Spatial and Temporal Development of Microorganisms and the Effect of Clogging in Up-Flow Sand Filter
Corresponding email: farhana.abdlahin@ums.edu.my
Published at : 10 Jul 2024
Volume : IJtech
Vol 15, No 4 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i4.6357
Lahin, F.A., Sarbatly, R., Ken, C.C., 2024. Spatial and Temporal Development of Microorganisms and the Effect of Clogging in Up-Flow Sand Filter. International Journal of Technology. Volume 15(4), pp. 870-879
| Farhana Abd Lahin | 1 Chemical Engineering, Faculty of Engineering, Universiti Malaysia Sabah, Jalan UMS, Kota Kinabalu 88400, Sabah, Malaysia 2 Nanofiber and Membrane Research Laboratory, Faculty of Engineering, Univer |
| Rosalam Sarbatly | 1 Chemical Engineering, Faculty of Engineering, Universiti Malaysia Sabah, Jalan UMS, Kota Kinabalu 88400, Sabah, Malaysia 2 Nanofiber and Membrane Research Laboratory, Faculty of Engineering, Univer |
| Chiam Chel Ken | 2 Nanofiber and Membrane Research Laboratory, Faculty of Engineering, Universiti Malaysia Sabah,Jalan UMS, Kota Kinabalu 88400, Sabah, Malaysia 3 Oil and Gas Engineering, Faculty of Engineering, Univ |
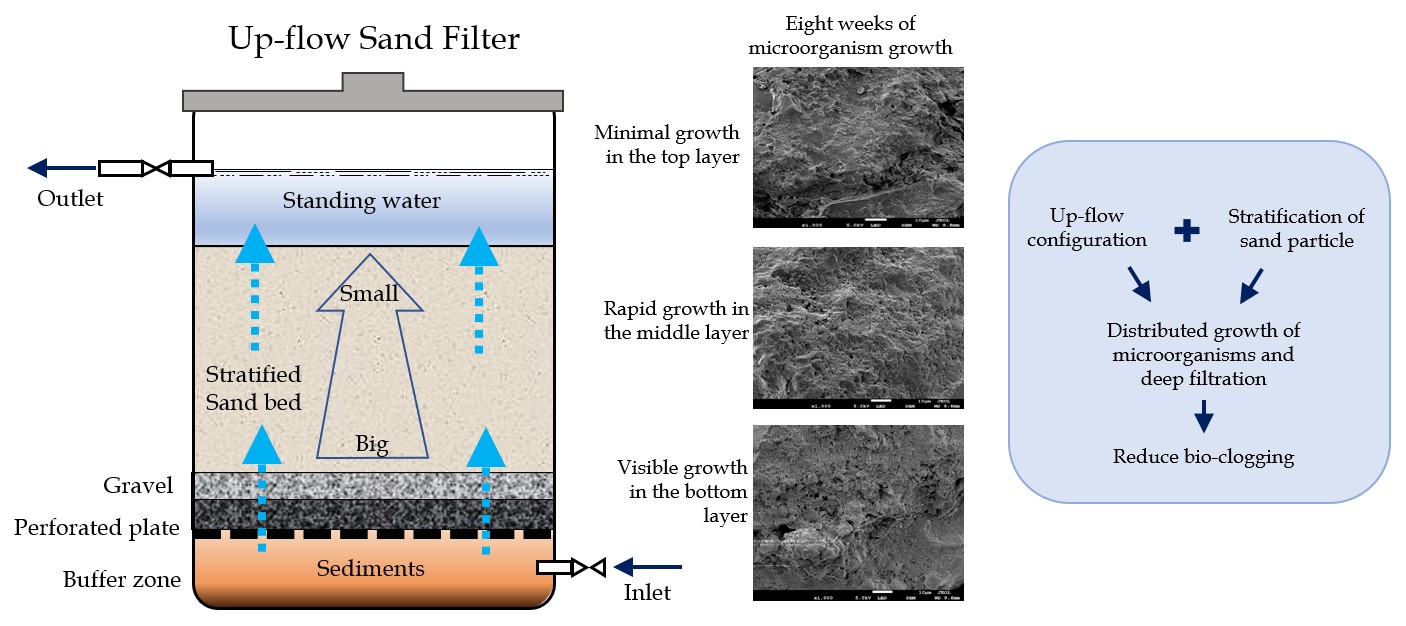
Up-flow sand filtration is an excellent alternative
to point-of-use treatment for rural water supply. However, the sites and
interval of microorganism growth in filter beds and the effect on bio-clogging
remain unknown. This study aimed to assess microorganism growth based on
biological activity levels using Dissolved Oxygen (DO) consumption and Field
Emission Scanning Electron Microscopy (FE-SEM) imaging in various zones of the
filter bed. Additionally, pressure drop was monitored to evaluate bio-clogging.
The results showed that microorganism activities differed within the 0.50 m
up-flow sand filter bed during 8 weeks of acclimatization. Exponential growth
started after day 35, and DO levels declined to a minimum of 2 mg/L at 0.10 m
bed height (measured from the bottom) after day 46. At 0.30 m and 0.50 m
height, the DO decreased to 0.8 mg/L and 0.3 mg/L after days 35 and 46,
respectively. FE-SEM images confirmed microorganism growth on samples from 0.1
m after 4 weeks of acclimatization. Substantial growth was seen on samples of
0.10 and 0.30 m height at 8 weeks, while lesser growth was observed on samples
of 0.50 m. The pressure drop showed no significant increase, signifying that
clogging did not occur during the 135-day operational period. In conclusion,
the up-flow configuration prevented bio-clogging in sand filters, reducing
maintenance requirements.
Acclimatization; Clogging; Microorganism; Sand filter; Up-flow
Up-flow sand filter is
recognized as a highly promising alternative for water remediation,
particularly for point-of-use treatment in rural settings. This is due to the
ease of material procurement and simplicity of construction, operation, and
maintenance (Lahin, Sarbatly, and Chel-Ken, 2021).
Up-flow filtration has been used to remove turbidity, suspended solids,
phosphorus, nitrogen, heavy metals, bacteria, and algae in multiple water
treatment
processes (Al-Saedi, Smettem,
and Siddique, 2019; Heikal, Wagdy, and Eldidamony, 2017). In this
method, feed water entering from the bottom of the filter and initially flowing
through the gravel layers improves the treatment process by eliminating the
susceptibility to clogging. Additionally, filter cleaning is conducted more
rapidly,
Up-flow sand filter similar to a down-flow
configured sand filter removes pollutants using physical and biological mechanisms.
Approximately 70% of suspended particles and pathogenic contents in water are
physically separated through straining and adsorption (Shreya
et al., 2023; Wu et al., 2020; Polyakov et al., 2019).
However, with the aid of biological methods, such as predation, elimination,
natural death or inactivation, and microorganism metabolism, a pathogen removal
efficiency of up to 99% is achievable (Andreoli and
Sabogal-Paz, 2020; Budhijanto et al., 2015;
CAWST, 2012). Microorganisms growing in sand
filter beds play an essential role in water remediation by consuming pathogens
and bacteria in the water passing through the filter beds (Duran-Romero et al., 2020). The
establishment of microorganisms in the filter bed is an essential step in
commissioning sand filter operations (Bozorg, Gates,
and Sen, 2015). Inoculation can either be performed by introducing an
existing microorganism from an established sand filter or through inherent
inoculation, where the sand filter is exposed to the intended feed water
containing microorganisms and nutrients. The microorganisms will then be
allowed to grow naturally over time (Ramsay, Breda,
and Soborg, 2018). The latter method may require a more extended period
but is most feasible for setting up sand filters in isolated areas. Typically,
6-8 weeks of acclimatization is required to establish a newly constructed sand
filter, although some studies suggest that ripening might require close to 6
months (Cai et al., 2016).
As microorganisms grow in sand filter beds, Dissolved
Oxygen (DO) concentration decreases. This allows for monitoring of the growth
of microorganisms using DO concentrations. Studies reported lower
concentrations in areas where biological processes occurred in the beds,
typically below the top surface of a down-flow sand filter. DO concentrations
vary across sand bed levels, reflecting the biological activities (Andreoli and Sabogal-Paz, 2020; Ramsay, Breda, and Soborg, 2018; Young-Rojanschi and
Madramootoo, 2015). Therefore, observation of low DO concentrations will
signify the establishment of microorganisms as oxygen is consumed during
biological treatment processes.
As time progresses, the biological layer build-up
could cause a considerable yield reduction due to pore clogging (Mohamed et al., 2023; Kurniawan et al.,
2022; Mutsvangwa and Matope, 2017). This necessitates subsequent filter
cleaning, which disrupts the microorganism population and results in lower
filtration efficiency and risk of pathogen breakthrough. Depending on filter
size, the recovery process through ripening requires 4–8 weeks (De-Souza et al., 2021; Saravanan and Gobinath,
2015). Bio-clogging occurs more in slow sand filters as microorganism
growth is concentrated more on the top layer of the filter bed (schmutzdecke),
which is also the site for straining and sedimentation (Donda
Paranita, and Simatupang, 2024; Segismundo et al., 2016; Wakelin et
al., 2010). However, bio-clogging is expected to be significantly
reduced with up-flow configured sand filters that eliminate the schmutzdecke
layer and use a deep filtration mechanism (Lahin, Sarbatly,
and Chel-Ken, 2022, Zeng, Chen, et al. 2020).
2.1. Sand Filter Bed
The up-flow sand filter bed was constructed using 0.10 mm D10 sand media and ranged in size from < 0.075–2 mm. Sand media was obtained from the Tamparuli River, Sabah, Malaysia, and prepared according to the sand filter manual (CAWST, 2012). Two layers of gravel support were used, each 0.05 m thick. The effective size of each gravel support was 0.49 and 2.10 mm, respectively. The support section comprised a series of small layers that were evenly distributed and compacted to promote uniform water circulation during operation. Subsequently, when the support was even, sand media was poured in small quantities before being leveled and distributed across the filter surface. This process was repeated until sand bed height reached 0.40 m.
Figure 1 Up-flow Sand Filter Design
The
up-flow sand filter was activated by allowing the bed to fluidize for 30
minutes before being left to resettle. Fluidization allowed sand particles to
stratify based on size. Larger and denser particles settled at the bottom,
while lighter and smaller grains rose to the top of the sand bed.
The filter was monitored for 8
weeks after commissioning to allow for media maturation. During this period,
untreated feed water was circulated in the filter for 8 hours daily at 0.072
m/h surface velocity. The sand bed was then submerged in water while the sand
filter was not operational to ensure the survival of the developed
microorganisms.
2.2. The Pilot Up-flow Sand Filter Setup
The pilot plant used to monitor filter clogging was based on the design
described by Lahin, Sarbatly, and Chel-Ken (2022).
During the pilot up-flow sand filter operation, three surface velocities were
used, 0.072, 0.1805, and 0.4813 m/h, denoted by Q1, Q2, and Q3, respectively.
The total monitoring period was 135 days.
2.3. Feed Water
The feed water used during the study
contained natural pollutants found in surface water. The purpose was to promote
microorganism growth organically by introducing polluted feed water. Lahin, Sarbatly, and Chel-Ken (2022) provided
details on the feed water content.
2.4. DO Concentration
DO concentration in the up-flow sand
filter bed was measured as a reference for microorganism growth. It was
documented using sampling valves installed at 0.20 m intervals across the
filter. The concentration was assessed onsite with a HI98193 Hanna Portable DO
Meter. DO sampling was conducted in the morning, 1 hour after the daily
start-up and operation of the filter.
2.5. Physical Microorganism Growth
Microorganism growths on sand grains
were examined using FE-SEM. Samples from different filter bed heights were
carefully washed with distilled water and air-dried without being fixed,
dehydrated, or frozen. These samples were mounted on a metal stub with carbon
conductive paint before platinum coating was sputtered on the sample with a
JEOL JEC-3000FC Auto Fine Coater. FE-SEM images were then captured at a 5 kV
accelerating voltage using JEOL JSM-7900F FE-SEM.
2.6. Pressure Drop Monitoring
Pressure drops across the filter bed
was measured using manometer tubes connected to manometer outlets installed
lateral to the up-flow sand filter column (Figure 1). The bottom manometer
channel was located at the base of the sand filter bed, while the top indicator
was 0.05 m from the surface. The height differences between the two manometers
were documented after the readings stabilized.
3.1. Microorganism Activity
DO concentration across the filter bed was monitored at 3 – 4 day intervals to assess the microbial activity. The microorganisms were established during the 8-week observation period following the activation of the up-flow sand filter. Filter bed depth was divided into three sections, lower (0.10 m from filter bottom), middle (0.30 m from filter bottom), and upper part (0.50 m from filter bottom), denoted by V5, V3, and V1, respectively (Figure 1). The influent and effluent water DO concentrations were also recorded as a reference, with labels In and Effl., respectively.
Figure 2 DO Concentration across Filter Bed at 8 Weeks during
Acclimatization Period
Figure 2 shows
DO concentration over the 8-week acclimatization period. Initial observations
showed a varying decline in concentration between sand bed sections. An average
of 8.5%, 17.48%, and 4.02% reduction was observed in V5, V3, and V1,
respectively. This pattern persisted until day 34 when the reduction changed
significantly. From day 35, a further reduction in DO concentration was
observed in V5, with an average decrease of 27.2% and a concentration of 3.4
mg/L compared to 4.0 mg/L during the first 34 days. Subsequently, the value
dropped from day 46 to 58, falling in the 2 – 3.3 mg/L range. In V3, DO
concentration dramatically decreased to 0.8 mg/L after day 35, remaining below
1 mg/L until after the commissioning. The lowest V3 reading was 0 mg/L on days
46 and 55. V1 decreased to 0.3 mg/L on day 46 and was maintained at a lower
concentration until day 58.
Microbial
population growth started immediately after commissioning, as observed by DO
consumption in the filter bed. Based on the general microbial growth curve, the
lag phase lasted until day 34, when a minimal increase in DO consumption was
observed. The exponential phase began on day 35, marked by a rapid reduction in
DO concentration. Meanwhile, the stationary phase began around days 46 to 49,
when DO concentration reduction appeared to stabilize. This phase continued
until the end of the experiment. Table 1 summarizes the average oxygen
consumption observations during non-rapid (1-34 days) and rapid (35 – 58 days)
DO reduction.
Table
1 The Average
DO Concentration in Different Sand Filter Sections ± Standard Deviation
|
Time (Day) |
Average DO
Concentration (mg/L) ± S.dev | ||||
|
In |
V5 |
V3 |
V1 |
Out | |
|
1 - 34 |
4.43 ± 0.24 |
4.05 ±0.27 |
3.71 ±0.45 |
4.04 ±0.38 |
4.28 ±0.24 |
|
35 - 58 |
4.71 ±1.35 |
3.42 ± 1.11 |
1.32 ±1.19 |
1.84 ±1.56 |
3.49 ±1.69 |
The observed oxygen consumption patterns suggested that the
microbial population was primarily concentrated in the middle layer of the sand
bed, supported by the lowest level of DO in V3. Since the water flowed from the
bottom, microbes and nutrients were
introduced to the lowest layer of the filter first. The larger pores in the
bottom layer allowed most pollutants and particulate matter to stream through
before entering the sand bed. Additionally, rapid growth at the bottom section
was also hindered by the elevated shearing caused by the increased flow rate
and pressure in this section. Straining occurred in the deeper layer of the
filter due to smaller pore sizes, resulting in the retention of most
microorganisms and attachment taking place in the deeper section of the filter
bed (V3). This led to a larger population of microorganisms. Lower nutrients
and DO availability in the top layer of the filter prevented the rapid growth
of microorganisms at this site.
3.2. Physical Growth of
Microorganisms
FE-SEM images (Figure 3 – 5)
show that the physical growth of microorganisms on the sand surface started
from the bottom and progressively moved upwards. Figure 4 shows that at week 4,
growth was observed in V5, with less growth in V3, and no significant growth in
V1. The images from week 8 show significant microorganism growth in V5 and V3.
Meanwhile, in V1, a more negligible growth was observed, consistent with DO
consumption results.
Figure 3 FE-SEM Images of Sand Media at Start-up
Based on DO consumption and
microorganism growth images, this study found that the microorganisms in the
up-flow sand filter were established 7–8 weeks post-commissioning. The sand
filter bed was established simply by inoculating the up-flow sand filter with
natural microorganisms and exposing it to the intended feed water for
acclimatization. However, other studies found that using the same inoculation
technique might require over 90 days to achieve stable microorganism growth in
down-flow granulated media filters (Duran Romero et
al., 2020; Gibert et al., 2013). The results also show that
the up-flow sand filter required a shorter acclimatization period due to the
up-flow configuration and sand media stratification (Salkar
and Tembhurkar, 2016).
Aside from flow configuration, the type
of filter media could influence the structure of the microorganism community in
granular filters. For instance, Wakelin et al.
(2010) reported no significant variation between bacteria and archaea
compositions at different depths for anthracite and Granulated Activated Carbon
(GAC). Meanwhile, sand media exhibited a high level of bacterial richness at
0.40 m deep in the filter bed. In another study, nitrifiers were identified in
the zone of the filter bed prone to clogging, confirming that heterotrophs were
the primary contributors to organic removal and biofilm development (Freitas et al., 2021; Bassin et al., 2012).
This study provided good insight into
spatial and temporal information on microorganism growth in up-flow sand
filters but lacked a detailed analysis of the morphological diversity of the
microorganisms. The complexity of microorganism species in filter beds is a
crucial indicator for sand filter performance as different types of
microorganisms, such as heterotrophs, nitrifiers, and oxidizers, play different
roles in pollutant removal (Chan et al.,
2018).
3.3. Clogging
Post-acclimatization of the up-flow sand
filter, a pilot system was used to monitor the clogging effect for 135 days
through pressure drop measurements. Figure 6 shows that a higher pressure drop
was recorded at increased surface velocity. In the operation, the pressure drop
ranged from 0.08 to 0.20 kPa in Q1, 0.23–045 kPa in Q2, and 0.61–1.05 kPa in
Q3. During Q3, the pressure drop increased initially due to an algae bloom.
Following backwashing, the pressure drops stabilized at 0.61–0.76 kPa. Although slight increments of pressure drops were observed in Q3 compared to Q1 and Q2, the increase was insignificant, with R2 of 0.476, 0.0339, and 0.4273, respectively. This shows that the length of the operational period did not considerably affect the pressure drop of the up-flow sand filter bed.
Figure 6 Pressure Drop for 135 Days of Operation in
Varied Flowrate
Various
factors contribute to clogging in sand filters. These include sand particle and
pore size, particulate concentration, and nutrient contents in the feed water (Al-Saedi, Smettem, and Siddique, 2019). In
addition, microorganism growth and death rate affect biomass accumulation (Wang et al., 2023; Abukhanafer et al.,
2021) in the filter bed, other than the time elapsed. These factors
influence the amount of pore size reduction caused by accumulated attached
biomass and strained particles in the sand filter. Clogging primarily occurs in
the first few centimeters of downflow configured sand filters (Chen et al., 2021; Altmann et al., 2016),
where sand particles are typically smaller, and the schmutzdecke layer
develops. The treatment mechanisms in downflow sand filters also rely heavily
on the top layer, where most of the straining and biological treatment occurs (Chen et al., 2021).
Based on the results, the
direction of water flow and sand grain stratification were the most essential
factors in reducing clogging in the up-flow sand filter. In addition to the
distribution of microorganisms in the filter bed, the water flow corresponded
with the stratification of sand filter grains, which ranged from coarse to
fine, enabling deeper penetration and distribution of the particles. The
results were similar to studies by Suryawan et
al. (2021) and Altmann et al.
(2016). Furthermore, the support gravel at the bottom caused some
particles to become trapped before entering the sand filter bed. The up-flow
sand filter in this study was designed with a buffer zone at the bottom to
promote sedimentation of larger particles before the water entered the support
gravel layer, acting as a particulate pre-removal mechanism.
In conclusion, after 8 weeks of monitoring
post-commissioning of the up-flow sand filter, microorganism growth started at
the bottom and progressed upwards into the deeper sites of the filter bed.
FE-SEM imaging confirmed that consistent DO levels below 1 mg/L resulted in
exponential levels of microorganism activities at 0.3 m from the base of the
filter. Furthermore, the development of microorganisms deeper in the filter bed
was influenced by the increased availability of nutrients and oxygen due to the
flow direction of the feed water. Higher shearing at the bottom of the filter
bed and insufficient nutrients and oxygen supplies at the top layer prevented
microorganism growth. This study successfully showed that up-flow sand filters
prevented clogging through the pre-removal of higher-density sediments by its
bottom buffer zone, pre-filtration by layers of gravel support, and the
distribution of filtration sites deeper in the bed. Although clogging was not
significant during the 135 days of monitoring, the effects in the up-flow sand
filter over a long-term period could be further explored.
The authors would like to thank the Ministry of Higher
Education Malaysia for funding this research.
Abd-Lahin,
F., Sarbatly, R., Chel-Ken, C., 2021. Point-of-use Up-flow Sand Filter for
Rural Water Treatment using Natural Local Sand: Understanding and Predicting
Pressure Drop. In: IOP Conference Series Material Science Engineering,
Volume 1192 (1), p. 012008
Abukhanafer, G., Al-Fatlawi, A.H., Joni, H.H., Salman, H.M., 2021. A Laboratory Investigation
to Remove the Responsible for Clogging In the Filtration Process. Environmental
Technology & Innovation, Volume 21, p. 101345
Al-Saedi,
R., Smettem, K., Siddique, K.H.M., 2019. The Impact of Biodegradable Carbon
Sources on Microbial Clogging of Vertical Up-flow Sand Filters Treating
Inorganic Nitrogen Wastewater. Science of the Total Environment, Volume
691, pp. 360–366
Altmann,
J., Rehfeld, D., Träder, K., Sperlich, A., Jekel, M., 2016. Combination of
Granular Activated Carbon Adsorption and Deep-bed Filtration as a Single
Advanced Wastewater Treatment Step for Organic Micropollutant and Phosphorus
Removal. Water Research, Volume 92, pp. 131–139
Andreoli,
F.C., Sabogal-Paz, L.P., 2020. Household Slow Sand Filter to Treat Groundwater
with Microbiological Risks in Rural Communities. Water Research, Volume
186, pp. 1–11
Bassin,
J.P., Kleerebezem, R., Rosado, A.S., Van Loosdrecht, M.C.M., Dezotti, M., 2012.
Effect of Different Operational Conditions on Biofilm Development,
Nitrification, and Nitrifying Microbial Population in Moving-Bed Biofilm
Reactors. Environmental Science and Technology, Volume 46(3), pp.
1546–1555
Bozorg,
A., Gates, I.D., Sen, A., 2015. Impact of Biofilm on Bacterial Transport and
Deposition in Porous Media. Journal of Contaminant Hydrology, Volume
183, pp. 109–120
Budhijanto,
W., Deendarlianto, D., Kristiyani, H., Satriawan, D., 2015. Enhancement of
Aerobic Wastewater Treatment by the Application of Attached Growth
Microorganisms and Microbubble Generator. International Journal of
Technology, Volume 6, pp. 1101–1109
Cai,
Y.A., Li, D., Liang, Y., Zeng, H., Zhang, J., 2016. Operational parameters
required for the start-up process of a biofilter to remove Fe, Mn, and NH3-N
from low-temperature groundwater. Desalination Water Treatment, Volume
57, pp. 3588–3596
Centre
for Affordable Water and Sanitation Technology (CAWST), 2012. Biosand Filters
for Technician: Construction Manual. Centre for Affordable Water and
Sanitation Technology, pp. 1–50
Chan,
S., Pullerits, K., Riechelmann, J., Persson, K.M., Rådström, P., Paul, C.J.,
2018. Monitoring Biofilm Function in New and Matured Full-Scale Slow Sand
Filters Using Flow Cytometric Histogram Image Comparison (CHIC). Water
Research, Volume 138, pp. 27–36
Chen, S.,
Dougherty, M., Chen, Z., Zuo, X., He, J., 2021. Managing Biofilm Growth and
Clogging to Promote Sustainability in an Intermittent Sand Filter (ISF). Science
of The Total Environment, Volume 755 (1), p. 142477
De-Souza,
F.H., Roecker, P.B., Silveira, D.D., Sens, M.L., Campos, L.C., 2021. Influence
of Slow Sand Filter Cleaning Process Type on Filter Media Biomass: Backwashing
Versus Scraping. Water Research, Volume 189, pp. 1–12
Donda, D., Paranita, D., Simatupang, D.F., 2024. Analysis of Pressure Loss for Treatment
Process of Demineralized Water at the Water Treatment Plant Unit at PT. ABC
North Sumatra. Justek: Jurnal Sains dan Teknologi, Volume 7 (1), pp. 11–17
Duran-Romero,
D.A., de Almeida Silva, M.C., M. Chaúque, B.J., D. Benetti, A., 2020. Biosand
Filter as a Point-of-Use Water Treatment Technology: Influence of Turbidity on
Microorganism Removal Efficiency. Water, Volume 12, p.
2302
Freitas,
B.L.S., Terin, U.C., Fava, N.deM.N., Sabogal-Paz, L.P., 2021. Filter media
depth and its effect on the efficiency of Household Slow Sand Filter in
continuous flow. Journal of Environmental Management. Volume 288, p.
112412
Gibert,
O. Lefèvre, B., Fernández, M., Bernat, X., Paraira, M., Calderer, M.,
Martínez-Lladó, X., 2013. Characterizing Biofilm Development on Granular
Activated Carbon used for Drinking Water Production. Water Research,
Volume 47, pp. 1101–1110
Heikal,
G., Wagdy, R., Eldidamony, G., 2017. Bacteriophage Removal Using Up-flow
Biosand filter: A Laboratory Study. American Scientific Research Journal for
Engineering, Technology, and Science, Volume 1, pp. 118–125
Kurniawan,
A., Yamamoto, T., Ekawati, A.W., Salamah, L.N., Amin, A.A., Yanuar, A.T., 2022.
Characteristics of Cd (II) Biosorption into Streamer Biofilm Matrices. International
Journal of Technology, Volume 13, pp. 367–377
Lahin,
F.A., Sarbatly, R., Chel-Ken, C., 2022. Performance of an Upflow Sand Filter as
a Point-of-Use Treatment System in Rural Areas. American Society for
Microbiology (ASM) Science Journal, Volume (17), pp. 2–7
Mohamed,
A.Y.A., Tuohy, P., Healy, M.G., hUallacháin, D.Ó., Fenton, O., Siggins, A.,
2023. Effects of Wastewater Pre-Treatment on Clogging of An Intermittent Sand
Filter, Science of The Total Environment, Volume 876, p. 162605
Mutsvangwa,
C., Matope, E., 2017. Use of an External Organic Carbon Source in the Removal
of Nitrates in Bio-sand Filters (BSFs). Drinking Water Engineering and
Science, Volume 10(2), pp. 119–127
Polyakov,
V., Kravchuk, A., Kochetov, G., Kravchuk, O., 2019. Clarification Of Aqueous
Suspensions with a High Content of Suspended Solids in Rapid Sand
Filters. EUREKA: Physics and Engineering, Volume 1, pp. 28–45
Ramsay,
L., Breda, I.L., Soborg, D.A., 2018. Comprehensive Analysis of the Start-up
Period of a Full-scale Drinking Water Biofilter Provides Guidance for
Optimization, Drinking Water Engineering and Science, Volume 11(2), pp.
87–100
Salkar,
V.D., Tembhurkar, A.R., 2016. Experimental Evaluation of Ripening Behavior:
Down-Flow vs. Up-Flow Rapid Sand Filters. KSCE Journal of Civil
Engineering, Volume 20, pp. 1221–1227
Saravanan, S.P., Gobinath, R., 2015. Drinking Water
Safety through Bio Sand Filter - A Case Study of Kovilambakkam Village,
Chennai. International Journal of Applied Engineering Research, Volume
10(53), pp. 973 –4562
Segismundo,
E.Q., Lee, B.S., Kim, L.H., Koo, B.H., 2016. Evaluation of the Impact of Filter
Media Depth on Filtration Performance and Clogging Formation of a Stormwater
Sand Filter. Journal of Korean Society on Water Environment, Volume
32(1), pp. 36–45
Shreya,
A.T., Doris, V.H., Jan W.F., Jan, P.V.D.H., 2023. The Contribution of Deeper
Layers in Slow Sand Filters to Pathogens Removal. Water Research, Volume
237, p. 119994
Suryawan,
I.W.K., Septiariva, I.Y., Helmy, Q., Notodarmojo, S., Wulandari, M., Sari,
N.K., Sarwono, A., Pratiwi, R., Lim, J.W., 2021. Comparison of Ozone
Pre-Treatment and Post-Treatment Hybrid with Moving Bed Biofilm Reactor in
Removal of Remazol Black 5. International Journal of Technology, Volume
12(2), pp. 727–738
Wakelin,
S.A., Page, D.W., Pavelic, P., Gregg, A.L., Dillon, P.J., 2010. Rich Microbial
Communities Inhabit Water Treatment Biofilters and are Differentially Affected
by Filter Type and Sampling Depth. Water Science and Technology: Water
Supply, Volume 10(2), pp. 145–156
Wang,
H., Xin, J., Zheng, X., Fang, Y., Zhao, M., Zheng, T., 2023. Effect of Biofilms
on the Clogging Mechanisms of Suspended Particles in Porous Media During
Artificial Recharge. Journal of Hydrology, Volume 619, p. 129342
Wu,
Z., Qi, Y., Kang, A., Li, B., Xu, X. 2020. Evaluation of Particulate Matter
Capture and Long-Term Clogging Characteristics of Different Filter Media for
Pavement Runoff Treatment. Advances in Materials Science and Engineering,
Volume 2020, p. 5012903
Young-Rojanschi, C., Madramootoo, C., 2015. Comparing the Performance of Biosand Filters Operated with Multiday Residence Periods. Journal of Water Supply: Research and Technology–AQUA, Volume (64), pp. 157–167
Zeng, J., Chen, S., Wan, K., Li, J., Hu, D., Zhang, S., Yu, X., 2020. Study Of Biological Up-Flow Roughing Filters Designed for Drinking Water Pretreatment in Rural Areas: Using Ceramic Media as Filter Material. Environmental Technology (United Kingdom), Volume (41), pp. 1256–1265
