Study of Solid–Liquid Extraction Kinetics of Oil from Dried Avocado (Persea Americana) Flesh Using Hexane as A Solvent
Corresponding email: satriana@unsyiah.ac.id
Published at : 28 Jul 2023
Volume : IJtech
Vol 14, No 5 (2023)
DOI : https://doi.org/10.14716/ijtech.v14i5.6370
Satriana, Maulida, A., Qardhawi, R., Lubis, Y.M., Moulana, R., Mustapha, W.A.W., Arpi, N., 2023. Study of Solid–Liquid Extraction Kinetics of Oil from Dried Avocado (Persea Americana) Flesh Using Hexane as A Solvent. International Journal of Technology. Volume 14(5), pp. 982-992
| Satriana | 1. Department of Agricultural Product Technology, Universitas Syiah Kuala, Banda Aceh 23111, Indonesia, 2. Halal Research Center, Universitas Syiah Kuala, Banda Aceh 23111, Indonesia |
| Amrina Maulida | Natural and Energy Resource Laboratory, Department of Chemical Engineering, Universitas Syiah Kuala, Banda Aceh 23111, Indonesia |
| Rizky Qardhawi | Department of Agricultural Product Technology, Universitas Syiah Kuala, Banda Aceh 23111, Indonesia |
| Yanti Meldasari Lubis | Department of Agricultural Product Technology, Universitas Syiah Kuala, Banda Aceh 23111, Indonesia |
| Ryan Moulana | Department of Agricultural Product Technology, Universitas Syiah Kuala, Banda Aceh 23111, Indonesia |
| Wan Aida Wan Mustapha | Department of Food Sciences, Faculty of Science and Technology, Universiti Kebangsaan Malaysia 43600 UKM Bangi, Selangor DE, Malaysia |
| Normalina Arpi | Department of Agricultural Product Technology, Universitas Syiah Kuala, Banda Aceh 23111, Indonesia |
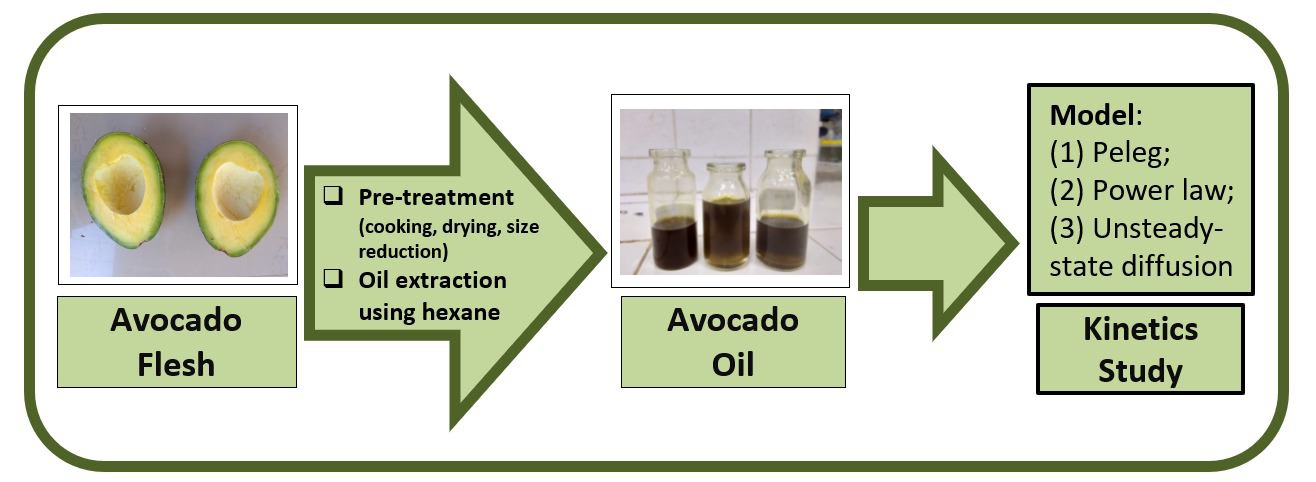
In the present study, solid–liquid extraction of
dried avocado flesh was investigated using hexane as a solvent for three
pre-treatment methods (uncooked, cooked with CaCO3, and cooked with
water) and two material sizes (8 mm × 5 mm × 5 mm and 1.19 mm). Based on the
highest yield of avocado oil obtained, the kinetics of oil extraction for a
material size of
1.19 mm for the various pre-treatment methods was studied.
Two stages, namely rapid oil extraction and slow oil extraction, were observed.
Three kinetics models (Peleg, power law, and unsteady-state diffusion model)
were used to describe the extraction of avocado oil. At conditions with the
highest oil yield, the Arrhenius equation was used to calculate the activation
energy at three different temperatures (25, 40, and 50°C). Based on the R2 value obtained, the
unsteady-state diffusion model (R2 = 0.6236–0.8752) is most
suitable to describe the avocado oil extraction process. A positive value of
the activation energy (47.51 kJ/mol) confirmed that the avocado oil extraction
process is endothermic.
Avocado oil; Kinetics model; Pre-treatment; Solid–liquid extraction
Avocado (Persea americana) belongs to the
Lauraceae family and originated in Mexico. It is widespread and farmed around
the world in tropical and subtropical climates. Avocados are edible, and their
oil yield is significantly higher than that of other edible oil crops, including
rapeseed, sunflower, sesame, and jatropha (Satriana
et al., 2019). Avocados have the potential to produce valuable
and useful products, one of which is avocado oil. Avocado oil finds common use
in both food and non-food industries, such as cooking oil and cosmetic
formulations. It contains a high level of monounsaturated and polyunsaturated
fatty acids, making it a natural ingredient suitable for various applications (Ge et al., 2021). Avocado oil contains a
mixture of compounds, including antioxidants, vitamins, and phytosterols, that
are beneficial for a healthy diet (Satriana et
al., 2019). Demand for avocado oil has grown significantly over time
as consumers have become aware of its health advantages.
Several studies report that
the development of extraction techniques can produce avocado oil with a high
content of bioactive compounds in addition to maintaining its properties and
nutritional value after extraction and during storage (Satriana
et al., 2019; dos Santos et al., 2014). Avocado oil has characteristics
similar to olive oil (Barros et al., 2016)
and is extracted from the dried
fruit's fleshy pulp. Although solvent-free extraction is a currently trending
environmentally-friendly process, the traditional lipid extraction method of
solid-liquid solvent extraction is still widely applied in industrial-scale
processes due to its efficiency (Mgoma, Basitere, and
Mshayisa,
2021). This
method involves the penetration of the solvent into the lipid membrane while
matching the polarity of the target chemicals. However, since the solvents come
into direct contact with the lipids, it is necessary to pre-treat the raw
material before adding the solvent to improve the extraction yield.
Pre-treatment, including size reduction
and heat treatment, of raw material is one of the primary methods for enhancing
oil recovery during the extraction process. Size reduction is used because the contact area between the material and
the solvent increases as the size of the material decreases (Yusuff, 2021). Heat treatment, on the other hand,
increases the yield of extracted oil by destroying the oil cells, thereby
lowering the viscosity and moisture content of the oil and causing the flour
proteins to coagulate. It also inactivates enzymes in ingredients that may
affect the quality of the extracted oil (Evangelista, Isbell, and
Cermak, 2012). Treatment with water combined with
processing aids, such as enzymes and calcium carbonate (CaCO3), also
weakens the oily cell walls and helps prepare the plant matrix for optimal oil
extraction (Satriana et al., 2019). Squeo et al. (2016) reported that CaCO3
could be used as a coadjuvant to increase the extraction yield of extra
virgin olive oil. However, there was no clear trend regarding the impact on oil
quality. More recently, Arpi et al. (2023) reported
the effect of a precooking treatment with water and CaCO3 on the
properties of dried avocado pulp and its oil extract.
Solid–liquid extraction of avocado oil
is a multiphase and unsteady-state mass transfer process in which the solute
concentration in the solid is continuously varied. A model of avocado oil
extraction and an estimate of its extraction rate is necessary for analyzing
and designing extraction processes on an industrial scale. The available
literature contains limited information on the kinetics of avocado oil
extraction. This is likely due to the fact that many avocado oil manufacturers
still rely on traditional technology and may not prioritize process
optimization. Mgoma Basitere, and Mshayisa, (2021) studied the kinetics and
thermodynamics of oil extraction of Hass avocados from South Africa. Arimalala, Herve, and Rafihavanana (2022) reported
that the second-order kinetics model provides a good representation of the
experimental results of the oil extraction of avocados from Madagascar.
Understanding the kinetics of avocado oil extraction is essential for
conducting technical and economic analyses of the process. Kinetic knowledge
aids in fundamental comprehension of the process, allowing for better process
control and higher efficiency. Additionally, studying kinetics is crucial for
scaling up the process, as noted by Prihutami et
al. (2021).
Previous studies suggested that avocado
oil extraction could be improved using various pre-treatment methods (Arpi et al., 2023; Santana et al.,
2015). The novelty of this work resides in the investigation of the
kinetics of avocado oil extraction using raw material cooking pre-treatment and
the addition of a coadjuvant. To the best of the authors' knowledge, there is
little information in the published literature about the pre-treatment of raw
material using coadjuvant in avocado oil extraction processes. The primary goal
is to examine the solid–liquid extraction yield and kinetics of oil from dried
avocado flesh using hexane as a solvent. The experimental data obtained will be
compared to published kinetics models.
2.1. Materials
A fully ripe Hass avocado fruit was
obtained from agricultural resources in Central Aceh, Indonesia.
Analytical-grade hexane and CaCO3 of 99.95% purity were procured
from Merck (Indonesia). All compounds were utilized without further
purification.
Figure 1 Schematic of the
experimental procedure.
2.2.1. Sample
Preparation
The experiment required approximately 8
kilograms of ripe avocado. Three different pre-treatment methods, as reported
by Arpi et al. (2023), were applied
to the avocado flesh. In the first sample group, the avocado flesh was cooked
in water. In the second sample group, the avocado flesh was cooked with water
containing 5% CaCO3 (w/w of flesh). The last sample group was
uncooked as a control. After completing the three pre-treatment methods, the
resulting dried avocado was shaped into two sizes: 8 mm × 5 mm × 5 mm and 1.19
mm.
2.2.2. Avocado
Oil Extraction
The influence of
the three pre-treatment methods (uncooked, cooked with CaCO3, and
cooked with water) and two material sizes (8 mm × 5 mm × 5 mm and 1.19 mm) on
the avocado oil yield were investigated in the first series of trials.
Evaluation of the extraction kinetics was carried out based on the highest
yield of avocado oil obtained in the first series of trials for the various
pre-treatment methods. The influence of temperature (25, 40, and 50°C) on the
extraction yield was investigated using the highest avocado oil yield obtained
in the first series of trials.
2.3. Kinetic Model of Avocado Oil Extraction
Various kinetic models have been developed
to describe the oil extraction mechanism. The phenomena of mass transfer
through solid plant materials and from their surfaces into the bulk of the
solvent are the basis for physical kinetic models. Several kinetic models for
avocado oil extraction have been reported, including second-order, Peleg,
logarithmic (Arimalala, Herve, and Rafihavanana 2022), first-order reaction, Fick's law, and Van't
Hoff's (Mgoma, Basitere, and Mshayisa, 2021). The most
frequently used models are based on film theory and the concept of
unsteady-state diffusion through particles. The Peleg model is an empirical and classical
hyperbolic model that was initially designed to describe moisture sorption
curves. The Peleg model has been modified and applied to represent the
solid–liquid extractions of numerous plant metabolites in general and in
phenolics, particularly due to the similarity between the extraction and
sorption curves (Milicevic et al., 2021).
2.3.1. Peleg
Model
The Peleg model can provide an accurate
estimation of the kinetics of the solid–liquid extraction process (Liao, Guo, and Yu, 2021). The mathematical model introduced by Peleg (Eq. [2]) was adopted as
the kinetic model for the extraction of plant material.
In Eq. (2), t
is the extraction time (min), C0 is the initial concentration
of avocado oil (g/g), Ct is the concentration of avocado oil
at t (g/g), k1 is Peleg’s rate constant (min.g/g), and
k2 is Peleg’s capacity constant (g/g). The constants k1
and k2 indicate the initial extraction rate and the maximum
solute concentration achieved during the process, respectively (Anbalagan et al., 2019). A modified Peleg
equation to plot solute concentration in the extraction solvent is presented in
Eq. (3).
2.3.2. Power Law Model
The
power law model (Eq. [4]) has previously been applied to the extraction process
(Natolino and Porto, 2020; Alara and Abdurahman,
2019).
In Eq. (4), Ct is the concentration (g/L) of avocado oil at any time
t (s), B denotes the extraction coefficient (L/g.s), and n denotes the power law exponent.
Equation (4) can be further simplified to Eq. (5).
Plotting ln Ct
against ln (t) gives n and ln B as the slope and intercept, respectively.
2.3.3. Unsteady-state
Diffusion Model
The unsteady-state
diffusion model was developed based on a solvent extraction technique that
included concurrent evaporation and diffusion processes. The model defined by
Eq. (6) contains two parameters: the quick oil extraction stage, represented by
b (evaporation coefficient), and the slow oil extraction stage,
represented by k (diffusion coefficient). This model can be applied to
simulate the oil extraction kinetics of any plant material.
In Eq. (6), q0 represents the initial oil content (g/g), q represents the oil yield (g/g) at time
t, b represents the evaporation coefficient, k represents the diffusion coefficient (1/min), and t represents the extraction period
(min). The linearized form of Eq. (7) can be used to determine b and k.
2.4. Activation Energy (Ea)
The
activation energy (Ea) is the minimum energy required to initiate the
extraction process (Abed et al., 2019),
and it can be calculated using the Arrhenius equation. Equation (8) presents
the correlation between the extraction rate constant (k) and the temperature of extraction (T).
where k
represents the diffusion coefficient (min-1), Ea represents
the activation energy (kJ/mol), A represents the Arrhenius constant (s-1),
T represents the absolute temperature (K), and R represents the
universal gas constant (kJ/mol.K). A linear relationship between ln k
and 1/T can be obtained (Eq. 9).
Plotting ln k
against 1/T gives -Ea/R and ln A as the slope and intercept, respectively.
3.1. Influence of Process
Variables
The avocado oil obtained from each experiment
was similar in appearance; it was dark green and had a distinctive viscosity.
The avocado oil yield was significantly affected by extraction time. Figure 2
depicts the influence of time on the oil yield for each pre-treatment method
and material size. There was a marked correlation between extraction time,
pre-treatment method, material size, and oil yield under the given conditions.
The avocado oil yield increased as the
extraction time increased. This was because longer extraction times led to
longer contact between the solvent and plant material, thereby increasing mass
transfer. The yield of oil increased significantly initially, then gradually
decreased as the extraction progressed. As shown in Figure 2, increasing the
extraction time after approximately 30 min did not further increase the oil
yield. It may be inferred that 30 min after extracting the material, the
remaining oil in the avocado was reduced to a minimum value. Many studies have
observed similar trends, such as in the extraction of alpha-glucosidase
inhibitors from lemongrass (Widiputri et al.,
2020) and the extraction of bioactive compounds from avocado seeds (Corral-Perez, and Almajano, 2016).
As expected, the yield increased as the material size decreased.
The oil yield obtained by material size 1.19 mm was higher than that obtained
by the 8 mm × 5 mm × 5 mm material. By reducing the size of the materials, the
surface area increases, which increases
the mass transfer of the active principle from the plant material to the
solvent and makes extraction more efficient. In addition, the extraction yield
increases because the dispersion distance of the solute in the solid decreases
when the particle size is small; therefore, a shorter time is required for the
solute to reach the surface. It should also be noted that very small particle
sizes can cause difficulties during the screening process (Makanjuola, 2017). Santos et al. (2015) discovered a similar pattern when
using ethanol as a solvent to extract oil from Jatropha curcas L.
However, as the size of the material increased, the influence of pre-treatment
on oil yield became more significant. It has been suggested that pre-treatment
of the material provides several advantages, such as easier penetration of the
solvent into the plant cell to release the oil from the cell (Arpi et al., 2023).
Figure 2 Influence of extraction time on avocado oil yield at various
extraction conditions (extraction temperature of 25°C; a solid-to-solvent ratio
of 1:15 g/mL; 400 rpm).
Figure 3 depicts the
influence of temperature on avocado oil yield. Extraction temperature was
critical to maximize the extraction yield, and the results obtained indicate
that the amount of oil extracted increased with extraction temperature. This
relationship was predicted because the driving force for extraction increases
as temperature increases. As the temperature increases, oil solubility and
solvent diffusivity increase, and the viscosity of both solute and solvent
decreases, facilitating mass transfer processes and resulting in a change in
oil yields (Mgoma, Basitere, and Mshayisa, 2021; Anbalagan et
al., 2019). Moreover, increased temperature promotes the
spread of oil and reduces its viscosity (Meziane
and Kadi, 2008).
Figure 3 Influence of temperature on avocado oil yield (experimental
conditions: cooking pre-treatment with water and material size 1.19 mm).
Figures
2 and 3 depict the time course of the change in avocado oil yield during the
extraction process for various process variables. There is a typical curve for
extracting oil from plants. Avocado oil extraction can
be divided into two stages: rapid oil extraction and slow oil extraction.
The rapid oil extraction stage occurred during the early stages of extraction,
during which oil was rapidly released and evaporated from the outer surface of
the plant material. During this time, the yield of avocado oil increased
rapidly. As the extraction process progressed, the extraction rate decreased
until a nearly constant oil yield was reached. During the slow oil extraction
stage, oil extraction was followed by slow molecular diffusion of the oil from
the undamaged cells to the surfaces of the plant materials. Oil production
slowed down because the oil spread slower. This mechanism was critical for
further extraction kinetics modeling. Some researchers, including Anbalagan et al. (2019) in the mangiferin
extraction from Mangifera indica
leaves and Santos et al. (2015) in
the oil extraction from Jatropha curcas,
reported a similar trend.
3.2. Kinetic Model of Avocado Oil Extraction
The kinetic model is a method used to
describe the process of mass transfer and diffusion in the extraction process (Alara and Abdurahman, 2019). Figure 4 displays
graphs of the kinetics of the Peleg, power law, and unsteady-state diffusion
models for various cooking pre-treatment methods. The slope and intercept of
each curve were utilized to calculate the kinetic parameters of each model and
are presented in Table 1.
The
coefficient of determination (R2)
was used to select the best mathematical model for avocado oil extraction (Arimalala, Hervé, and Rafihavanana, 2022).
In the present study, the unsteady-state diffusion model had the highest R2
value, indicating that this model provided the most accurate prediction of the
results of the extraction process. Both kinetic parameters of the
unsteady-state diffusion model were greater for the cooking pre-treatment than
for the control sample. These results are in line with the obtained oil yield
trend. It was also discovered that the value of k was less than the
value of b for all experimental variables, implying that the slow oil
extraction stage influenced the process rate. The coefficients for the rapid
oil extraction periods were 86–120 times larger than those for the slow oil
extraction periods, indicating that diffusion is much slower than evaporation.
This trend has also been reported for oil extraction from avocado seeds (Segovia, Corral-Perez, and Almajano, 2016) and sunflower seeds (Perez, Carelli, and Crapiste, 2011). The performance of an
extraction process can be represented by the k value. It was observed
that the increase in temperature led to an increase in extraction rate. The k
value obtained was comparable to those published in the available literature
for avocado oil extraction (Table 2).
Table 1 Kinetic parameter for Peleg, power law, and unsteady-state
diffusion models.
Figure 4 (A) Peleg model, (B) power law model, and
(C) unsteady-state diffusion model (experimental
conditions: material size 1.19 mm and extraction temperature of 50°C).
Table 2 Comparison
of kinetic model parameters for avocado oil extraction.
3.3. Activation Energy
Figure
5 depicts the relationship between k and 1/T. The estimated
Arrhenius parameters are presented in Table 3. The value of
R2 from the graph of ln k versus 1/T in Figure 5 is
0.9014, and the value of Ea was
calculated from the linear correlation between ln k and 1/T. The Arrhenius parameters of the process for
material size 1.19 mm and the cooking pre-treatment with water were 47.51
kJ/mol and 21,111,692 min-1 for Ea and A,
respectively. Table 3 provides a comparison of Ea values for various vegetable oils. It can be observed that the value
of Ea obtained is low and reasonable compared with the value reported in
the literature. A high Ea reduces the extraction process rate, while a
low Ea helps to increase the extraction process rate. The lower the Ea value, the less energy is required
for oil extraction to begin (Ramesh, Yasin, and
Arshad,
2020).
Furthermore, a positive Ea indicates that the process is endothermic (Rahma and Hidayat, 2023). The energy required to
overcome the endothermic nature of the process is provided by heating during
extraction. Previous work has demonstrated that Ea values of certain vegetable oils are in the range of 79 to 104
kJ/mol, indicating that they have varying fatty acid profiles (Tan et al., 2001). Oils with lower Ea values are expected to require higher
temperatures to trigger certain changes to the oxidation rate (Aktar and Adal, 2019).
Figure 5 Plot of ln k against
1/T to determine the activation energy.
Table 3 Comparison of the estimated activation energy of various vegetable
oils.
Kinetic studies contribute to a fundamental understanding of the
process and enable better process control and process scaling. In this study,
the solid-liquid extraction kinetics of dried avocado flesh using hexane as a
solvent was investigated. Experimental results showed that there was a clear
correlation between extraction time, pre-treatment method, material size,
extraction temperature and oil yield. Avocado oil extraction can be divided
into two stages, rapid oil extraction and slow oil extraction. The coefficients
for the rapid oil extraction stages were 86–120 times larger than those for the
slow oil extraction stages, suggesting that diffusion is much slower than
evaporation. Based on the magnitude of R2 values, the
unsteady-state diffusion model was shown to adequately represent the rate of
avocado oil extraction. A positive Ea value of 47.51 kJ/mol was
obtained, indicating that the extraction process is endothermic. This research
is necessary to develop and implement a complete and improved industrial
avocado oil extraction process.
The
authors are thankful for the support of the Universitas Syiah Kuala through Lektor Kepala
research grant (No. 145/UN11/SPK/PNBP/2022).
Abed, K.M., Kurji, B.M., Rashid, S.A., Abdulmajeed, B.A., 2019.
Kinetics and Thermodynamics of Peppermint Oil Extraction from Peppermint Leaves.
Iraqi Journal of Chemical and Petroleum
Engineering, Volume 20(4), pp. 1–6
Aktar, T., Adal, E., 2019. Determining the Arrhenius Kinetics of Avocado
Oil: Oxidative Stability under Rancimat Test Conditions. Foods, Volume 8(7), p. 236
Alara, O.R., Abdurahman, N.H., 2019. Microwave-assisted Extraction
of Phenolics from Hibiscus sabdariffa
Calyces: Kinetic Modelling and Process Intensification. Industrial Crops and Products, Volume 137, pp. 528–535
Anbalagan, K., Kumar, M.M., Ilango, K., Mohankumar, R., Priya,
R.L., 2019. Prelusive Scale Extraction of Mangiferin from Mangifera Indica Leaves: Assessing Solvent Competency, Process Optimization,
Kinetic Study and Diffusion Modelling. Industrial
Crops and Products, Volume 140, p. 111703
Arimalala, A.F., Herve, R.P.,
Rafihavanana, R., 2022. Modeling and Kinetics Study of Avocado Oil
Extraction from Madagascar Using Different Mathematical Models. South
African Journal of Chemical Engineering, Volume 41, pp. 93–97
Arpi, N., Satriana, Mustapha, W.A.W., Syamsuddin, Y., Putra, T.W.,
Supardan, M.D., 2023. Effect of Cooking Pre-Treatment on The Properties of
Dried Avocado Flesh and Its Oil Extract. South
African Journal of Chemical Engineering, Volume 43, pp. 1–8
Barros, H.D.F.Q., Coutinho, J.P., Grimaldi, R., Godoy, H.T.,
Cabral, F.A., 2016. Simultaneous Extraction of Edible Oil from Avocado and
Capsanthin from Red Bell Pepper Using Supercritical Carbon Dioxide as Solvent. Journal of Supercritical Fluids, Volume
107, pp. 315–320
dos Santos, M.A.Z., Alicieo, T.V.R., Pereira, C.M.P., Ramis-Ramos,
G., Mendonca, C.R.B., 2014. Profile of Bioactive Compound in Avocado Pulp Oil: Influence
of The Drying Processes and Extraction Methods. Journal of the American Oil Chemists’ Society, Volume 91, pp. 19–27
Evangelista, R.L., Isbell, T.A., Cermak, S.C., 2012. Extraction of Pennycress
(Thlaspi arvense L.) Seed Oil by Full Pressing. Industrial Crops and Products, Volume 37, pp. 76–81
Ge, Y., Dong, X., Liu, Y., Yang, Y., Zhan, R., 2021. Molecular and Biochemical
Analyses of Avocado (Persea Americana)
Reveal Differences in The Oil Accumulation Pattern Between The Mesocarp and
Seed During The Fruit Developmental Period. Scientia
Horticulturae, Volume 276, p. 109717
Liao, J., Guo, Z., Yu, G., 2021. Process Intensification and
Kinetic Studies of Ultrasound-Assisted Extraction of Flavonoids from Peanut
Shells. Ultrasonics Sonochemistry,
Volume 76, p. 105661
Makanjuola, S.A., 2017. Influence of Particle Size and Extraction
Solvent on Antioxidant Properties of Extracts of Tea, Ginger, and Tea–Ginger
Blend. Food Science & Nutrition, Volume 5, pp. 1179–1185
Meziane, S., Kadi, H., 2008. Kinetics and Thermodynamics of Oil
Extraction from Olive Cake. Journal of the American Oil Chemists' Society,
Volume 85, pp. 391–396
Mgoma, S.T., Basitere, M., Mshayisa, V.V., 2021. Kinetics and Thermodynamics
of Oil Extraction from South African Hass Avocados Using Hexane as A Solvent. South African Journal of Chemical
Engineering, Volume 37, pp. 244–251
Milicevic, N., Kojic, P., Sakac, M., Misan, A., Kojic, J.,
Perussello, C., Banjac, V., Pojic, M., Tiwari, B., 2021. Kinetic Modelling of
Ultrasound-Assisted Extraction of Phenolics from Cereal Brans. Ultrasonics
Sonochemistry, Volume 79, p. 105761
Natolino, A., Porto, C.D., 2020. Kinetic Models for Conventional and
Ultrasound Assistant Extraction of Polyphenols from Defatted Fresh and
Distilled Grape Marc and Its Main Components Skins and Seeds. Chemical Engineering Research and Design,
Volume 156, pp. 1–12
Perez, E.E., Carelli, A.A., Crapiste, G.H., 2011. Temperature-Dependent
Diffusion Coefficient of Oil from Different Sunflower Seeds during Extraction with
Hexane. Journal of Food Engineering,
Volume 105(1), 180–185
Prihutami, P., Sediawan, W.B., Prasetya, A., Petrus, H.T.B.M.,
2021. A Product Diffusion Model for The Extraction of Cerium and Yttrium from
Magnetic Coal Fly Ash Using Citric Acid Solution. International Journal of Technology, Volume 13(4), pp. 921–930
Rahma, F.N., Hidayat, A., 2023. Biodiesel Production from Free
Fatty Acid Using Zro2/Bagasse Fly Ash Catalyst. International Journal of Technology, Volume 14(1), pp. 206–218
Ramesh, N., Yasin, N.M., Arshad, Z.M., 2020. Kinetics Studies and
Thermodynamics of Oil Extraction from Immobilized Microalgae Cells of Chlorella Vulgaris: The Effect of The
Different Matrix System. IOP Conference
Series: Materials Science and Engineering, Volume 991, p. 012003
Santana, I., dos Reis, L.M., Torres, A.G., Cabral, L.M. and
Freitas, S.P., 2015. Avocado (Persea americana Mill.) Oil Produced by
Microwave Drying and Expeller Pressing Exhibits Low Acidity and High Oxidative
Stability. European Journal of Lipid
Science and Technology, Volume 117 (7), pp. 999–1007
Santos, S.B., Martins, M.A., Caneschi, A.L., Aguilar, P.R.M.,
Coimbra, J.S.R., 2015. Kinetics and Thermodynamics of Oil Extraction from Jatropha Curcas L. Using
Ethanol as A Solvent. International
Journal of Chemical Engineering, Volume 2015, p. 871236
Satriana, Supardan, M.D., Arpi, N., Mustapha, W.A.W., 2019.
Development of Methods Used in The Extraction of Avocado Oil. European Journal of Lipid Science and
Technology, Volume 121, p. 1800210
Segovia, F.J., Corral-Pérez, J.J., Almajano, M.P., 2016. Avocado Seed:
Modeling Extraction of Bioactive Compounds. Industrial
Crops and Products, Volume 85, pp. 213–220
Squeo, G., Silletti, R., Summo, C., Paradiso, V.M., Pasqualone, A.,
Caponio, F., 2016. Influence of Calcium Carbonate on Extraction Yield and
Quality of Extra Virgin Oil from Olive (Olea europaea L. cv. Coratina). Food
Chemistry, Volume 209, pp. 65–71
Supardan, M.D., Misran, E., Mahlizar, Satriana, Mustapha,
W.A.W., 2019. Effect of Material Length on Kinetics of Essential Oil
Hydrodistillation from Lemongrass (Cymbopogon
citratus). Journal of Engineering
Science and Technology, Volume 14(2), pp. 810–819
Tan, C.P., Che Man, Y.B., Selamat, J., Yusoff, M.S.A., 2001. Application of
Arrhenius Kinetics to Evaluate Oxidative Stability in Vegetable Oils by Isothermal Differential Scanning Calorimetry. Journal of the American Oil
Chemists' Society, Volume 78(11), pp. 1133–1138
Widiputri, D.I., Julisantika, I., Kartawiria, I.S., Gunawan-Puteri,
M.D.P.T. Ignatia, F., 2020. Upscaling the Cymbopogon
Citratus (Lemongrass) Extraction Process to Obtain Optimum
Alpha-Glucosidase Inhibitor (AGI) Levels. International Journal of Technology, Volume 11(3), pp.532–543
Yusuff, A.S., 2021. Parametric Optimization of Solvent Extraction of
Jatropha Curcas Seed Oil Using Design of Experiment and Its Quality
Characterization. South African Journal
of Chemical Engineering, Volume 35, pp. 60–68
