Effect of Oxidants in the Utilization of Polysulfone Hollow Fiber Membrane Module as Bubble Reactor for Simultaneously Removal of NOx and SO2
Corresponding email: sutrasno@che.ui.ac.id
Published at : 25 Jan 2024
Volume : IJtech
Vol 15, No 1 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i1.6415
Kartohardjono, S., Karamah, E.F., Hayati, A.P., Talenta, G.N., Ghazali, T.A., Lau, W.J., 2024. Effect of Oxidants in the Utilization of Polysulfone Hollow Fiber Membrane Module as Bubble Reactor for Simultaneously Removal of NOx and SO2 . International Journal of Technology. Volume 15(1), pp. 63-74
| Sutrasno Kartohardjono | Process Intensification Laboratory, Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok 16424 |
| Eva Fathul Karamah | Process Intensification Laboratory, Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok 16424 |
| Adinda Puspa Hayati | Process Intensification Laboratory, Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok 16424 |
| Grace Natalie Talenta | Process Intensification Laboratory, Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok 16424 |
| Thoriq Ahmad Ghazali | Process Intensification Laboratory, Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok 16424 |
| Woei Jye Lau | Advance Membrane Technology Research Center, Universiti Teknologi Malaysia, 81310 Skudai, Johor, Malaysia |
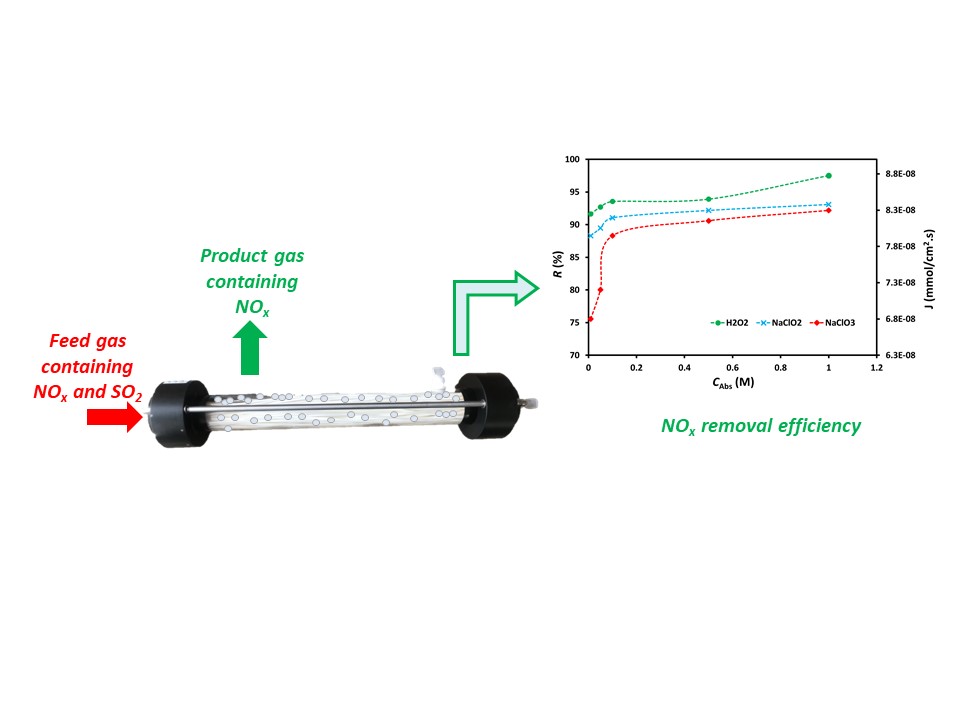
Air pollution has become a global issue and
contributes significantly to climate change, mainly due to the massive energy
consumption in industry and the transportation sector. Emissions of harmful gases from burning fuels such
as NOx and SO2 are the most significant sources of
environmental pollution, which have negative impacts on the environment, such
as the greenhouse effect, damage to the ozone layer, photochemical smog, and
acid rain, and can interfere with the respiratory system in humans. This study
utilizes hollow fiber membrane modules, which act as a reactor on the shell
side of the membrane module and a gas distributor by the membrane fiber to
remove NOx and SO2 spontaneously. The oxidant solutions
used were a pair of hydrogen peroxide and sodium hydroxide (H2O2-NaOH)
solutions, a pair of sodium chlorite and sodium hydroxide (NaClO2-NaOH)
solutions, and a pair of sodium chlorate and sodium hydroxide (NaClO3-NaOH)
solutions. Based on the results of experiments, SO2 can be removed
entirely in the process, while NOx depends on the feed gas flow rate and the concentration of the oxidant
solution used. H2O2 is the most effective oxidizing agent
in removing NOx and SO2 because of its higher oxidative
properties than NaCLO2 and NaClO3. The increase in feed
gas flow rate resulted in a decrease in the efficiency of NOx
removal even though the NOx mass transfer flux and NOx
loading increased. Meanwhile, an increase in the concentration of oxidants
increases the efficiency of NOx removal and mass transfer flux but
decreases NOx loading. Based on the experimental results, the
maximum NOx removal efficiency achieved by the oxidant solutions is
93.9, 91.1, and 88.3% for H2O2-NaOH, NaClO2-NaOH, and NaClO3-NaOH, respectively.
Climate change; Harmful gases; NOx; Removal efficiency; SO2
Air pollution has become a global issue and contributes significantly to
climate change due to the industry and transportation sector's massive energy
consumption (Manisalidis et al., 2020). In many countries, such as
Indonesia, the emission of air pollutants from industrial and transportation
activities is increasing due to the burning of fossil fuels. Emissions of
harmful gases from the fuels burning process, such as NOx and SO2,
are the most significant causes of environmental pollution, which have negative
impacts on the environment, such as the greenhouse effect, damage to the ozone
layer, photochemical smog, and
acid rain, and can interfere with the respiratory system in humans (Kartohardjono et al., 2019; Ma et al., 2019). One promising strategy to reduce exhaust
gas pollutants such as NOx and SO2 is to control the
source of the pollution (Zhu et al., 2023). Removing
NOx and SO2 in flue gases, such as those from coal burn
boilers and marine diesel engines, is currently attracting much attention (Zhao et al., 2022; Yan et
al., 2020). In 2020, analysis tools revealed a
significant increase in the trend of NOx and SO2 emissions from
Indonesian coal-fired power plants. The emissions for SO2 and NOx
were reported as 798.5 kton/year and 120.02 kton/year, respectively (Sunarno, Purwanto, and Suryono, 2021).
Considering the losses that SO2 and NOx gases can cause,
the Indonesian Government has set various regulations related to the quality
standard of the two gases, which is 200 or 0.16 and 0.076 ppm for NO and SO2, respectively (Ministry of Environment and Forestry, 2019).
Several
technologies have been developed to control pollutant emissions in many
industries, including Selective Catalytic Reduction (SCR) and Selective
Non-Catalytic Reduction (SNCR) for NOx removal (Karamah et al., 2021)
and Flue Gas Desulfurization for SO2 removal (Sharma et al., 2012). The
conventional technology of SCR for NOx and FGD for SO2
has been widely adopted in various countries (Xu et al., 2022). With increasing environmental
awareness, the government and society need strict legislation and regulations
to minimize NOx and SO2 emissions into the air (Jia et al., 2022; Chen et
al., 2021). Although the removal rate is relatively
high, several problems are faced, such as the catalyst used in the SCR system
is quite expensive, must be replaced periodically, and requires a large area of
land for its application (Guo et al., 2018). Therefore,
it has prompted the search for suitable alternative technologies to remove SO2
and NOx simultaneously. The simultaneous removal of SO2 and
NOx through two different technologies needs high operational and
investment costs because the process is becoming more complex (Zhao et al., 2021b; Cheng and
Zhang, 2018) and has a high working area (Zhao et al., 2021a). NOx
and SO2 are both acidic gases, but the solubility of NOx
in water is less than SO2, so a different technique is needed to
remove the two gases (Fang et al., 2011).
Several
approaches that can be used to remove NOx and SO2
simultaneously include the oxidation approach, the reduction approach, the
absorption or adsorption approach, and the microbial approach (Chen et al., 2021). Oxidation
approaches include gas-liquid oxidation, gas-liquid oxidation, and gas-solid
oxidation. The gas oxidation approach can use ozone (Sun et al., 2013), oxygen (Atkinson et al., 2004), the
oxidant chlorine (Cl2) and chlorine dioxide (ClO2) (Mostafa et al., 2018), and
non-thermal plasma (Feng et al., 2018). The
gas-liquid oxidation approach, also known as the wet process, includes
gas-liquid oxidation using H2O2 (Kartohardjono et al., 2023; Waclawek et
al., 2017), Peroxydisulfate/Peroxymonosulfate (Matzek and Carter, 2016), and
NaClO/NaClO2 (Zhitao et al., 2019). Meanwhile,
for the gas-solid oxidation approach through a photocatalytic process using
catalysts such as TiO2 (Su et al., 2013), ZnO (Boyjoo et al., 2017), CeO2 (Tsang et al., 2019), Bi2WO6
(Wang et al., 2017), and BiOX
(Cl, Br, I) (Xia et al., 2015). The reduction approach includes gas-liquid
reduction, gas-liquid reduction, and gas-solid reduction. Reduction of gases
can use reductants such as CO (Makeev and Peskove, 2013), H2
(Ge et al., 2018), and CxHy (Pan et al., 2015). The
gas-liquid reduction can use ammonia, urea, and sodium sulfide (Na2S)
(Mok and Lee, 2006), while
gas-solid reduction can use carbon materials (Ma et al., 2013). Absorption/adsorption
approaches include Alkaline solution absorption (Sun et al., 2015), complex
absorption (Guo et al., 2014), carbon-based adsorption (Xiong et al., 2015),
zeolite-based adsorption (Rezaei et
al., 2015), metal oxide-based adsorption (Vikrant et al., 2017). Meanwhile,
the microbial approach uses autotrophic micro-organisms under anoxic conditions (Xiao et al., 2017).
The wet
method approach is becoming more commonly applied to remove NOx and
SO2 simultaneously because of its high efficiency and low cost (Johansson, Normann, and
Andersson, 2021). The wet method includes wet scrubbing
technology, widely used in SO2 gas removal processes, and a bubble
reactor to remove NOx (Zhang et al., 2021). Bubble reactors are multiphase
reactors widely used in various industries, such as the chemical,
petrochemical, and biochemical industries. These reactors play a
pivotal role in numerous chemical processes encompassing oxidation,
chlorination, alkylation, polymerization, and hydrogenation reactions. In these
reactors, the feed gas is introduced into the system and then dispersed into
bubbles as part of the technical process. Meanwhile, the liquid phase or
liquid-solid suspension can be operated in batch mode or flowed in the
direction/opposite direction of the gas flow so that contact or reaction will
occur in the reactor column (Jakobsen, Linborg, and Dorao, 2005).
The wet
method facilitates the removal of gaseous pollutants through contact between pollutant
gas and oxidant liquid, which triggers a reaction between pollutant gas and
oxidant liquid, becoming other species (Jin et al., 2006). The main
obstacle in removing NOx gas through the wet method is that NOx
gas is a species that cannot be dissolved in the oxidant (Kang et al., 2020). To address
this issue, an oxidizing agent is introduced to convert the NOx
species into more soluble forms, such as hydrogen peroxide (H2O2), sodium
chlorite (NaClO2), and sodium chlorate (NaClO3). At the
same time, an alkaline solution such as NaOH can be applied to remove SO2 (Purnawan et al., 2021).
Membrane
technology is a non-conventional technique that can simultaneously remove NOx
and SO2. The membrane is a porous medium in the form of a thin film
that can diffusely transfer certain gas compounds due to a driving force in the
form of concentration toward the solvent through the membrane pore (Wang and Yu, 2017). A membrane
contactor has several advantages, such as ease of operation and scale-up, low
separation costs and energy consumption, and high efficiency (Kartohardjono et al.,
2020). This study utilized a hollow fiber membrane module (HFMM) that
functions as a reactor and gas distributor to remove NOx and SO2
spontaneously. Using an HFMM as a bubble reactor enhances the area for
gas-liquid contact, providing a better removal reaction between NOx
and SO2 gases with the applied oxidant solutions. The oxidant
solutions used were a pair of H2O2-NaOH solutions, a pair
of NaClO2-NaOH solutions, and a pair of NaClO3-NaOH
solutions.
The
reactions between NOx and SO2 with a pair of H2O2
and NaOH solutions are presented in Equations
(1) – (4) (Purnawan et al., 2021; Sun, Zwoli?ska,
and Chmielewski, 2016):
The reactions that occur between NOx and SO2 with a pair of NaClO2 and NaOH solutions are presented in Equations (5) – (8) (Zhao et al., 2010; Chien, Chu, and Hsueh, 2003):
The CV Bandung
Indonesia supplied the polysulfone hollow fiber membrane module consisting of
50 fibers used in the study. The analytic grade H2O2,
NaClO2, NaClO3, and NaOH are provided by Merck Indonesia.
Meanwhile, the feed gas in the form of a gas mixture of 600 ppm NOx
and 500 ppm SO2 in nitrogen was provided by PT EIN Indonesia. The
feed gas flow rate was regulated during the experiments using the CX Series
mass flow controller, which can precisely control the gas flow rate. In
addition, the concentration of gases entering and leaving the membrane was
measured using an ECOM-D Gas analyzer.
The HFMM
operates on a principle similar to that of a bubble reactor. The oxidant, which
contains a pair of 200 mL solutions of H2O2-NaOH, NaClO2-NaOH,
or NaClO3-NaOH, is located on the shell side of the HFMM. The feed
gas stream containing SO2 and NOx entered the membrane module
through a silicone hose connection to the lumen fibers. A CX Series mass flow
controller regulated the gas flow rates and made contact with oxidant solutions
in the shell side of HFMM. The ECOM-D Gas Analyzer measured the NOx
and SO2 composition, as it leaving the
membrane module.
The NOx
or SO2 removal efficiency, flux, and gas loading were calculated by Equations (11-14) (Kartohardjono et al.,
2020):
Cin and Cout are the NOx
or SO2 concentrations in the feed gas and gas left from the HFMM,
respectively. Meanwhile, GasAbs,
Am, Coxidant, QG,
P, T, and R are NOx
or SO2 absorbed by the oxidant, membrane area, concentration of H2O2,
NaClO2, or NaClO3, feed gas flowrate, pressure,
temperature, and ideal gas constant, respectively. The series of experimental
equipment is shown in Figure 1. All experiments were conducted three times, and
the experimental results' standard deviation was less than 6%.
Figure 1 Experimental equipment set up: 1. Feed gas
tank, 2. Gas regulator, 3. Mass flow controler, 4. HFMM, 5. Gas Analyzer, 6.
Data storage
This study
used a feed gas with initial concentrations of NOx and SO2
of 600 ppm and 500 ppm, respectively. The oxidant solutions used were H2O2-NaOH
solutions, NaClO2-NaOH solutions, and NaClO3-NaOH
solutions with a concentration of 0.1M and 0.5M of 200 mL each. The gas flow in
the experiments varied from 0.1 to 0.2 L/minute at a constant temperature and
pressure of 28? and 1 atm, respectively. The process of NOx and SO2
gases transfer through the HFMM during the experiment occurred in three stages:
(i) gas diffusion to the inner surface of the fiber membrane; (ii) gas
diffusion through the membrane pores to the outer surface of the membrane
fibers; and (iii) gas absorption by the oxidant (Kartohardjono et al.,
2019).
For all
experiments, the SO2 removal efficiency is generally 100%, as it has
a high solubility in water and better chemical reactivity (Liu, Shi, and Wang, 2022), so its presence in the feed gas
will be examined to see the influence on NOx removal. Figure 2 shows
the impact of varying feed gas flow rates on NOx gas's absorption
efficiency (%R) with various oxidants.
As
demonstrated in Figure 2, the removal efficiency of NOx for all
oxidants decreases with increasing feed gas flow. Increasing the feed gas flow
causes an increase in the NOx absorbed by the oxidant solutions,
thereby increasing the efficiency of NOx removal. However,
increasing the feed gas flow led to less gas residence time in the HFMM, which
caused a decrease in the removal efficiency of NOx. The decline in
the removal efficiency of NOx to the gas flow indicates that the
effect of gas residence time in the membrane module is more influential than
the increase in the adsorbed NOx (Xu et al., 2022). The removal efficiency of NOx
decreased from 93.9 to 81.3%, 91.1 to 79.5%, and 88.3 to 71.0% for H2O2-NaOH,
NaClO2-NaOH, and NaClO3-NaOH adsorbents, respectively.
Oxidant solutions containing H2O2 have the highest
removal efficiency because of their higher
oxidative properties than NaClO2 and NaClO3. The standard reduction potentials for H2O2, NaClO2,
and NaClO3 are 1.77, 0.76, and 0.62 Volt, respectively (Purnawan et al., 2021; Lide, 2004). Previous
studies showed a slight decrease in the removal efficiency of NOx
from about 99.8 to 98.8%, 99.4 to 98.6%, and 99.3 to 98.3% for H2O2-HNO3,
NaClO2-NaOH, and NaClO3-NaOH oxidant pairs, respectively,
under the same conditions as this study using feed gas containing 600 ppm NOx
without SO2 and flow rates from 100 to 200 mL/min (Purnawan et al., 2021). Thus, it
is clear that the presence of SO2 in the feed gas reduces the NOx
removal efficiency due to the influence of competition in consuming the oxidant
solution (Kartohardjono et al., 2023), as shown in Equations (3), (8), and (10). In addition, the wet
method has the disadvantage that it can only be used indirectly if the exhaust
gas temperature is high enough because the wet process is only adaptable to
operate at ambient temperature.
Figure 2 NOx
removal efficiency, R-NOx,
at various feed gas flow rates, QG
The NOx mass transfer flux, as
presented in Figure 3, rises with increasing the feed gas flow, indicating that
increasing gas flow contributes to an increase in oxidant performance in
absorbing NOx passing through the membrane. With the feed gas flow
increase from 100 CC/min to 200 CC/min, the NOx mass transfer flux
rose from 4.9 to 8.4×10-8 mmol/cm2.s, 4.7 to 8.2 ×10-8
mmol/cm2.s, and 4.6 to 7.4 ×10-8 mmol/cm2.s, for the H2O2-NaOH,
NaClO2-NaOH, and NaClO3-NaOH oxidant pairs, respectively.
Increasing the gas flow enhances the absorbed NOx, as presented in
Figure 3, so it increases the flux in the end. A similar phenomenon also occurs
for NOx loading, the ratio between NOx absorbed and the
amount of oxidant (H2O2, NaClO2, or NaClO3),
where the NOx loading appears to increase with the higher feed gas
flow rate, indicating that the feed gas flow also contributes to the rise in
the uptake of NOx by the oxidant solutions, as presented in Figure
4. When the feed flow raised from 100 to 200 CC/min, the NOx loading
increased from 0.0019 to 0.0033 mmol/mol.s, 0.0019 to 0.0032 mmol/mol.s, and
0.0018 to 0.0026 mmol/mol.s, for the H2O2-NaOH, NaClO2-NaOH,
and NaClO3-NaOH solvent pairs, respectively. In previous studies,
under the same conditions using feed gas containing 600 ppm NOx
without SO2 and flow rates from 100 to CC mL/min, the mass transfer
flux increased from about 0.54 to 1.1 ×10?7 mmol/cm2.s
for all pairs of oxidants as their NOx removal efficiency only
slightly different. Meanwhile, NOx loading increased from 0.002 to
0.004 mmol/mol.s for all pairs of oxidants (Purnawan et al., 2021). It reveals that the NOx
mass transfer flux and NOx loading using feed gas without SO2
is higher than that in the feed gas with SO2 due to the competition
in oxidant consumption, as shown in Equations (5), (6), (11), (14), and (19).
Figure 4 NOx Loading at various feed
gas flow rates, QG
Figure 5
shows the effect of oxidant concentration on NOx removal efficiency
and mass transfer flux. The absorption efficiency of NOx by the
oxidant solution increases with raising the oxidant solution concentration. The
higher the concentration of the oxidant solution, the more chemical compounds
are available to react with NOx; thereby, it can increase the number
of chemical reactions between NOx and chemical compounds in the
oxidant to boost the NOx removal efficiency. The increase in NOx
mass transfer flux is also proportional to the increase in NOx
removal efficiency, as the feed gas flow rate used is the same for each
concentration of the oxidant solution (Zhao et al., 2020). NOx
removal efficiency and flux increased significantly at oxidant concentrations
between 0.01 and 0.1 M while only slightly increased at oxidant concentrations
greater than 0.1 M. The efficiency of NOx removal is still
relatively low, around 75.6, 88.3, and 91.6% for NaClO3, NaClO2,
and H2O2, respectively, with a concentration of around
0.01 M. Hence, an increase in oxidant concentration up to 0.1 M still gives a
significant increase. However, at 0.1 M oxidant concentration, the NOx
removal efficiency was relatively high, around 88.3, 91.1, and 93.5% for NaClO3,
NaClO2, and H2O2, respectively. Hence, an
increase in oxidant concentration above 0.1 M gave a not as sharp rise in NOx
removal efficiency as in the oxidant concentration area between 0.01 and 0.1 M.
Similar findings were also reported in the previous studies using NOx
feed gas without SO2, where NOx removal increased with
increasing oxidant concentration using a PVDF HFMM consists of 40 fibers. The
NOx removal efficiency increased from 93.3 to 99.0%, 98.7 to 99.2%,
and 98.9 to 99.7% with the raised of oxidant concentration from 0.05 to 0.25M,
0.01 to 0.05M, and 0.015 to 0.075 M, for the oxidants NaClO3, NaClO2,
and H2O2, respectively (Purnawan et al., 2021). Shi et
al. reported a rise in NOx removal from about 34.5 to 91.7% when
the concentration of NaClO3 solution as an oxidant increased from
0.005 to 0.1 M in a bubble column reactor (Shi, Sun, and Cui, 2019). Meanwhile, Zhitao et al.
reported that increasing the NaClO2 concentration from 0.005 to 0.15
M could improve the efficiency of the NO removal process with an initial
concentration of 800 ppm
through a cyclic scrubbing process from 62.5 to 85% (Zhitao et al., 2019). It is seen that the presence of SO2 in the feed gas
affects reducing the efficiency of NOx removal.
Figure 5 NOx
removal efficiency, R, and NOx mass transfer flux, J, at various concentration of oxidant
present in oxidant solutions, CAbs
NOx
loading in the NOx removal process using an oxidant solution is the
ratio between the absorbed NOx by the oxidant solution and the
number of moles of oxidant in the oxidant solution. As presented in Figure 6,
an increase in the concentration of oxidants in the NOx removal
process decreases gas loading because more oxidants are used, while the
increase in NOx absorbed is much smaller (Karamah et al., 2021). These
results indicate that a low oxidant concentration is preferable because it
provides a high NOx loading. However, the desired NOx
removal target also influences the decision to determine the oxidant
concentration in the oxidant solution used. In this study, the NOx
loading declined from around 0.015 to 0.0002 mmol/mol.s, 0.018 to 0.0002
mmol/mol.s, and 0.019 to 0.0002 mmol/mol.s for NaClO3, NaClO2,
and H2O2, respectively, when the oxidant concentration in
the oxidant solutions was increased from 0.01 to 1 M. Figure 6 also
demonstrates that the three oxidants used have almost the same NOx
loading, so the images coincide. It indicates
that the type of oxidant used does not have a significant effect on NOx
loading due to the insignificant difference in the amount of NOx
absorbed, as also reported previously (Purnawan et al., 2021). Table 1 summarizes the experimental results at a feed gas flow rate of 100
mL/min and an oxidant concentration of 0.1 M.
Figure 6 NOx
loading at various concentration of oxidant
Table 1 The results of NOx
removal efficiency, flux, and NOx loading at the concentration of
the oxidant 0.1 M and feed gas flow rate of 100 mL/min
|
Oxidants |
NOx Removal efficiency (%) |
Flux (mmol/cm2.s) |
NOx loading (mmol/mol.s) |
|
H2O2 |
93.9 |
4.9 x 10-8 |
0.0019 |
|
NaClO2 |
91.1 |
4.7 x 10-8 |
0.0019 |
|
NaClO3 |
88.3 |
4.6 x 10-8 |
0.0018 |
H2O2,
NaClO2, and NaClO3 are all capable of removing NOx
and SO2 from flue gases, but their effectiveness depends on feed gas
flow and concentration. All experimental results show that the efficiency of SO2
removal is generally 100% due to its high solubility in water and better
chemical reactivity. H2O2 is a highly effective oxidizing
agent and has been shown to be capable of removing both NOx and SO2
because of its higher oxidative properties than NaClO2 and NaClO3.
Based on the experimental results, it can be seen that a rise in the feed gas
flow rate decreases the NOx removal efficiency even though the NOx
mass transfer flux and NOx loading increase. Meanwhile, increasing
the oxidant concentration increases NOx removal efficiency and mass
transfer flux but decreases NOx loading. The three oxidant solutions
used relatively have the same NOx loading at the same oxidizing
concentration.
The authors
wish to acknowledge the funding of this research by The Directorate General of
the Higher Education Republic of Indonesia through Universitas Indonesia with
contract No. NKB 858 /UN2.RST/HKP.05.00/2022.
Atkinson, R., Baulch, D., Cox,
R. A., Crowley, J., Hampson, R., Hynes, R., Jenkin, M.E., Rossi, M.J., Troe, J., 2004. Evaluated Kinetic and Photochemical
Data for Atmospheric Chemistry: Volume I-Gas Phase Reactions of Ox, HOx, NOx
and SOx Species. Atmospheric Chemistry and Physics, Volume 4(6), pp. 1461–1738
Boyjoo, Y., Sun, H., Liu, J.,
Pareek, V.K., Wang, S., 2017. A Review on Photocatalysis for Air Treatment:
From Catalyst Development to Reactor Design. Chemical
Engineering Journal, Volume 310, pp. 537–559
Chen, R., Zhang, T., Guo, Y.,
Wang, J., Wei, J., Yu, Q., 2021. Recent Advances in Simultaneous Removal of SO2
and NOx from Exhaust Gases: Removal Process, Mechanism and Kinetics. Chemical
Engineering Journal, Volume 420, p. 127588
Cheng, G., Zhang, C., 2018.
Desulfurization and Denitrification Technologies of Coal-fired Flue Gas. Polish
Journal of Environmental Studies, Volume 27(2), pp.
481–489
Chien, T.W., Chu, H., Hsueh,
H.T., 2003. Kinetic Study on Absorption of SO2 and NO x with acidic
NaClO2 Solutions Using the Spraying Column. Journal
of Environmental Engineering, Volume 129(11), pp. 967–974
Fang, P., Cen, C., Tang, Z.,
Zhong, P., Chen, D., Chen, Z., 2011. Simultaneous Removal of SO2 and
NOx by Wet Scrubbing Using Urea Solution. Chemical
Engineering Journal, Volume 168(1), pp. 52–59
Feng, X., Liu, H., He, C., Shen,
Z., Wang, T., 2018. Synergistic Effects and Mechanism of a Non-Thermal Plasma
Catalysis System in Volatile Organic Compound Removal: a Review. Catalysis
Science & Technology, Volume 8(4), pp. 936–954
Ge, T.,
Zuo, C., Wei, L., Li, C. 2018. Sulfur Production from
Smelter Off-Gas Using CO–H2 Gas Mixture as The Reducing Agent Over
Modified Fe/?-Al2O3 Catalysts. Chinese
Journal of Chemical Engineering, Volume 26(9), pp. 1920–1927
Guo, L., Han, C., Zhang, S.,
Zhong, Q., Ding, J., Zhang, B., Zeng, Y., 2018. Enhancement Effects of O2?
and OH Radicals on NOx Removal in the Presence of SO2 by Using
an O3/H2O2 AOP System with Inadequate O3
(O3/NO Molar Ratio= 0.5). Fuel, Volume 233, pp. 769–777
Guo, Q., He, Y., Sun, T., Wang,
Y., Jia, J., 2014. Simultaneous Removal of NOx and SO2 from Flue Gas
Using Combined Na2SO3 Assisted Electrochemical Reduction and Direct
Electrochemical Reduction. Journal of Hazardous Materials, Volume
276, pp. 371–376
Jakobsen, H.A., Linborg, H., Dorao, C.A., 2005. Modelling of Bubble
Column Reactors: Progress and Limitation. Industrial
and Engineering Chemistry Research,
Volume 44, pp. 5107–5151
Jia, S.,
Pu, G., Gao, J., Yuan, C., 2022. Oxidation-Absorption
Process for Simultaneous Removal of NOx and SO2 over Fe/Al2O3@SiO2 Using Vaporized
H2O2. Chemosphere, Volume 291,
p. 133047
Jin, D.-S., Deshwal, B.-R.,
Park, Y.-S., Lee, H.-K., 2006. Simultaneous Removal of SO2 And NO By
Wet Scrubbing Using Aqueous Chlorine Dioxide Solution. Journal
of Hazardous Materials, Volume 135(1-3), pp. 412–417
Johansson, J., Normann, F.,
Andersson, K., 2021. Techno-Economic Evaluation of Co-Removal of NOx and SOx
Species from Flue Gases via Enhanced Oxidation of NO by ClO2—Case
Studies of Implementation at a Pulp and Paper Mill, Waste-to-Heat Plant and a
Cruise Ship. Energies, Volume 14(24), p.
8512
Kang, M.S., Shin, J., Yu, T.U.,
Hwang, J., 2020. Simultaneous Removal of Gaseous NOx and SO2 by Gas-Phase
Oxidation with Ozone and Wet Scrubbing with Sodium Hydroxide. Chemical
Engineering Journal, Volume 381, p. 122601
Karamah, E.F., Arbi, D.S.,
Bagas, I., Kartohardjono, S., 2021. Hollow Fiber Membrane Modules for NOx
Removal using a Mixture of NaClO3 and NaOH Solutions in the Shell Side as
Absorbents. International Journal of Technology, Volume 12(4), pp. 690–699
Kartohardjono, S., Karamah,
E.F., Talenta, G.N., Ghazali, T.A., Lau, W.J., 2023. The Simultaneously Removal
of NOx and SO2 Processes through a Polysulfone Hollow Fiber Membrane Module. International
Journal of Technology, Volume 14(3), pp. 576–583
Kartohardjono, S., Merry, C.,
Rizky, M.S., Pratita, C.C., 2019. Nitrogen Oxide Reduction Through Absorbent
Solutions Containing Nitric Acid and Hydrogen Peroxide in Hollow Fiber Membrane
Modules. Heliyon, Volume 5(12), p. e02987
Kartohardjono, S., Rizky, M.S.,
Karamah, E.F., Lau, W., 2020. The Effect of the Number of Fibers in Hollow
Fiber Membrane Modules for NOx Absorption. International Journal of
Technology, Volume 11(2), pp. 269–277
Lide, D.R., 2004. CRC
Handbook of Chemistry and Physics. Volume 85. CRC press
Liu, Y., Shi, S., Wang, Z.,
2022. A Novel Double Metal Ions-Double Oxidants Coactivation System for NO and
SO2 Simultaneous Removal. Chemical Engineering Journal, Volume
432, p. 134398
Ma, C., Yi, H., Tang, X., Zhao,
S., Yang, K., Song, L., Zhang,
Y., Wang, Y. 2019. Improving
Simultaneous Removal Efficiency of SO2 and NOx from Flue Gas by
Surface Modification of MgO with Organic Component. Journal
of Cleaner Production, Volume 230, pp. 508–517
Ma, S.C., Yao, J., Ma, X., Gao,
L., Guo, M., 2013. Removal of SO2 and NOx Using Microwave Swing
Adsorption Over Activated Carbon Carried Catalyst. Chemical
Engineering & Technology, Volume 36(7), pp. 1217–1224
Makeev, A.G., Peskov, N.V.,
2013. The Reduction of NO by CO Under Oxygen-Rich Conditions in a fixed-bed
Catalytic Reactor: A Mathematical Model That Can Explain the Peculiar
Behavior. Applied Catalysis B: Environmental, Volume 132,
pp. 151–161
Manisalidis, I., Stavropoulou,
E., Stavropoulos, A., Bezirtzoglou, E., 2020. Environmental and Health Impacts
of Air Pollution: A Review. Frontiers in Public Health,
Volume 8, p. 14
Matzek, L.W., Carter, K.E.,
2016. Activated Persulfate for Organic Chemical Degradation: A Review. Chemosphere,
151, pp. 178–188
Ministry of Environment and Forestry,
R.I., 2019. Regulation of the Minister of Environment and
Forestry Number P.15/MENLHK/SETJEN/KUM.1/4/2019 concerning Quality Standards
for Thermal Power Generation Emissions. Jakarta
Mok, Y.S.,
Lee, H.-J., 2006. Removal Of Sulfur
Dioxide and Nitrogen Oxides by Using Ozone Injection and Absorption–Reduction
Technique. Fuel Processing Technology, Volume 87(7),
pp. 591–597
Mostafa, E., Reinsberg, P.,
Garcia-Segura, S., Baltruschat, H., 2018. Chlorine Species Evolution During
Electrochlorination on Boron-Doped Diamond Anodes: In-Situ Electrogeneration of
Cl2, Cl2O and ClO2. Electrochimica
Acta, Volume 281, pp. 831–840
Pan, H., Guo, Y., Jian, Y., He,
C., 2015. Synergistic Effect of Non-Thermal Plasma on NOx Reduction by CH4
Over an In/H-BEA Catalyst At Low Temperatures. Energy &
Fuels, Volume 29(8), pp. 5282–5289
Purnawan, I., Kartohardjono, S.,
Wibowo, L., Ramadhani, A. F., Lau, W. J., Febriasari, A., 2021. Effect of
Absorbents on NOx Removal through Polyvinylidene Fluoride (PVDF)
Hollow Fiber Membrane Modules. International Journal of Chemical
Engineering, Volume 2021, pp. 1–8
Rezaei, F., Rownaghi, A.A.,
Monjezi, S., Lively, R.P., Jones, C.W., 2015. SOx/NOx Removal from Flue Gas
Streams by Solid Adsorbents: A Review of Current Challenges and Future
Directions. Energy & Fuels, Volume 29(9),
pp. 5467–5486
Sharma, A.K., Prasad, D.,
Acharya, S., Sharma, R., 2012. Utility and Application of FGD System (Flue Gas
Desulphurization) In Chemical and Environmental Engineering. International
Journal of Chemical Engineering and Applications, Volume 3(2), p.
129
Shi, D., Sun, G., Cui, Y., 2019.
Study on The Removal of NO from Flue Gas by Wet Scrubbing Using NaClO3. Journal
of the Serbian Chemical Society, Volume 84(10), pp. 1183–1192
Su, C.,
Ran, X., Hu, J., Shao, C., 2013. Photocatalytic
Process of Simultaneous Desulfurization and Denitrification of Flue Gas by TiO2–Polyacrylonitrile
Nanofibers. Environmental Science & Technology, Volume
47(20), pp. 11562–11568
Sun, C., Zhao, N., Wang, H., Wu,
Z., 2015. Simultaneous Absorption of NOx and SO2 Using Magnesia
Slurry Combined with Ozone Oxidation. Energy & Fuels, Volume 29(5),
pp. 3276–3283
Sun, W.-Y., Wang, Q.-Y., Ding,
S.-l., Su, S.-J., 2013. Simultaneous Absorption of SO2 and NOx with
Pyrolusite Slurry Combined with Gas-Phase Oxidation of NO Using Ozone: Effect
of Molar Ratio of O2 (SO2+ 0.5 NOx) in Flue Gas. Chemical
Engineering Journal, Volume 228, pp. 700–707
Sun, Y., Zwoli?ska, E.,
Chmielewski, A.G., 2016. Abatement Technologies for High Concentrations of NOx
and SO2 Removal from Exhaust Gases: A Review. Critical Reviews
in Environmental Science and Technology, Volume 46(2), pp.
119–142
Sunarno, S., Purwanto, P.,
Suryono, S., 2021. Trend Analysis of NOx and SO2
Emissions in Indonesia from the Period of 1990-2015 using Data Analysis
Tool. Advances in Science, Technology and
Engineering Systems Journal, Volume 6(1), pp. 257–263
Tsang, C.H.A., Li, K., Zeng, Y.,
Zhao, W., Zhang, T., Zhan, Y., Xie,
R., Leung, D.Y.C., Huang, H., 2019.
Titanium Oxide Based Photocatalytic Materials Development and Their Role of in
The Air Pollutants Degradation: Overview and Forecast. Environment
International, Volume 125, pp. 200–228
Vikrant,
K., Kumar, V., Kim, K.-H., Kukkar, D., 2017. Metal–Organic Frameworks (MOFs): Potential and Challenges for
Capture and Abatement of Ammonia. Journal of Materials Chemistry A, Volume
5(44), pp. 22877–22896
Wac?awek, S., Lutze, H.V.,
Grübel, K., Padil, V.V., ?erník, M., Dionysiou, D.D., 2017. Chemistry of
Persulfates in Water and Wastewater Treatment: A Review. Chemical
Engineering Journal, Volume 330, pp. 44–62
Wang, L., Sun, B., Wang, W.,
Feng, L., Li, Q., Li, C. 2017. Modification of Bi2WO6 Composites with rGO for
Enhanced Visible Light Driven NO Removal. Asia?Pacific
Journal of Chemical Engineering, Volume 12(1), pp. 121–127
Wang, Y., Yu, X., 2017. Removal
of NO Research in A Polypropylene Hollow Fiber Membrane Contactor. In:
6th International Conference on Energy, Environment and Sustainable
Development (ICEESD 2017), pp. 1015–1022
Xia, D., Hu, L., He, C., Pan,
W., Yang, T., Yang, Y., Shu, D., 2015. Simultaneous Photocatalytic Elimination
of Gaseous NO and SO2 in a BiOI/Al2O3-Padded
Trickling Scrubber Under Visible Light. Chemical Engineering Journal, Volume 279,
pp. 929–938
Xiao, C., Ma, Y., Ji, D., Zang,
L., 2017. Review of Desulfurization Process for Biogas
Purification. In: IOP Conference Series: Earth and
Environmental Science, Volume 100, p. 012177
Xiong, Y., Tang, C., Yao, X.,
Zhang, L., Li, L., Wang, X., Deng,
Y., Gao, F., Dong, L. 2015. Effect
Of Metal Ions Doping (M= Ti4+, Sn4+) on The Catalytic
Performance of MnOx/CeO2 Catalyst for Low Temperature Selective
Catalytic Reduction of NO with NH3. Applied Catalysis A: General, Volume
495, 206–216
Xu, X.-J., Wu, Y.-N., Xiao,
Q.-Y., Xie, P., Ren, N.-Q., Yuan, Y.-X., Lee, D.J.,
Chen, C., 2022. Simultaneous Removal of NOx
and SO2 from Flue Gas in an Integrated FGD-CABR System by Sulfur
Cycling-Mediated Fe (II) EDTA Regeneration. Environmental
Research, Volume 205, p. 112541
Yan, Y.-G.,
Mao, Z.-J., Luo, J.-J., Du, R.-P., Lin, J.-X., 2020. Simultaneous Removal of SO2, NOx and Hg0 by O3
Oxidation Integrated with Bio-Charcoal Adsorption. Journal
of Fuel
Chemistry and Technology, Volume 48(12), pp. 1452–1460
Zhang, Z., Zhou,
S., Xi, H., Shreka, M., 2021. A Prospective Absorption System for Marine NOx
Removal from Simulated Gas Using Na2SO3/urea Composite
Absorbents in Bubble Reactor. Fuel, Volume
288, p. 119709
Zhao, J., Wei, Q.,
Bi, D., Liu, L., Wang, S., Ren, X., 2022. A Brand New Two-Phase Wet Oxidation
Absorption System for The Simultaneous Removal of SO2 and NOx From Simulated Marine Exhaust
Gas. Chemosphere, Volume 307,
p. 135830
Zhao, K., Sun, X.,
Wang, C., Song, X., Wang, F., Li, K., Ning, P., 2021a. Supported Catalysts for
Simultaneous Removal of SO2, NOx, and Hg0 from Industrial Exhaust
Gases: A Review. Chinese Chemical Letters, Volume
32(10), pp. 2963–2974
Zhao, L., Sun, Y.,
Chmielewski, A.G., Pawelec, A., Bu?ka, S., 2020. NO Oxidation with NaClO, NaClO2,
and NaClO3 Solution Using Electron Beam and A One-Stage Absorption
System. Plasma Chemistry and Plasma
Processing, Volume 40, pp. 433–447
Zhao, M., Xue, P.,
Liu, J., Liao, J., Guo, J., 2021b. A Review of Removing SO2 and NOx by Wet Scrubbing. Sustainable Energy Technologies and Assessments, Volume 47,
p. 101451
Zhao, Y., Guo,
T.-X., Chen, Z.-Y., Du, Y.-R., 2010. Simultaneous Removal of SO2 and
NO Using M/NaClO2 Complex Absorbent. Chemical Engineering Journal, Volume 160(1),
pp. 42–47
Zhitao, H., Yu, G., Shaolong, Y., Jingming, D., Xinxiang, P., Tian, L., Liguo, S., Zhijun, Y., Deping, S., Kaixuan, N., 2019. NO Removal from Simulated Diesel Engine Exhaust Gas by Cyclic Scrubbing Using NaClO2 Solution in A Rotating Packed Bed Reactor. Journal of Chemistry, Volume 2019, p. 3159524
Zhu, C., Ru, J., Gao, S., Li, C. 2023. The Simultaneous Removal of NOx and SO2 from Flue Gas by Direct Injection of Sorbents in Furnace of Waste Incinerator. Fuel, Volume 333, p. 126464
