 13, no. 3, pp. 496-507, https://doi.org/10.14716/ijtech.v13i3.5507
Gasc, F, Thiebaud-roux, S & Mouloungui, Z 2009, 'Methods for synthesizing diethyl carbonate from ethanol and supercritical carbon dioxide by one-pot or two-step reactions in the presence of potassium carbonate', The Journal of Supercritical Fluids, vol. 50, no. 1, pp. 46-53, https://doi.org/10.1016/j.supflu.2009.03.008
Hawashi, M, Aparamarta, H, Widjaja, T &Gunawan, S 2019, 'Optimization of solid state fermentation conditions for, International Journal of Technology, vol. 10, no. 3, pp. 624-633, https://doi.org/10.14716/ijtech.v10i3.2923
Iida, H, Kawaguchi, R & Okumura, K 2018, 'Production of diethyl carbonate from ethylene carbonate and ethanol over supported fluoro-perovskite catalysts, Catalysis Communications, vol. 108, pp. 7-11, https://doi.org/10.1016/j.catcom.2018.01.019
Jawad, AH, Abdulhameed, AS, Wilson, LD, Syed-hassan, SSA, Alothman, ZA & Khan, MR 2021, 'High surface area and mesoporous activated carbon from KOH-activated dragon fruit peels for methylene blue dye adsorption?: Optimization and mechanism study', Chinese Journal of Chemical Engineering, vol. 32, pp. 281-290, https://doi.org/10.1016/j.cjche.2020.09.070
Jawad, AH, Awad, I & Abdulhameed, AS 2020a, 'Tuning of fly ash loading into chitosan ? ethylene glycol diglycidyl ether composite for enhanced removal of reactive red 120 dye?: optimization using the box – behnken design', Journal of Polymers and the Environment, vol. 28, pp. 2720-2733
Jawad, AH, Bardhan, M, Islam, A, Islam, A, Syed-hassan, SSA & Surip, SN 2020b, 'Insights into the modeling, characterization and adsorption performance of mesoporous activated carbon from corn cob residue via microwave- assisted H3PO4 activation', Surfaces and Interfaces, vol. 21, p. 100688, https://doi.org/10.1016/j.surfin.2020.100688
Leino, E, Kumar, N, Mäki-Arvela, P, Rautio, A-R, Dahl, J, Roine, J & Mikkola, J-P 2017, 'Synthesis and characterization of ceria-supported catalysts for carbon dioxide transformation to diethyl carbonate', Catalysis Today, vol. 306, pp.128-137, https://doi.org/10.1016/j.cattod.2017.01.016
Leino, E, Mäki-Arvela, P, Eränen, K, Tenho, M, Murzin, DY, Salmi, T & Mikkola, J-P 2011, 'Enhanced yields of diethyl carbonate via one-pot synthesis from ethanol, carbon dioxide and butylene oxide over cerium (IV) oxide', Chemical Engineering Journal, vol. 176, pp.124-133, https://doi.org/10.1016/j.cej.2011.07.054
Leino, E, Mäki-Arvela, P, Eta, V, Kumar, N, Demoisson, F, Samikannu, A, Leino, AR, Shchukarev, A, Murzin, DY & Mikkola, JP 2013, 'The influence of various synthesis methods on the catalytic activity of cerium oxide in one-pot synthesis of diethyl carbonate starting from CO2, ethanol and butylene oxide', Catalysis Today, vol. 210, pp. 47-54, https://doi.org/10.1016/j.cattod.2013.02.011
Lian, GUO, Xinqiang, Z, Hualiang, AN & Yanji, W 2012, 'Catalysis by lead oxide for diethyl carbonate synthesis from ethyl carbamate and ethanol, Chinese Journal of Catalysis, vol. 33, no. 4–6, pp. 595-600, https://doi.org/10.1016/S1872-2067(11)60373-2
Lubis, MR, Fujianti, DS, Zahara, R & Darmadi 2019, The optimization of the electrocoagulation of palm oil mill effluent with a box-behnken design', International Journal of Technology, vol. 10, no. 1, pp.137-146, https://doi.org/10.14716/ijtech.v10i1.838
Murugan, C & Bajaj, HC 2011, Synthesis of diethyl carbonate from dimethyl carbonate and ethanol using KF / Al 2 O3 as an ef fi cient solid base catalyst, Fuel Processing Technology, vol. 92, no. 1, pp. 77-82, https://doi.org/10.1016/j.fuproc.2010.08.023
Qi, Y, Wu, T, He, J & King, DA 2013, 'China ’ s carbon conundrum', Nature Geoscience, vol. 6, pp. 507-509
Raissi, S & Farsani, R-E 2009, 'Statistical process optimization through multi-response surface methodology', International Journal of Mathematical, Computational, Physical, Electrical and Computer Engineering, vol. 3, no. 3, pp. 197-201
Saud, A, Nadiah, N, Firdaus, M, Rangabhashiyam, S, Jawad, H, Wilson, LD, Mundher, Z, Al-kahtani, AA & Alothman, ZA 2021, Statistical modeling and mechanistic pathway for methylene blue dye removal by high surface area and mesoporous grass-based activated carbon using K2CO3 activator, Journal of Environmental Chemical Engineering, vol. 9, no. 4, p. 105530, https://doi.org/10.1016/j.jece.2021.105530
Shukla, K & Srivastava, VC 2017, Diethyl carbonate synthesis by ethanolysis of urea using Ce-Zn oxide catalysts, Fuel Processing Technology, vol. 161, pp.116-124, https://doi.org/10.1016/j.fuproc.2017.03.004
Petrus, HTBM, Olvianas, M & Astuti, W 2021, Valorization of geothermal silica and natural bentonite through geopolymerization?: a characterization study and response surface design, International Journal of Technology, vol.12, no. 1, pp. 195-206, https://doi.org/10.14716/ijtech.v12i1.3537
Variyana, Y, Muchammad, RSC & Mahfud, M 2019, 'Box-behnken design for the optimization using solvent-free microwave gravity extraction of garlic oil from Allium sativum L', IOP Conference Series: Materials Science and Engineering, vol. 673, pp.1-13, DOI 10.1088/1757-899X/673/1/012005
Wang, L, Li, H, Xin, S, Li, F, 2014, 'Generation of solid base catalyst from waste slag for the ef fi cient synthesis of diethyl carbonate from ethyl carbamate and ethanol', Catalysis Communications, vol. 50, pp. 49-53, https://doi.org/10.1016/j.catcom.2014.02.028
Wang, D, Yang, B, Zhai, X & Zhou, L 2007, 'Synthesis of diethyl carbonate by catalytic alcoholysis of urea', Fuel Processing Technology, vol. 88, pp. 807-812, https://doi.org/10.1016/j.fuproc.2007.04.003
Wang, L, Ammar, M, He, P, Li, Y, Cao, Y, Li, F, Han, X & Li, H 2017, The efficient synthesis of diethyl carbonate via coupling reaction from propylene oxide, CO2 and ethanol over binary PVEImBr / MgO catalyst, Catalysis Today, vol. 281, pp. 360-370, https://doi.org/10.1016/j.cattod.2016.02.052
Wang, L, Li, H, Xin, S, He, P, Cao, Y, Li, F & Hou, X 2014, 'Highly efficient synthesis of diethyl carbonate via one-pot reaction from carbon dioxide , epoxides and ethanol over KI-based binary catalyst system', Applied Catalysis A: General, vol. 471, pp.19-27, https://doi.org/10.1016/j.apcata.2013.11.031
Wang, Z, Zheng, X, Wang, Y, Lin, H & Zhang, H 2021, 'Evaluation of phenanthrene removal from soil washing effluent by activated carbon adsorption using response surface methodology', Chinese Journal of Chemical Engineering, vol. 42, pp.399-405, https://doi.org/10.1016/j.cjche.2021.02.027
Xin, S, Wang, L, Li, H, Huang, K & Li, F 2014, 'Synthesis of diethyl carbonate from urea and ethanol over lanthanum oxide as a heterogeneous basic catalyst', Fuel Processing Technology, vol. 126, pp. 453-459, https://doi.org/10.1016/j.fuproc.2014.05.029
Yan, C, Lu, B, Wang, X, Zhao, J & Cai, Q 2011, Electrochemical synthesis of dimethyl carbonate from methanol, CO2 and propylene oxide in an ionic liquid, Journal of Chemical Technology and Biotechnology, vol. 86, pp. 1413-1417, https://doi.org/10.1002/jctb.2647
Wang, Y, Jia, D, Zhu, Z & Sun, Y 2016, Synthesis of diethyl carbonate from carbon dioxide, propylene oxide and ethanol over KNO3-CeO2 and KBr-KNO3-CeO2 catalysts, Catalyst, Volume 52(6), pp.1–11,
Zhang, M, Xiao, M, Wang, S, Han, D, Lu, Y & Meng, Y 2015a, Cerium oxide-based catalysts made by template-precipitation for the dimethyl carbonate synthesis from Carbon dioxide and methanol, Journal of Cleaner Production, vol. 103, pp. 847-853, https://doi.org/10.1016/j.jclepro.2014.09.024
Zhang, P & Ma, X, 2010, 'Catalytic synthesis of diethyl carbonate by oxidative carbonylation of ethanol over PdCl 2 / Cu-HMS catalyst', Chemical Engineering Journal, vol. 163(1–2), pp.93-97, https://doi.org/10.1016/j.cej.2010.07.025
Zhang, P, Wang, S, Chen, S, Zhang, Z & Ma, X 2008, 'The effects of promoters over PdCl2 -CuCl2 / HMS catalysts for the synthesis of diethyl carbonate by oxidative carbonylation of ethanol', Chemical Engineering Journal, vol. 143, no. 1–3, pp. 220-224, https://doi.org/10.1016/j.cej.2008.04.008
Zhang, P, Zhou, Y, Fan, M & Jiang, P 2015b, Catalytic synthesis of diethyl carbonate with supported Pd ? Cu bimetallic nanoparticle catalysts?: Cu ( I ) as the active species, Chinese Journal of Catalysis, vol. 36, no. 11, pp.2036-2043, https://doi.org/10.1016/S1872-2067(15)60973-1
Zhang, X, Jia, D & Zhang, J 2014, Direct synthesis of diethyl carbonate from CO2 and ethanol catalyzed by ZrO 2 / molecular sieve, Catalysis Letters, vol. 144, pp.2144-2150,
Zhang, Y, Ji, Z, Xu, R & Fang, Y 2011, 'Experimental and kinetic studies on a homogeneous system for diethyl carbonate synthesis by transesterification', Chemical Engineering Technology, vol. 35, no. 4, pp. 693-699, https://doi.org/10.1002/ceat.201100276
Zhang, Y, Zou, X & Sui, L 2006, 'The effects of potassium halides on catalytic activities of CeO2 – K based catalysts for diesel soot oxidation', Catalysis Communications, vol. 7, pp. 855-859, https://doi.org/10.1016/j.catcom.2006.03.013
Zhao, L, Hou, Z, Liu, C, Wang, Y & Dai, L 2014, 'A catalyst-free novel synthesis of diethyl carbonate from ethyl carbamate in supercritical ethanol', Chinese Chemical Letters, vol. 25, no. 10, pp.1395-1398, https://doi.org/10.1016/j.cclet.2014.05.012
Zhao, Y, Zhang, Z, Zhao, X & Hao, R 2016, 'Catalytic reduction of carbon dioxide by nickel-based catalyst under atmospheric pressure', Chemical Engineering Journal, vol. 297, pp.11-18, https://doi.org/10.1016/j.cej.2016.03.108
Zhu, D, Mei, F, Chen, L, Mo, W, Li, T & Li, G 2011, 'An efficient catalyst Co ( salophen ) for synthesis of diethyl carbonate by oxidative carbonylation of ethanol', Fuel, vol. 90, no. 6, pp.2098-2102, https://doi.org/10.1016/j.fuel.2011.02.023
