Stability and Kinetic Study of Vitamin C containing Hydrogenated and Middle-Chain Triglyceride Coconut Oil-Based Double Emulsion
Corresponding email: lanny.sapei@staff.ubaya.ac.id
Published at : 24 Dec 2024
Volume : IJtech
Vol 15, No 6 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i6.6371
Sapei, L., Savitri, E., Jati, I.R.A., Indrawanto, R., Darsono, H.E., Anggraeni, Y., Sumampouw, C., 2024. Stability and Kinetic Study of Vitamin C containing Hydrogenated and Middle-Chain Triglyceride Coconut Oil-Based Double Emulsion. International Journal of Technology. Volume 15(6), pp. 1982-1993
| Lanny Sapei | 1. Department of Chemical Engineering, Faculty of Engineering, University of Surabaya, Jl Raya Kalirungkut, Surabaya 60293, East Java, Indonesia. 2. Center of Excellence for Food Products and Health S |
| Emma Savitri | Department of Chemical Engineering, Faculty of Engineering, University of Surabaya, Jl Raya Kalirungkut, Surabaya 60293, East Java, Indonesia |
| Ignasius Radix A.P. Jati | Department of Food Technology, Faculty of Agricultural Technology, Widya Mandala Surabaya Catholic University, Jl Dinoyo 42-44, Surabaya 60265, East Java, Indonesia |
| Rochmad Indrawanto | PT. Lautan Natural Krimerindo, Jl. Raya Mojosari – Pacet KM. 4, Pesanggrahan Ketidur - Kutorejo, Mojokerto 61383, East Java, Indonesia |
| Hillary Emmanuella Darsono | Department of Chemical Engineering, Faculty of Engineering, University of Surabaya, Jl Raya Kalirungkut, Surabaya 60293, East Java, Indonesia |
| Yenni Anggraeni | Department of Chemical Engineering, Faculty of Engineering, University of Surabaya, Jl Raya Kalirungkut, Surabaya 60293, East Java, Indonesia |
| Cindy Sumampouw | Department of Chemical Engineering, Faculty of Engineering, University of Surabaya, Jl Raya Kalirungkut, Surabaya 60293, East Java, Indonesia |
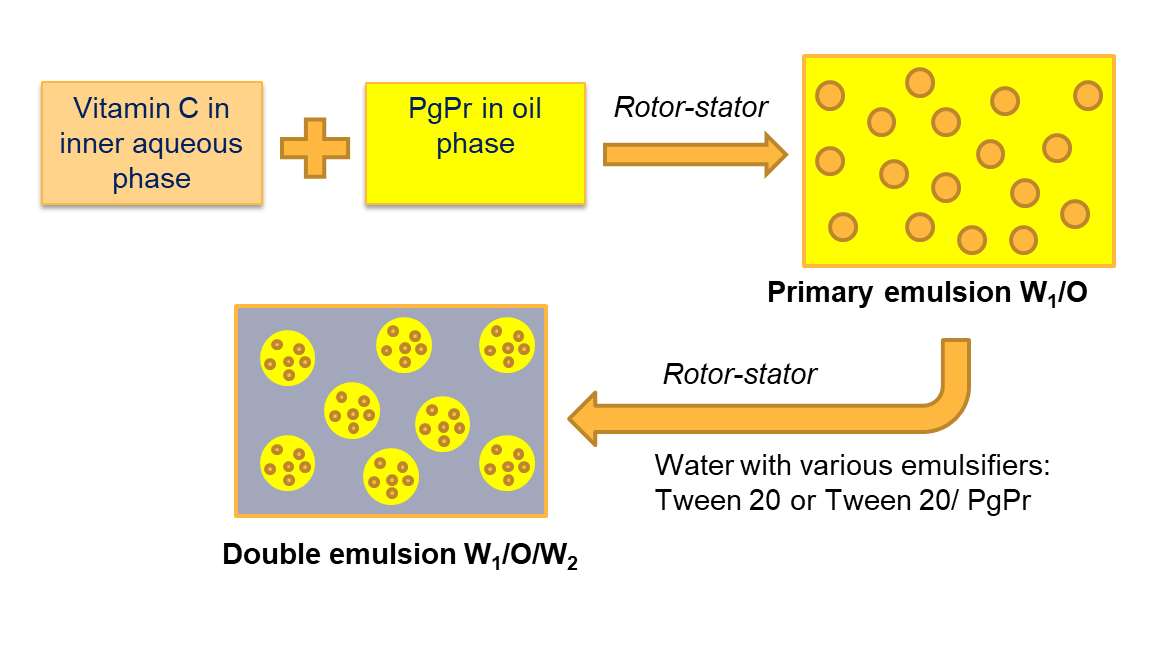
Vitamin C or ascorbic acid, is an organic compound that is highly required by human since it acts as antioxidant, help increase metabolism, and improves the immune system. Vitamin C is easily oxidized and damaged during storage due to several external factors such as light, metal, shear, etc. The encapsulation technique is able to improve the stability as well as the activity of Vitamin C in order to protect the bioactive compound from deleterious external factors. Coconut oil which is composed of about 50% lauric acid, has an antiviral property. This research aimed to obtain the stable water-in-oil-in-water (W1/O/W2) double emulsion using coconut oil upon the incorporation of Vitamin C. Hydrogenated (HCNO) and Medium-Chain Triglycerides (MCT) coconut oil was used as the oil phase, and various emulsifiers such as Tween-20 0.5%, Tween-20 1%, and Tween-20 0.5%/ PgPr 0.5% were applied to strengthen the outer interfacial layer. The double emulsion stability was monitored macroscopically, and the destabilization kinetics was studied using the zero and first-order kinetic models. It turned out that the HCNO-based double emulsion demonstrated higher stability compared to the MCT-based double emulsion. The lowest destabilization rate constants of 4.5 x 10-3 h-1 and 6.8 x 10-3 h-1 were obtained for HCNO and MCT-based double emulsions both stabilized with Tween-20 0.5%, respectively. The coconut oil-based double emulsion enriched with Vitamin C would be potentially developed for various functional food applications.
Double emulsion; HCNO; Kinetics; Tween-20; Vitamin C
Vitamin C is an organic compound that is mainly present in citrus fruit and green vegetable and belongs to essential nutrients required by the body to maintain the metabolism system. Vitamin C cannot be synthesized in the body; therefore, it must be regularly supplied to fulfill its daily requirement. Furthermore, Vitamin C is easily destroyed or oxidized during storage due to the presence of oxygen, light, metal, etc. (Carita et al., 2020; Sapei and Hwa, 2014). Encapsulation of Vitamin C would protect the stability and availability of its bioactive compound. Encapsulation offers some advantages, such as improving product stability, increasing material safety, assisting the handling of the bioactive compound, and facilitating the controlled release (Carita et al., 2020). The Recommended Dietary Allowances (RDAs) for Vitamin C are 75-90 mg/ day and 45-75 mg/day for adults (>19 years old) and children/ teenagers (? 18 years old) (Yan et al., 2021). A severe lack in Vitamin C causes scurvy due to suspended collagen formation, which formation has to be aided by Vitamin C (Carita et al., 2020; Abbas et al., 2012).
There are several techniques to encapsulate
Vitamin C, such as spray drying, spray cooling/ chilling, fluidized bed
coating, liposomes, and extrusion (Comunian et
al., 2022). Spray drying
has been used commercially because it is continued in operation and readily
scalable. Encapsulation of water-soluble materials derived from the extraction
process containing a polysaccharide matrix in solution has been widely carried
out using the spray drying technique (Matsunaga et
al., 2014). Other
techniques except for liposomes also, were also applied to ascorbic acid in
water-based solution. However, research has shown that ascorbic acid has a
longer shelf-life when encapsulated within a lipophilic matrix, such as
liposomes or in the form of an emulsion (Comunian
et al., 2022).
Additionally, the Vitamin C-containing emulsion can be spray dried to further
improve its stability. Emulsion is a mixture containing at least two immiscible
phases, mostly oil, and water, whereas one phase is dispersed in another phase
in the form of small droplets (McClements, 2016). Double emulsion is a more complex emulsion
system whereby two interfacial layers exist in the system, such as
oil-in-water-in-oil (O/W/O) or water-in-oil-in-water (W/O/W). Both aqueous and
oil dispersed phases of double emulsions could be potentially used as the
encapsulation vehicle of water based and oil based bioactive materials. The
presence of surfactants or emulsifiers was crucial in order to obtain stable
emulsions. Emulsifiers are active ingredients that are responsible for reducing
the surface tension between the immiscible phases, whereby the need of
renewable and environmentally friendly emulsifiers to replace synthetic ones
has been soaring (Qadariyah et al., 2022).
Coconut oil has been recognized as one of the
healthiest oil despite its high saturated fatty acids, up to 90% (Lima and Block, 2019; Boateng et al., 2016). However, more than 50% of their fatty acid
belongs to medium chain triglyceride (MCT), which is easily absorbed and
digested in the body. MCT consists of caproic acid, caprylic acid, and capric
acid. Since this oil is not accumulated in the body, it can be readily used as
a source of energy (Boateng et al., 2016). It also possesses good oxidative stability
and can extend the shelf life of end products. On the other hand, hydrogenated
coconut oil (HCNO) primarily consists of long-chain saturated fatty acids
resulting from the hydrogenation process. HCNO oil is highly stable and can
also prolong the shelf life of products while maintaining their solid texture,
even at high temperature.
Double emulsion has been attractive due to
the increasing demand for nutritious food. It has been fascinating for the food
industry due to its capability to encapsulate compounds, fabricate
polymersomes, and act as fat replacers or sweetness enhancers in different
foods (Loffredi and Alamprese, 2024; Kumar
et al., 2022; Mudric et al., 2019). Recent studies demonstrated the use of W1/O/W2
double emulsion as a vehicle for the co-delivery of both hydrophilic and
hydrophobic bioactive compounds such as curcumin/ catechin (Aditya et al., 2015), insulin/ quercetin (Han et al., 2022), and ascorbic acid/ tocopherol (Khan et al., 2023). Tania and
Kuswahyuning (2020) investigated
the stability of paraffin-based W/O/W double emulsion whereby Span-80 and
sodium carboxymethyl cellulose were varied to strengthen the inner and outer
interfacial layers. Ying et al.
(2021) studied the
encapsulation of soy peptide in W/O/W stabilized with PgPr and octenyl succinic
anhydride (OSA) starch/ maltodextrin as lipophilic and hydrophilic emulsifiers,
respectively. Sapei et
al. (2022a; 2022b; 2018) improved the stability of W1/O/W2
double emulsion using rice husk biosilica and chitosan-modified rice husk ash
on the outer interfacial layer. Molet-Rodriguez,
Martín-Belloso, and Salvia-Trujillo (2021) used lipid gelling agent along
with PgPr and sodium alginate/ Tween 80 to increase the stability of W/O/W
emulsion. Chevalier, Gomes, and Cunha
(2022) investigated
different hydrophilic emulsifiers such as sodium caseinate, whey protein
isolate, and Tween-80 on the stability of W1/O/W2 double
emulsion. Sodium caseinate produced the most stable emulsion by favoring the
formation of small droplets. Su et al.
(2022) studied the
encapsulation of amino acids in W1/O/W2 double emulsion
stabilized with PgPr and gum arabic/ xanthan gum as lipophilic and hydrophilic
emulsifiers. Snoussi et al. (2020) investigated the encapsulation of catechin
in W1/O/W2 double emulsion with the addition of chitosan/
sodium caseinate and lactose/ sodium caseinate in W1 and W2,
respectively. Encapsulation of Vitamin C
using W/O/W double emulsions as vehicles has been attempted by several
investigations (Sapei et al., 2023; Khan
et al., 2023; Dai et al., 2022; Hu et al., 2022; Kheynoor et
al., 2022; Fraj et al., 2019). PgPr was extensively used as lipophilic emulsifier to
form a highly stable W/O emulsion, whereas protein such as whey protein
isolate, sodium caseinate, and soluble protein was mainly added in the outer
continuous phase to stabilize the outer interfacial layer of W/O/W double
emulsions containing Vitamin C (Khan et al.,
2023; Dai et al., 2022; Hu et al., 2022; Fraj et al.,
2019). Moreover, some previous investigations combined
non-ionic emulsifiers such as PgPr and Tween to strengthen the inner and outer
W/O/W interfacial layers, respectively (Sapei et
al., 2023; Kheynoor et al., 2022). The use of non-ionic emulsifiers
is preferred over proteins in food applications, as proteins are inherently
complex and their behavior is highly sensitive to pH and ionic strength due to
the presence of both positive and negative charges in their structure.
However, there has been no publication yet investigating the effect of different concentration of external emulsifiers using Tween-20 and combined Tween-20/ PgPr on the stability of coconut oil-based W/O/W double emulsion containing Vitamin C in liquid form. There is also no study related to the double emulsion stability with time and the evaluation of its destabilization kinetics. The kinetics data could be useful for predicting the emulsion stability behavior in the long term. In this research, the double emulsion stability prepared with two different coconut oil derivatives, namely HCNO and MCT were compared. The product would be of importance, innovative functional food which helps boost the immunity and maintain overall health.
2.1. Materials
Hydrogenated coconut oil
(HCNO) comprised of 0.5% caproic acid, 5% caprylic acid, 6% capric acid, 45%
lauric acid, 20% myristic acid, 11% palmitic acid, 12% stearic acid, and trace
amounts of oleic, linoleic, and linoleic acids; middle chain triglycerides
(MCT) comprised of 60% caprylic acid (C8) and 40% capric acid (C10); Vitamin C/
ascorbic acid (Sigma-Aldrich, UK); Tween-20 (Merck, Germany), Polyglycerol
Polyricinoleate/ PgPr 4120 (Palsgaard, Denmark), and demineralized water.
2.2. Preparation
of primary emulsion/ water-in-oil (W1/O) emulsion
The
internal aqueous phase (W1) was prepared by adding 25% (w/w) of
ascorbic acid into the remaining water and then mixed at 100 rpm for 3 minutes
using a magnetic stirrer until homogeneous. The oil phase was prepared by
adding 6% (w/w) PgPr into the oil phase. The mixture was stirred using a
magnetic stirrer at 800 rpm for 7 minutes. Afterward, the aqueous phase (W1)
with a fraction of 30% (w/w) was dispersed in the oil phase and homogenized
using a rotor-stator (IKA T25 digital ULTRATURRAX, Germany) at 20,000 rpm for 6
minutes. To prepare the primary emulsion using HCNO, the aqueous phase and oil
phase were heated to an elevated temperature of 60°C. This was necessary
because HCNO has a higher melting point compared to MCT oil.
2.3. Preparation of double emulsion/ water-in-oil-in-water
(W1/O/W2) emulsion
The
external aqueous phase (W2) was prepared by mixing emulsifiers at
certain amounts, as depicted in Table 1. The concentrations of emulsifiers were
in % (w/w) relative to the outer aqueous phase (W2). The emulsifiers
were added to water and mixed using a magnetic stirrer at 300 rpm for 7
minutes. The fractions of primary emulsion (W1/O) dispersed into the
outer aqueous phase were 30% (w/w) and 40% (w/w) for HCNO and MCT oil,
respectively. The mixtures were homogenized using a rotor-stator (IKA T25
digital ULTRATURRAX, Germany) at 8,000 rpm for 3 minutes until homogeneous. The
resulting double emulsion was poured into the vial (inner diameter = 25 mm and
height = 95 mm) at room temperature (~28-30?C). The stability of each double emulsion was
continuously monitored for up to 4 days.
Table 1 Variation of emulsifiers used for the secondary emulsion
|
Emulsion |
Oil |
Emulsifiers |
|
H1 |
|
PgPr/Tween-20 (0.5%/0.5%) |
|
H2 |
HCNO |
Tween-20 (0.5%) |
|
H3 |
|
Tween-20 (1%) |
|
M1 |
|
PgPr/Tween-20 (0.5%/0.5%) |
|
M2 |
MCT |
Tween-20 (0.5%) |
|
M3 |
|
Tween-20 (1%) |
2.4. Determination of the stability of double
emulsion and its destabilization kinetics
The
stability of the double emulsion was evaluated macroscopically by measuring the
ratio of the emulsion height after a certain time to its initial height
immediately after secondary emulsification. The monitoring was conducted until
4 days, whereby a significant phase separation between the emulsion and aqueous
phase was distinctly seen. Liquid with a milky and homogeneous appearance
demonstrated a stable emulsion without any emulsion instabilities such as
flocculation, sedimentation, or creaming. The emulsion stability (%S) was
calculated according to equation (1).
whereby ht = the height of the double
emulsion at a particular time and h0 = the height of the initial
double emulsion right after the preparation.
Kinetic
models of zero order and first order, according to equations (2) and (3),
respectively, were used to study the destabilization kinetics of the double
emulsion quantitatively. The coefficients of determination (R2) of
both models were also determined. The experimental data used for the kinetic
study was within the range of 0 to 10 hours and 0 to 24 hours for HCNO and
MCT-based double emulsion, respectively.
Whereby S is the percentage of emulsion
stability at a certain time (t) in hours; S0 is the initial
stability of the double emulsion in percentage; k0 and k1
are the destabilization rate constants derived from the zero-order (%
stability/hour) and first-order model (per hour), respectively.
3.1. Stability of double emulsion prepared using HCNO and MCT coconut oil
All double emulsions
prepared according to Table 1 were completely stable right after the
preparation. However, the stability of double emulsions decreased with time, as
seen in Figure 1. The double emulsions stability was monitored for up to 4
days. Interestingly, the double emulsion prepared
with HCNO demonstrated a much-delayed destabilization at the beginning and
tended to be leveled off after approximately 24 hours with much higher emulsion
stability percentages. In contrast, the double emulsion prepared with MCT
demonstrated a continuous decrease in stability with time.
Figure 1 Stability of the double emulsions with time monitored
until 4 days. (A) HCNO; (B) MCT
Furthermore, the macroscopic stability of HCNO and MCT-based double
emulsion after 4-day storage can be seen in Figure 2.
The percentages of double emulsion stability prepared using HCNO and MCT
observed on days 0, 1, and 4 were depicted in Table 2 and Table 3,
respectively.
Figure 2 Macroscopic
stability of double emulsions prepared using various emulsifiers’ types and
concentrations after 4-day storage. A) HCNO; B) MCT
Table 2 Stability of double
emulsions prepared using HCNO
|
Time
(day) |
Stability
(%) | ||
|
Emulsion
H1 |
Emulsion
H2 |
Emulsion
H3 | |
|
0 |
100 |
100 |
100 |
|
1 |
86.11 |
95.83 |
90 |
|
4 |
86.11 |
95.83 |
90 |
Table 3 Stability of double
emulsions prepared using MCT
|
Time
(day) |
Stability
(%) | ||
|
Emulsion
M1 |
Emulsion
M2 |
Emulsion
M3 | |
|
0 |
100 |
100 |
100 |
|
1 |
84.72 |
85.71 |
74.65 |
|
4 |
70.83 |
67.53 |
67.53 |
As seen in Figure 2,
it was obvious that double emulsions were subjected to instability indicated by
the separation of the aqueous phase from the double emulsion phase. The inner
aqueous phase droplets dispersed in the oil globules could agglomerate and
coalesce and finally diffuse into the external aqueous phase (Hu et al., 2022; Leister and Karbstein, 2020; Schuch et al., 2013). This
was strongly influenced by differences in osmotic pressure and Laplace pressure
between the aqueous phases (Heidari et al., 2022). The increased volume of the external aqueous phase (W2) would,
in turn, trigger the phase separation between the aqueous phase and emulsion
phase due to the difference in densities. The water-rich phase underwent
sedimentation out from the whole emulsion due to its higher density. The
partial detachment of the hydrophilic emulsifiers initially present at the
interface between oil and outer aqueous phase into the outer aqueous phase with
time could be another factor of this instability. This would lead to
flocculation and, thus, coalescences of oil globules in order to minimize their
surface tension. However, the creaming of primary emulsions was hardly seen in
all double emulsions. Furthermore, the separation of the external aqueous phase
was remarkably seen in double emulsions prepared using MCT compared to those
prepared using HCNO, albeit a much lower W1/O fraction was used in
MCT-based double emulsion. The W1/O fraction used for the MCT-based
double emulsion was 40%, while it was only 30% when HCNO was used. The increase
in the dispersed phase fraction would definitely increase the stability of
double emulsions due to an increase in the overall viscosity. However, the
MCT-based double emulsions still demonstrated much lower stability compared to
the HCNO-based double emulsions. The fatty acid profiles of both oils did make
this difference. HCNO is composed of entirely saturated fatty acids due to
hydrogenation ranging from short to long fatty acid chains, whereas MCT is
comprised of middle chain fatty acids of C8 and C10,
imparting a much higher melting point to HCNO with a melting point of about 31 -
33?C. On the other hand, the melting point of MCT is relatively low,
about 5 - 7?C. The HCNO oil tended to turn into gel or solid upon
storage reducing the destabilization rate of the double emulsion. The formed
crystal network reduced the permeability of the oil phase due to increased
tortuosity of the diffusive path between inner and outer aqueous phases (Nelis et al., 2019). The solidified HCNO also increases the
viscosity of the dispersed phase, resulting in higher viscosity of the double
emulsion and thereby increasing its stability. Vice versa, the dispersed MCT
oil remained liquid upon storage and thus facilitating the diffusion of the
inner aqueous phase into the outer aqueous phase. Besides that, the oil
globules were prone to flocculation and coalescence, leading to a higher rate
of double emulsion destabilization.
Based on the
microscopic structures of double emulsions prepared using HCNO and MCT, as
depicted in Figure 3, it was confirmed that the double emulsion prepared using
HCNO seemed to be very thick in contrast to the liquid MCT-based double
emulsion. The oil globule sizes in HCNO-based double emulsion seemed bigger
than those in MCT-based double emulsion. This again confirmed the higher
release rate of the inner aqueous phase into the outer aqueous phase reducing
the MCT oil globule sizes and inferring higher instability of MCT-based double
emulsion (Schuch
et al., 2013). This instability could be
dominated by the coalescence of inner droplets followed by the coalescence of
both inner and outer aqueous phase (Leister and Karbstein, 2020). However, the double emulsion formation was still partially retained after
4 days storage demonstrated by the presence of inner aqueous phase droplets
inside the oil globules.
Figure 3 Microscopic structure of double emulsions after 4 days storage. (A)
HCNO; (B) MCT
The
double emulsion with HCNO demonstrated quite high stability of above 85% after
4 days, whereas the double emulsion with MCT retained about 70% stability after
4 days (Table 2 vs. Table 3). This was in line with the previous investigation
whereby the stability of MCT-based W/O/W emulsions were much lower than those
prepared using HCNO upon the addition of thickeners such as
isomalto-oligosaccharides (IMO) and inulin (Sapei et al.,
2023). The stability of these double emulsions prepared with HCNO was a bit
higher compared to the stability of Pickering palm oil-based W/O/W emulsions
stabilized with rice husk silica/ chitosan particles (Sapei et al., 2022b). However, the stability of Pickering palm oil-based W/O/W double
emulsions stabilized using Tween 20/ rice husk silica demonstrated a much
superior stability of more than 96% after 4 days (Sapei
et al., 2018). Moreover, the
double emulsion (H2) prepared using Tween-20 0.5% showed the highest stability.
In the case of MCT-based emulsion, sample M1 which was prepared using mixed
emulsifiers PgPr/ Tween-20 (0,5%/0,5%), showed the highest stability In order
to strengthen the interfacial layer between the oil and external aqueous phase
and suppress the desorption rate of emulsifiers from the interface, it is
generally desirable to use emulsifier mixtures with low and high HLB values,
such as PgPr (HLB = 4) and Tween-20 (HLB = 16.7). However, the use of a single
emulsifier in HCNO-based double emulsion seemed to be more efficient. The use
of Tween-20 only could be superior to the use of mixed PgPr/ Tween-20 due to
the interaction or competition amongst the emulsifiers adsorbed at the inner
interfacial and outer interfacial layers (Schuch et al., 2013). Previous investigation, on the contrary,
showed the lowest stability on palm oil-based W/O/W emulsion stabilized with
Tween-20 only, which demonstrated synergism when being combined with rice husk
silica (Sapei et al., 2018). This implied a different mechanism between
mixed polymeric emulsifiers and polymeric emulsifiers/ particles in
strengthening the interfacial layers, thus stabilizing the entire emulsion. The
polymeric emulsifier was easily adsorbed but easily desorbed from the
interfaces, whereas particles could form a multilayer barrier once adsorbed at
the interfaces (Zheng et al., 2022; Sapei et al.,
2018). Creaming has been a common
instability phenomenon of oil in water emulsion whereby oil-rich phase moves
upward due to its lower density (Sapei et al.,
2022b; McClements, 2016). In all resulting
double emulsions, the emulsions part appeared on the upper layer, with an
increasing portion of the aqueous layer at the bottom during the double
emulsion destabilization with time. One of the major causes of the distinction
of these two layers between the double emulsion layer and water-rich layer was
the release of the inner aqueous phase into the outer aqueous phase inducing
water-rich phase sedimentation. Furthermore, increasing Tween 20 concentrations
up to 1% tended to deteriorate the emulsion stability. The excess hydrophilic
emulsifier would be, in turn, dispersed in the outer aqueous phase instead of
being adsorbed at the interfacial layer attracting the emulsifiers attached to
the interfacial layers into the outer aqueous phase, thus promoting the
destabilization of the double emulsion. Moreover, the excess of Tween 20 could
possibly modify the antioxidant efficacy by improving the antioxidant oxidative
stability (Yamamoto and Misawa, 2018) and modifying the crystallization as well as
melting behavior of coconut oil (Maruyama et al.,
2014).
3.2. Kinetics of
double emulsion destabilization according to zero and first-order models
It has been pronounced that the stability of HCNO-based
double emulsion was higher than the MCT-based double emulsion with time. The
short-term W/O/W double emulsions stability would be furthermore quantitatively
proved through the determination of destabilization rate constants according to
zero and first-order kinetic models, as could be seen in Tables 4 and 5. The first-order kinetic model appeared to be
more suitable in determining the destabilization rate constants compared to the
zero-order based on the R2 values. The lowest destabilization rate
constant of about 4.5 x 10-3 h-1 was obtained for
HCNO-based double emulsion stabilized with Tween-20 0.5% (emulsion H2). The
highest destabilization rate was observed when mixed emulsifiers Tween 20 0.5%/
PgPr 0.5% were used for HCNO-based double emulsion (emulsion H1). The
destabilization rate constant became double when Tween-20 used was doubled,
inferring that the excessive use of hydrophilic emulsifier did not increase the
stability of the double emulsion. This short-term destabilization kinetic study
within the first 24 hours was in line with the long-term stability data shown
in Table 2.
In the case of double emulsion prepared with
MCT, the lowest destabilization constant of about 6.8 x 10-3 h-1
was obtained for that stabilized with Tween 20 0.5% (emulsion M2). Emulsion M1,
which was stabilized with Tween-20 0.5%/ PgPr 0.5%, demonstrated a slightly
higher destabilization rate constant than that of M2 even though the M1 sample
appeared to be the most stable in the long-term, i.e., after 4 days as seen in
Table 2 and Figure 3. This again implied that the use of mixed polymeric
emulsifiers of different HLB values did not significantly improve the stability
of the double emulsion due to the complexity of the presence of two interfacial
layers whereby the diffusion and interaction of emulsifiers between the
interfaces could possibly occur. Similarly with the HCNO-based double emulsion,
the use of doubled Tween-20 of 1% also resulted in a doubled destabilization
rate constant for the MCT-based double emulsion (M3), which seemed to be
consistent.
Table 4 Destabilization rate
constants and R2 values of HCNO-based double emulsions according to
zero and first-order kinetic models
|
|
Order 0 |
Order 1 | ||
|
ko (%stability/h) |
R2 |
k1 (h-1) |
R2 | |
|
Emulsion H1 |
1.0623 |
0.892 |
0.0111 |
0.895 |
|
Emulsion H2 |
0.4387 |
0.807 |
0.0045 |
0.809 |
|
Emulsion H3 |
0.8784 |
0.931 |
0.0091 |
0.932 |
Table 5 Destabilization rate
constants and R2 values of MCT-based double emulsions according to
zero and first-order kinetic models
|
|
|
Order 0 |
Order 1 | |||
|
|
ko (%stability/h) |
R2 |
k1 (h-1) |
R2 |
| |
|
Emulsion M1 |
|
0.7041 |
0.925 |
0.0076 |
0.918 | |
|
Emulsion M2 |
|
0.6386 |
0.965 |
0.0068 |
0.963 | |
|
Emulsion M3 |
|
1.2066 |
0.961 |
0.0136 |
0.957 | |
The
stabilization of double emulsion was more difficult to be achieved not only due
to its inherent thermodynamically unstable, but also due to other tremendous
complexity related to the double emulsion. The presence of inner and outer
interfaces between oil and the aqueous phase of which adsorbed emulsifiers
could be easily altered when there were some changes such as temperature, pH,
shear, viscosity, the presence of other constituents, etc. (Sapei et al., 2022b; 2018; Schuch et
al., 2013). The temperature changes would influence the emulsion stability. The
desorption of emulsifiers from the interfaces was faster, leading to a faster
rate of flocculation or coalescences of the oil globule and, finally, the
increasing rate of phase separation (Schuch et al., 2013). The density differences between the oil phase
and aqueous phase and the viscosity of double emulsion would also affect the
creaming properties. HCNO tended to be less creaming than MCT due to its higher
density than MCT, besides its higher viscosity. Furthermore, modulation of the
W1 phase by the addition of NaCl or gelling agent could improve the
emulsion stability as well as the efficiency of Vitamin C encapsulation (Chevalier, Gomes, and Cunha, 2022; Hu et al., 2022). Furthermore, the W1/O ratio, bioactive concentration in the
inner W1, emulsification process parameters and operation condition
also definitely influence the stability of the resulting double emulsions (Kumar et al., 2022; Ying et al., 2021). Further investigations are necessary to elucidate the proper mechanisms
of the destabilization process of double emulsions and how to optimize the
process to achieve double emulsions with high kinetic stability suitable for
various applications in food industries.
Double emulsions prepared with different
coconut oil types, namely hydrogenated (HCNO) and MCT, were developed with the
incorporation of Vitamin C. The inner aqueous phase was potentially developed
for functional food products such as low-calorie creamers with high nutrients.
Various emulsifiers of combined Tween 20/ PgPr were used to strengthen the
outer interfacial layer. It turned out that the double emulsions stabilized
with Tween-20 0.5% seemed to sufficiently stabilize the double emulsion, both
derived from HCNO and MCT. The HCNO-based double emulsions demonstrated
remarkable long-term stability of more than 85% owing to their higher melting
point compared to MCT. The lowest destabilization rate constants according to
the first-order model kinetics were 4.5 x 10-3 h-1 and 6.8 x 10-3 h-1 for HCNO
and MCT-based double emulsions stabilized with Tween-20 0.5%, respectively.
Both HCNO and MCT-based double emulsions have been promising to be developed
due to the superior health benefits of coconut oil, which exceed those of other
oils besides its great potential as an encapsulation vehicle for vitamins and
other antioxidant compounds in the inner aqueous phase. However, designing a
double emulsion with high kinetic stability has been a great challenge and needs
further investigation to unravel the complex factors affecting its stability.
We thank PT. Lautan Natural Krimerindo for
providing the hydrogenated and MCT coconut oil. We wish to thank Ms. Dyah Ayu
Ambarsari for the technical assistance during the experiments. The research was
funded by Indonesia Endowment Fund for Education (Lembaga Pengelola Dana
Pendidikan/ LPDP) in collaboration with Directorate General of Higher
Education, Research, and Technology, Ministry of Education, Culture, Research
and Technology of the Republic of Indonesia under the research grant scheme of
“Hibah Riset Keilmuan” 2022 (Contract number: 159/E4.1/AK.04.RA/2021).
Abbas, S., da Wei, C., Hayat, K., Xiaoming, Z., 2012. Ascorbic Acid: Microencapsulation Techniques and Trends-A Review. Food Reviews International, Volume 28 (4), pp. 343–374. https://doi.org/10.1080/87559129.2011.635390
Aditya, N.P., Aditya, S., Yang, H., Kim, H.W., Park, S.O., Ko, S., 2015. Co-delivery of Hydrophobic Curcumin and Hydrophilic Catechin by a Water-In-Oil-In-Water Double Emulsion. Food Chemistry, Volume 173, pp. 7–13.
https://doi.org/10.1016/j.foodchem.2014.09.131
Boateng, L., Ansong, R., Owusu, W., Steiner-Asiedu, M., 2016. Coconut Oil and Palm Oil’s Role in Nutrition, Health and National Development: A Review. Ghana Medical Journal, Volume 50 (3), pp. 189–196. http://dx.doi.org/10.4314/gmj.v50i3.11
Caritá, A.C., Fonseca-Santos, B., Shultz, J. D., Michniak-Kohn, B., Chorilli, M., Leonardi, G.R., 2020. Vitamin C: One Compound, Several Uses. Advances for Delivery, Efficiency and Stability. Nanomedicine: Nanotechnology, Biology and Medicine, Volume 24, p. 102117. https://doi.org/10.1016/j.nano.2019.102117
Chevalier, R.C., Gomes, A., Cunha, R.L., 2022. Role of Aqueous Phase Composition and Hydrophilic Emulsifier Type on The Stability of W/O/W Emulsions. Food Research International, Volume 156, p. 111123. https://doi.org/10.1016/j.foodres.2022.111123
Comunian, T., Babazadeh, A., Rehman, A., Shaddel, R., Akbari-Alavijeh, S., Boostani, S., Jafari, S.M., 2020. Protection and Controlled Release of Vitamin C by Different Micro/Nanocarriers. Critical Reviews in Food Science and Nutrition, Volume 62 (12), pp. 3301–3322. https://doi.org/10.1080/10408398.2020.1865258
Dai, Y., Lu, X., Li, R., Cao, Y., Zhou, W., Li, J., Zheng, B., 2022. Fabrication and Characterization of W/O/W Emulgels by Sipunculus nudus Salt-Soluble Proteins: Co-Encapsulation of Vitamin C and ?-Carotene. Foods, Volume 11(18), p. 2720.
https://doi.org/10.3390/foods11182720
Fraj, J.L., Petrovi?, L.B., Milinkovi? Budin?i?, J.R., Katona, J.M., Bu?ko, S., Spasojevi?, L.M., 2019. Properties of Double W/O/W Emulsions Containing Vitamin C and E Stabilized with a Gelatin/Sodium Caseinate Complex. Journal of the Serbian Chemical Society, Volume 84 (12), pp. 1427–1438. https://doi.org/10.2298/JSC190515075F
Han, L., Lu, K., Zhou, S., Qi, B., Li, Y., 2022. Co-delivery of Insulin and Quercetin in W/O/W Double Emulsions Stabilized by Different Hydrophilic Emulsifiers. Food Chemistry, Volume 369, p. 130918. https://doi.org/10.1016/j.foodchem.2021.130918
Heidari, F., Jafari, S.M., Ziaiifar, A.M., Malekjani, N., 2022. Stability and Release Mechanisms of Double Emulsions Loaded with Bioactive Compounds; a Critical Review. Advances in Colloid and Interface Science, Volume 299, p.102567.
https://doi.org/10.1016/j.cis.2021.102567
Hu, S., Zhuang, D., Gang, Z., Xiao, W., Yanna, Z., Fan, Z., Min, L., Jun, H., Wang, Z., 2022. Fabrication and Spray-Drying Microencapsulation of Vitamin C-loaded W1/O/W2 Emulsions: Influence of Gel Polymers in the Internal Water Phase on Encapsulation Efficiency, Reconstituted Stability, and Controlled Release Properties. LWT, Volume 170, p. 114113. https://doi.org/10.1016/j.lwt.2022.114113
Khan, M.A., Bao, H., Cheng, H., Feng, S., Wang, Y., Liang, L., 2023. Fabrication of Whey-Protein-Stabilized G/O/W Emulsion for the Encapsulation and Retention of l-ascorbic acid and ?-tocopherol. Journal of Food Engineering, Volume 341, p. 111335.
https://doi.org/10.1016/j.jfoodeng.2022.111335
Kheynoor, N., Hosseini, S.M.H., Yousefi, G.H., Gahruie, H.H., Mesbahi, G.R., 2018. Encapsulation of Vitamin C in a Rebaudioside-Sweetened Model Beverage Using Water in Oil in Water Double Emulsions. LWT, Volume 96, pp. 419–425.
https://doi.org/10.1016/j.lwt.2018.05.066
Kumar, A., Kaur, R., Kumar, V., Kumar, S., Gehlot, R., Aggarwal, P., 2022. New Insights into Water-in-Oil-in-Water (W/O/W) Double Emulsions: Properties, Fabrication, Instability Mechanism, and Food Applications. Trends in Food Science & Technology, Volume 128, pp. 22–37. https://doi.org/10.1016/j.tifs.2022.07.016
Leister, N., Karbstein, H.P., 2020. Evaluating the Stability of Double Emulsions—A Review of the Measurement Techniques for the Systematic Investigation of Instability Mechanisms. Colloids and Interfaces, Volume 4(1), p. 8.
https://doi.org/10.3390/colloids4010008
Lima, R.D.S., Block, J.M., 2019. Coconut Oil: What Do We Really Know About it so Far? Food Quality and Safety, Volume 3 (2), pp. 61–72. https://doi.org/10.1093/fqsafe/fyz004
Loffredi, E., Alamprese, C., 2024. Digestion Fate and Food Applications of Emulsions as Delivery Systems for Bioactive Compounds: Challenges and Perspectives. Food Reviews International, Volume 40(7), pp. 2103–2127.
https://doi.org/10.1080/87559129.2023.2249098
Maruyama, J.M., Soares, F.A.S.D.M., D’Agostinho, N.R., Gonc?alves, M.I.A., Gioielli, L.A. and Da Silva, R.C., 2014. Effects of Emulsifier Addition on the Crystallization and Melting Behavior of Palm Olein and Coconut Oil. Journal of Agricultural and Food Chemistry, Volume 62 (10), pp. 2253–2263. https://doi.org/10.1021/jf405221n
Matsunaga, Y., Machmudah, S., Wahyudiono, Kanda, H., Sasaki, M., Goto, M., 2014. Subcritical Water Extraction and Direct Formation of Microparticulate Polysaccharide Powders from Ganoderma Lucidum. International Journal of Technology, Volume 5(1), pp. 40–50. https://doi.org/10.14716/ijtech.v5i1.152
McClements, D.J., 2016. Food Emulsions. Boca Raton, London: CRC Press, Taylor & Francis Group. https://doi.org/10.1201/b18868
Molet-Rodríguez, A., Martín-Belloso, O., Salvia-Trujillo, L., 2021. Formation and Stabilization of W1/O/W2 Emulsions with Gelled Lipid Phases. Molecules, Volume 26(2), p. 312. https://doi.org/10.3390/molecules26020312
Mudric, J., Šavikin, K., Ibric, S., Duriš, J., 2019. Double Emulsions (W/O/W Emulsions): Encapsulation of Plant Bioactives. Lekovite sirovine, Volume 39, pp. 76–83. https://doi.org/10.5937/leksir1939076M
Nelis, V., Declerck, A., Vermeir, L., Balcaen, M., Dewettinck, K. and Van der Meeren, P., 2019. Fat Crystals: A Tool to Inhibit Molecular Transport in W/O/W Double Emulsions. Magnetic Resonance in Chemistry, Volume 57(9), pp. 707–718. https://doi.org/10.1002/mrc.4840
Qadariyah, L., Sahila, S., Sirait, C., Purba, C.P.E., Bhuana, D.S., Mahfud, M., 2022. Surfactant Production of Methyl Ester Sulfonate from Virgin Coconut Oil using Aluminum Oxide with Microwave Assistance. International Journal of Technology, Volume 13(2), pp. 378–388. https://doi.org/10.14716/ijtech.v13i2.4449
Sapei, L., Adiarto, T., Handomo, R., Chandra, S.H., 2018. Effect of pH on the Stability of W1/O/W2 Double Emulsion Stabilized by a Combination of Biosilica and Tween-20. In: MATEC Web of Conferences, Volume 215, p. 01028.
https://doi.org/10.1051/matecconf/201821501028
Sapei, L., Agustriyanto, R., Fitriani, E.W., Levy, Z., Sumampouw, C., 2022a. Rice Husk Ash for the Stabilization of the Outer Interfacial Layer of W/O/W Double Emulsion. In: AIP Conference Proceedings, Volume 2470(1), p. 040004.
https://doi.org/10.1063/5.0080285
Sapei, L., Agustriyanto, R., Fitriani, E.W., Levy, Z., Sumampouw, C., 2022b. Enhancement of the Stability of W/O/W Double Emulsion by Chitosan Modified Rice Husk Silica. International Journal of Technology, Volume 13(3), pp. 584–595. https://doi.org/10.14716/ijtech.v13i3.4752
Sapei, L., Hwa, L., 2014. Study on the Kinetics of Vitamin C Degradation in Fresh Strawberry Juices. Procedia Chemistry, Volume 9, pp. 62–68. https://doi.org/10.1016/j.proche.2014.05.008
Sapei, L., Savitri, E., Darsono, H.E. and Anggraeni, Y., 2023. Influence of Inulin and Isomalto-oligosaccharides as Thickener on the Stability of Vitamin C Containing W?/O/W? Double Emulsion. In: 4th International Conference on Informatics, Technology and Engineering 2023 (InCITE 2023), pp. 69–78. https://doi.org/10.2991/978-94-6463-288-0_8
Schuch, A., Deiters, P., Henne, J., Köhler, K., Schuchmann, H.P., 2013. Production of W/O/W (water-in-oil-in-water) Multiple Emulsions: Droplet Breakup and Release of Water. Journal of Colloid and Interface Science, Volume 402, pp. 157–164. https://doi.org/10.1016/j.jcis.2013.03.066
Snoussi, A., Chouaibi, M., Bouzouita, N., Hamdi, S., 2020. Microencapsulation of Catechin Using Water-in-Oil-in-Water (W1/O/W2) Double Emulsions: Study of Release Kinetics, Rheological, and Thermodynamic Properties. Journal of Molecular Liquids, Volume 311, p. 113304. https://doi.org/10.1016/j.molliq.2020.113304
Su, Y., Sun, Y., McClements, D.J., Chang, C., Li, J., Xiong, W., Sun, Y., Cai, Y., Gu, L., Yang, Y., 2022. Encapsulation of Amino Acids in Water-In-Oil-In-Water Emulsions Stabilized by Gum Arabic and Xanthan Gum. International Journal of Biological Macromolecules, Volume 220, pp. 1493–1500. https://doi.org/10.1016/j.ijbiomac.2022.09.150
Tania, D., Kuswahyuning, R., 2020. Water-in-Oil-in-Water (W/O/W) Double Emulsion Formulations using Variation Concentration of Carboxymethyl Cellulose Sodium. Journal of Food and Pharmaceutical Sciences, Volume 8 (20), pp. 284–293.https://doi.org/10.22146/jfps.739
Yamamoto, Y., Misawa, R., 2018. Effect of Emulsifier Concentration on the Oxidation of an O/W Emulsion Prepared from Canola Oil. Food and Nutrition Sciences, Volume 9(6), pp. 683–692. https://doi.org/10.4236/fns.2018.96052
Yan, B., Davachi, S.M., Ravanfar, R., Dadmohammadi, Y., Deisenroth, T.W., Van Pho, T., Odorisio, P.A., Darji, R.H., Abbaspourrad, A., 2021. Improvement of Vitamin C Stability in Vitamin Gummies by Encapsulation in Casein Gel. Food Hydrocolloids, Volume 113, p. 106414. https://doi.org/10.1016/j.foodhyd.2020.106414
Ying, X., Gao, J., Lu, J., Ma, C., Lv, J., Adhikari, B., Wang, B., 2021. Preparation and Drying of Water-in-Oil-in-Water (W/O/W) Double Emulsion to Encapsulate Soy Peptides. Food Research International, Volume 141, p. 110148.
https://doi.org/10.1016/j.foodres.2021.110148
Zheng, L., Cheng, X., Cao, L., Chen, Z., Huang, Q., Song, B., 2022. Enhancing Pesticide Droplet Deposition through O/W Pickering Emulsion: Synergistic Stabilization by Flower-Like ZnO Particles and Polymer Emulsifier. Chemical Engineering Journal, Volume 434, p. 134761. https://doi.org/10.1016/j.cej.2022.134761
