Investigation of Characteristics Whey Protein Isolate/Chitosan with Silica Addition in Biocomposite Film
Corresponding email: medyan_riza@unsyiah.ac.id
Published at : 31 Jan 2025
Volume : IJtech
Vol 16, No 1 (2025)
DOI : https://doi.org/10.14716/ijtech.v16i1.6335
Zuwanna, I, Riza, M, Aprilia, S, Syamsuddin, Y, Said, SD & Dewi, R 2025, 'Investigation of characteristics whey protein isolate/chitosan with silica addition in biocomposite film. International Journal of Technology, vol. 16, no. 1, pp. 359-369
| Ika Zuwanna | 1. Doctoral Program, School of Engineering, Post Graduate Program, Universitas Syiah Kuala, Banda Aceh 23111, Indonesia, 2. Department of Chemical Engineering, Faculty of Engineering, Universitas Syia |
| Medyan Riza | Department of Chemical Engineering, Faculty of Engineering, Universitas Syiah Kuala, Banda Aceh 23111, Indonesia |
| Sri Aprilia | Department of Chemical Engineering, Faculty of Engineering, Universitas Syiah Kuala, Banda Aceh 23111, Indonesia |
| Yanna Syamsuddin | Department of Chemical Engineering, Faculty of Engineering, Universitas Syiah Kuala, Banda Aceh 23111, Indonesia |
| Syahiddin Said | Department of Chemical Engineering, Faculty of Engineering, Universitas Syiah Kuala, Banda Aceh 23111, Indonesia |
| Rozanna Dewi | Department of Chemical Engineering, Faculty of Engineering, Universitas Malikussaleh, Aceh Utara 24351, Indonesia |
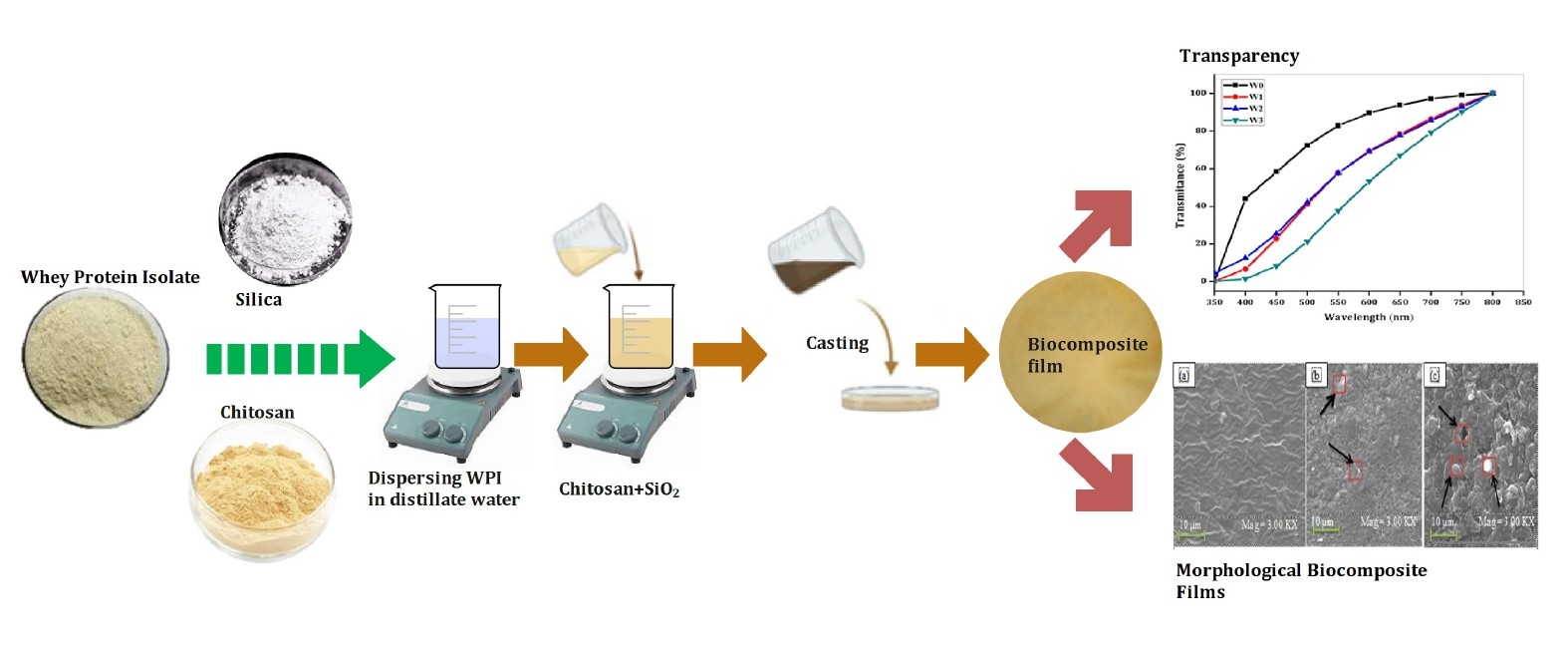
Biocomposites made from whey protein isolate (WPI), chitosan, and silica (SiO2) have the potential as an environmentally friendly biopolymer material. In this research, the WPI biocomposite film has been developed by casting method with the addition of chitosan and SiO2. The loading of SiO2 (1, 3, and 5 %w/w) and chitosan (1.5 %w/w) affects the characteristics of the biocomposite film. The functional groups, morphology, structure, mechanical, thermal, and transparency of the biocomposite films were evaluated by Fourier transform infrared (FTIR) spectroscopy, Scanning electron microscope (SEM), X-ray diffraction (XRD), tensile strength, thermogravimetric (TGA), differential scanning calorimetry (DSC), thickness, swelling, and UV-Vis. The results showed that the swelling value of WPI/chitosan 78.23% decreased to 69.35% with the use of 5% SiO2. Film transparency decreased with higher SiO2, while the film tensile strength increased with the addition of 5% SiO2 with a value of 28.83 MPa. The incorporation of SiO2 improves the thermal properties of biocomposite films. According to the results, increasing the amount of SiO2 in biocomposite films boosts their performance, suggesting that they could eventually replace synthetic polymers in packaging applications.
Biocomposite film; Chitosan; Film properties; Silica; Whey protein isolate
The worldwide issue of food product packaging and the negative long-term environmental impact of nondegradable plastic waste has prompted research into sustainable and green edible films (Berawi, 2023; Alizadeh-sani et al., 2018). Likewise, several biopolymer biocomposites have been employed to manufacture environmentally safe food components for packaging (Yusoff et al., 2021; Wang et al., 2014). Due to their biodegradability, high availability, and renewability, biopolymers such as protein structures, lipids, polysaccharides, and mixtures are accepted as the most commonly used materials (Kurek et al., 2014).
Chitosan is a biopolymer that can be extracted from various sources such as Scylla serrata shells, mud crab shells, shrimp shells, fungi, and mollusks (Narudin et al., 2022; Pakizeh et al., 2021; Narudin and Mahadi, 2020; Kaya et al., 2015). The extraction process of chitosan through the deacetylation of chitin at acidic or alkaline pH can produce valuable linear polysaccharides with significant potential (Zhang et al., 2016). Non-toxic, eco-friendly, biocompatible, and highly antimicrobial, chitosan is a promising biodegradable film material for food packaging (Khan et al., 2012). However, chitosan film has a few drawbacks, the most notable being its poor mechanical qualities and limited moisture resistance. The limitations of chitosan have led to numerous developments aimed at enhancing the characteristics of the materials, such as adding fillers.
Whey protein isolate (WPI) is a type of biopolymer that is noteworthy due to its unusual mechanical properties (Kurek et al., 2014). WPI is isolated from whey, a byproduct of the cheese-making sector. The films generated from WPI are transparent and act as highly effective barriers to gases. However, they do not demonstrate capabilities or significant moisture barrier properties (Sothornvit et al., 2009). Therefore, films made of WPI and chitosan have been developed to overcome these obstacles and to incorporate each components benefits into producing films with excellent characterization (Pereda et al., 2011). However, the incompatibility of WPI and chitosan has led to poor performance in terms of their mechanical and physical qualities. The proper method to address this compatibility problem is to integrate particles between the two polymers (Achachlouei and Zahedi, 2018).
Particles such as titanium dioxide (TiO2), zinc oxide (ZnO), clay, and silica (SiO2) have been studied as potential weakening agents for various polymers (Kusmono & Abdurrahim, 2019; Celebi and Kurt, 2015; Aryaei et al., 2013). SiO2 can be employed as a filler in biopolymer films due to its highly porous structure, high surface activity, and biocompatibility (Talebian and Zare, 2014). Incorporating the SiO2 into the matrix of the corn starch and LDPE biocomposite improved its mechanical properties (Datta and Halder, 2019). The concentration of filler incorporated into biocomposite films needs to be increased. Therefore, increasing the amount of SiO2 may affect the mechanical and thermal properties of WPI/chitosan-based biocomposite films due to their benefits and functional qualities.
WPI-based films reinforced with particles have been extensively studied by various researchers (Zhai et al., 2021; Alizadeh-sani et al., 2018; Azevedo et al., 2018). However, there is no traceable literature to combine chitosan with SiO2 as a reinforcing agent to obtain WPI-based biocomposites for packaging. The overall aim of this study is to produce biocomposite films with enhanced physical, chemical, mechanical, and thermal properties. To achieve this, WPI/chitosan will be blended with different amounts of SiO2 (1%, 3%, and 5% w/w) using the solution pouring process. The SiO2 used in this research was prepared from rice husk ash (RHA) using sodium hydroxide (NaOH). In addition, the effects on thermal durability, water resistance, morphology, and light transmission behavior of WPI/chitosan films after incorporating SiO2 were determined. The structure of the biocomposite film was characterized by X-ray diffraction (XRD) and Fourier transform infrared (FT-IR), while the tensile strength was determined using a tensile test.
2.1. Materials
The materials used to produce the biocomposite film for this study were: WPI (90% protein, specialities global USA). Chitosan from prawn shells (medical grade, CV. Chimultiguna, Indonesia), glacial acetic acid (KGAa, Germany), and glycerol (food grade, CV. Rudang Jaya, Indonesia). The SiO2 used in this study was prepared by isolating RHA using NaOH (Merck, Germany), which was titrated with hydrochloric acid (HCl, Merck, USA).
2.2. Fabrication of SiO2 from RHA
The SiO2 used in this study was produced from RHA using the formula published in our previous research (Zuwanna et al., 2021). After extracting SiO2 with a NaOH solution, the filtrate was titrated with 3 N HCl, with constant stirring, until the pH=7 in order to achieve complete precipitation. The silica gel was filtered and heated at 120°C for 12 hours. At 80°C, the resulting xerogel was rinsed with 100 mL of distillate water and agitated for 15 min. The mixture was then filtered, and the precipitate was heated at 120°C for 4 hours. After drying, the produced substance is ground into powder and stored at room temperature until further application. Figure 1 provide a schematic illustration of the production process of SiO2 from RHA.
Figure 1 The illustration to produce SiO2 from RHA.
2.3. Biocomposite Film Preparation
WPI suspension (5% w/v) was prepared by dispersing WPI in distillate water and heating it at 90°C for 30 minutes. To prepare the chitosan solution (1.5% w/v), chitosan was dispersed in a 1% v/v acetic acid solution and stirred continuously for 3 hours at 80°C. Based on preliminary experiments, the WPI-chitosan suspension was prepared by mixing two polymer suspensions and stirring magnetically for 15 minutes at 25°C. In the next step, SiO2 (1%, 3%, 5% w/w) was combined, and after mixing for 15 minutes, glycerol (70% w/w) was added to the biocomposite suspension and stirred for 30 minutes. The film suspension was placed at room temperature for 30 minutes to degas. The suspended biocomposite film was poured into petri dishes and dried at 55°C for 24 hours. The research work steps are schematically shown in Figure 2, and the code for the resulting WPI/chitosan-SiO2-based biocomposite is available in Table 1.
Figure 2 Schematic of the design of WPI biocomposite using the casting technique.
Table 1 Indexing of the formed WPI/chitosan based biocomposite film.
2.4. Biocomposite Film Preparation
The biocomposite film thickness was calculated using a micrometer (Mitutoyo, Japan) with an accuracy of ±0.001 mm. The mean of each sample was calculated after it completed five random tests. The dimensions of 2 x 2 cm pieces of biocomposite film were dried in a 105°C oven, weighed at their dry weight (Wi), and then submerged in 50 ml of distilled water at room temperature for 24 hr (Wf). Moisture absorption capacity is determined by applying the following formula (1) (Gohargani et al., 2020):
Swelling (%) = Wf-WiWi x 100 (1)
The chemical composition of the films was tested using an FTIR (IRPrestige-21, Shimadzu, Kyoto, Japan). The film spectrum was measured using a 25 scan resolution of 4 cm-1 for the sample, with a scan range of 4000 to 500 cm-1. The XRD of the biocomposite films was measured with an XRD (Philips, Malvern, UK) set with CuK radiation (? = 0.1541 nm) in the 2? zone of 5-30° with a step increment of 2? = 0.02 at 40 kV and 30 mA by scanning in the 2? mode. The morphology of biocomposite WPI/chitosan samples was visualized through scanning electron microscope (SEM) (VEGA II-550, TESCAN, Czech Republic) with an acceleration voltage of 10 kV. Before testing, samples were manually sliced in liquid nitrogen. The specimens were taped to a stub and thinly coated with gold. The samples were placed in the SEM chamber and viewed at an intensity of 5000 (surface area). The transparency of the films was analyzed using a UV-Vis spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan) at a wavelength of 350-850 nm. The tensile strength (TS) and elongation at break (EB) parameters of the biocomposite film were determined using a universal testing machine according to the ASTM standard procedure D638. The thermal behavior of biocomposites can be investigated based on a thermal analyzer. The biocomposite was trimmed 2 x 2 cm before being heated at 20°C per minute from a temperature of 50°C up to 500°C. Thermogravimetric (TGA) generates data on weight loss as a function of temperature in the form of TGA curves. The differential scanning calorimetry (DSC) curves specify the thermal properties of the emulsion film.
Thickness of biocomposite film
There was no visible difference in film thickness comparing the several WPI-based biocomposites. As listed in Table 2, when SiO2 was incorporated, the thickness increased. The addition of SiO2, W0, W1, W2, and W3 biocomposite films have a thickness between 0.18 to 0.23 mm. The increase in SiO2 concentration causes W3 to have a higher thickness than W0. The combination of SiO2 and chitosan with WPI solution impact the volume of the solution. The more the SiO2 concentration, the more significant the total dissolved solids in the biocomposite solution. It has been found that the thickness of a film increases as the filler concentration increases (Poerwadi et al., 2020; Marichelvam et al., 2019). The difference in thickness values between our work and Zhang et al. (2018) is due to the matrix, the type of SiO2 used, and the percentage concentration of bulking agent applied.
Table 2 Thickness of biocomposite film.
3.2. Swelling Performance of Biocomposite Film
The swelling value is an indicator that explains the amount of swelled material, which influences the performance of pore size. The degree of swelling in a material depends mostly on crystallinity and functional group. Furthermore, measuring the swelling level can determine the number of water molecules absorbed by the biocomposite mass. Due to the presence of SiO2, the swelling rate of W1, W2, and W3 films was observed to be slower than that of W0 films. Their ability to absorb moisture increased and limited their swelling rate (Table 3).
Table 3 The swelling of biocomposite film.
Adding SiO2 and chitosan can reduce the percentage of water absorption, as shown in Table 3. SiO2 and chitosan act as fillers to strengthen the resulting biocomposite. The percentage of water absorption for biocomposite W0 is 78.23%, while the percentages for W1, W2, and W3 are 75.15%, 71.13%, and 69.35%, respectively. W1, W2, and W3 demonstrate more excellent water absorption than W0. Increasing the concentration of biocomposite fillers causes the forces between the polymer matrices to become more concentrated, resulting in a more robust biocomposite structure. The biocomposite's resistance to water migration is enhanced due to the network's robust structure. The intermolecular hydrogen bonds between WPI, chitosan, and SiO2 enhance the cohesion of the polymer matrix. The mechanism of chitosan and its biocomposites involves interactions such as hydrogen bonding. Chitosan and its biocomposites have several hydroxyl groups and amino and carboxylic groups that are helpful for such interactions. Therefore, SiO2 and chitosan enhance the film's barrier properties and the water resistance (Hassannia-Kolaee et al., 2016).
3.3. FTIR analysis
The incorporation of chitosan with SiO2 caused changes in the spectrum of the resulting biocomposite, as depicted in Figure 3. The FTIR results of the biocomposite under various conditions of adding chitosan and SiO2 exhibited the same trend, except for a spectrum shift that occurred with increasing amounts of immobilized SiO2. The Si-O stretching vibration wavenumber of siloxane (Si-O-Si) decreases from 1058 cm-1 to a lower frequency. It was cross-linking between Si-O silanol groups and chitosan resulted in the formation of condensation. Figure 3 shows the absorption of the chitosan groups, such as the absorption at wave number 3346 cm-1, which shows -O-H stretching vibrations originating from chitosan, and at the peak of the 1660 cm-1 spectrum is an amide group remaining in chitosan, and the absorption at 1405 cm-1 is a -C-O bending vibration from WPI. This mixing did not result in the formation of chemical bonds, as evidenced by the appearance of each group in the infrared absorption spectrum (Imani et al., 2022; Sari et al., 2018).
Figure 3 FTIR spectrum of WPI/chitosan biocomposite film combined with SiO2.
3.4. Morphological Biocomposite Films
Figure 4 displays SEM taken from the surfaces of components W0, W2, and W3. According to the findings, the morphology of W0 is devoid of cracks and aggregates in its structure. On the other hand, incorporating SiO2 fillers and chitosan resulted in a rougher surface. Figure 4 shows that the fillers are evenly dispersed throughout the W2 biocomposite, forming small voids and agglomerates (rectangular marks in Figure 4b). Due to the inhomogeneous dispersion of fillers used in the W3 biocomposite film, its surface displays a large number of agglomerates as well as voids. This is because the WPI denatured during the process. The presence of SiO2 and chitosan fillers in suspension also tends to cause agglomeration in the biocomposite film (Giosafatto et al., 2019).
Figure 4 Surface SEM micrograph of (a) W0, (b) W2, and (c) W3.
3.5. Transparency
Transparency indicates polymer admixture and reflects the effect of combining chitosan with SiO2 as a filler in the matrix (Jiang et al., 2019). The biocomposite transparency test using UV-Vis light was carried out to determine the transmittance value passed through the biocomposite film in addition to 1.5% chitosan with 1%, 3%, and 5% SiO2 concentrations. The transparency of the biocomposite is shown in Figure 5.
Figure 5 The transparency of WPI/chitosan biocomposite film combined with SiO2.
The W0 biocomposite film had the highest transmittance value across the board for the various wavelength ranges measured. When SiO2 was added to the W1, W2, and W3 biocomposite films, these films' transparency was significantly reduced compared to W0. This decrease suggests that the concentration of SiO2 in the biocomposite film harms its transparency. The transparency of the W0 biocomposite at 600 nm was measured to be 89.68%, whereas that of W1 was only 53.21%. Interfacial interactions between chitosan/SiO2 and the WPI matrix can cause this. As a result of these interactions, the path of light can be blocked as it travels through the polymer matrix (Hejazi et al., 2018). In addition, the presence of SiO2 particles in the polymer matrix can increase the amount of light scattered in the matrix, which could also contribute to this decrease in transparency.
3.6. XRD Characterization
The X-ray diffraction pattern produced by a film is related to the shape of an amorphous crystal. It depends on the rate at which crystallization occurs in the biocomposite film. Figure 6 illustrates the XRD patterns of W0, W1, W2, and W3, respectively. The incorporation of chitosan and SiO2 in the biocomposite film resulted in several distinct peaks, including a peak at 19.09° which corresponds to chitosan. When the biocomposite film was formed using 1.5% chitosan, the resulting film exhibited an amorphous structure. However, it also provided a relatively good crystalline level, suggesting that it could be categorized as a semi-crystalline biopolymer. Because the formation of the protein's amorphous structure can change the chitosan film's physical state, this phenomenon can be explained by modifying the chitosan structure (Zhang et al., 2016).
Figure 6 XRD profile on biocomposite film in different concentration SiO2.
At W0, W1, W2, and W3 of the biocomposite film, the peaks appeared at 2? as 19.20°, 19.93°, and 19.97°, respectively. Through the associated with the addition of SiO2, a new peak appears between 2?= 37.77° and 38.03°. In the biocomposite film, the diffraction peaks that appear at 2? are attributed to SiO2 crystals. This is consistent with (Li et al., 2011), who reported that the amount of TiO2 and SiO2 particles increased the crystallinity of WPI. In addition, the presence of peaks indicates that the crystal structure of SiO2 is preserved during the production of biocomposite films. Compatibility between polymers and additives can lead to the formation of homogeneous films with desirable mechanical and physical properties, which are crucial for composite films (Mohammadian et al., 2021).
3.7. Tensile Strenght (TS) and Elongation of Breaks (EB)
The effect of chitosan and SiO2 on TS and EB is shown in Table 4. Compared to the W0 films, the incorporation of SiO2 increased the TS and EB values of the biocomposite films. The TS of the W0 film was 16.96 MPa, and W1, W2, and W3 TS increased to 17.65, 25.69, and 28.83 MPa, respectively. In comparison, the EB values of each biocomposite film were 21.17%, 25.41%, 25.90%, and 27.12%. An increase in TS and EB of biocomposite films with the addition of SiO2 can occur due to the uniform distribution of SiO2 through interfacial interactions (Jing et al., 2019).
Table 4 The mechanical properties of biocomposite film.
3.8 Thermal Properties
Thermal properties of WPI-based biocomposite films with the addition of chitosan and SiO2 are presented through TGA and DSC curves. As shown in Figure 7a, the TGA curve depicts a decrease in sample weight (%) against temperature (°C), while the DSC curve plots the derivative of the decrease in heat flow (mW) as a function of temperature (°C). The occurrence of multi-step thermal degradation events was found in both biocomposite films. The initial degradation step below 120°C results from eliminating water molecules. Furthermore, the weight loss at this stage can be attributed to the evaporation of low molecular weight compounds and water loosely bound in the biocomposite film. This weight loss was observed in all samples (W0, W1, W2, and W3). Further heating produces sharp peaks on the DSC W3. The evaporation of the adsorbed plasticizers and water molecules corresponds to the second stage of thermal degradation, which occurs between 130°C and 180°C. As indicated by the decrease in weight of the biocomposite film at temperatures above 285°C, the rate of thermal degradation was most significant at these temperatures. According to Figure 7b, the initial thermal degradation of the biocomposite film begins at approximately 285°C. This may result from the loss of hydrogen groups, depolymerization, and degradation of carbon chains. The maximum thermal decomposition of the W3 biocomposite film was 400°C, indicating that the thermal stability of the W3 biocomposite film was superior to W0. As demonstrated by previous research, adding fiber reinforcement increases the thermal stability of the biocomposite by reinforcing cellulose nanofibrils with chitosan/oregano essential oil and starch (Chen et al., 2020).
(b)
Figure 7 TGA (a) and DSC (b) of biocomposite films in different concentration.
In this study, chitosan and SiO2 were used to reinforce the matrix of WPI biocomposite films made by casting technique. WPI-based biocomposite films with the addition of fillers showed increased thermal and mechanical characteristics but decreased swelling properties. Based on SEM analysis, the morphology of W0 is homogeneous and smooth. The addition of filler makes the surface rougher and more heterogeneous, with small aggregates on the biocomposite surface. The maximum thermal decomposition of W3 biocomposite is 400°C, which indicates that W3 biocomposite film has better thermal stability than W0. WPI biocomposite films with improved thermal and mechanical properties can benefit the food packaging industry. A limitation of this study is that no assessment was made of the durability of the films under different environmental conditions. This research can be extended to evaluate the long-term durability of biocomposite films under various environmental conditions to establish their practical application in various industries. Future research can also evaluate the cost-benefit of producing WPI biocomposite films on a larger scale.
This work was supported by Universitas Syiah Kuala for ?nancial support granted via the PRUUPD Scholarship Program Number 478/UN11/SPK/PNBP/2022.
Author Contributions
Ika Zuwanna: Investigation, Writing-original draft, review & editing. Medyan Riza: Review & editing. Sri Aprilia: Performed the analysis. Yanna Syamsuddin: Visualization. Syahiddin Dahlan Said: Visualization. Rozanna Dewi: Conceptualization
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Achachlouei, BF & Zahedi, Y 2018, ‘Fabrication and characterization of CMC-based nanocomposites reinforced with sodiummontmorillonite and TiO2 nanomaterials’, Carbohydrate Polymers, vol. 199, pp. 415-425, https://doi.org/10.1016/j.carbpol.2018.07.031
Alizadeh-sani, M, Khezerlou, A & Ehsani, A 2018, ‘Fabrication and characterization of the bionanocomposite film based on whey protein biopolymer loaded with TiO2 nanoparticles, cellulose nanofibers and rosemary essential oil’, Industrial Crops and Products, vol. 124, pp. 300-315, https://doi.org/10.1016/j.indcrop.2018.08.001
Aryaei, A, Jayatissa, AH & Jayasuriya, A C 2013, ‘Mechanical and biological properties of chitosan/carbon nanotube nanocomposite films’, Journal of Biomedical Materials Research Part A, vol. 102, no. 8, pp. 2704–2712, https://doi.org/10.1002/jbm.a.34942
Azevedo, VM, Dias, MV, De Siqueira Elias, HH, Fukushima, KL, Silva, EK, Carneiro, JDDS, Soares, NDFF, & Borges, SV 2018, ‘Effect of whey protein isolate films incorporated with montmorillonite and citric acid on the preservation of fresh-cut apples’, Food Research International, vol. 107, pp. 306–313, https://doi.org/10.1016/j.foodres.2018.02.050
Berawi, MA 2023, ‘Smart cities: accelerating sustainable development agenda’, International Journal of Technology, vol. 14, no. 1, pp. 1-4, https://doi.org/10.14716/ijtech.v14i1.6323
Celebi, H & Kurt, A 2015, ‘Effects of processing on the properties of chitosan/cellulose nanocrystal films’, Carbohydrate Polymers, vol. 133, pp. 284-293, https://doi.org/10.1016/j.carbpol.2015.07.007
Chen, S, Wu, M, Wang, C, Yan, S, Lu, P & Wang, S 2020, ‘Developed chitosan/oregano essential oil biocomposite packaging film enhanced by cellulose nanofibril’, Polymers, vol. 12, no. 8, p. 1780, https://doi.org/10.3390/polym12081780
Datta, D & Halder, G 2019, ‘Effect of rice husk derived nanosilica on the structure, properties and biodegradability of corn-starch/LDPE composites’, Journal of Polymers and the Environment, vol. 27, pp. 710-727, https://doi.org/10.1007/s10924-019-01386-2
Giosafatto, CVL, Sabbah, M, Al-Asmar, A, Esposito, M, Sanchez, A, Santana, RV, Cammarota, M, Mariniello, L, Pierro, PD & Porta, R 2019, ‘Effect of mesoporous silica nanoparticles on glycerol-plasticized anionic and cationic polysaccharide edible films’, Coatings, vol. 9, no. 3, p. 172, https://doi.org/10.3390/coatings9030172
Gohargani, M, Lashkari, H & Shirazinejad, A 2020, ‘Study on biodegradable chitosan-whey protein-based film containing bionanocomposite TiO2 and zataria multiflora essential oil’, Journal of Food Quality, vol. 2020, pp. 1-11, https://doi.org/10.1155/2020/8844167
Hassannia-Kolaee, M, Khodaiyan, F, Pourahmad, R & Shahabi-Ghahfarrokhi, I 2016, ‘Development of ecofriendly bionanocomposite: whey protein isolate/pullulan films with nano-SiO2’, International Journal of Biological Macromolecules, vol 86, pp. 139–144, https://doi.org/10.1016/j.ijbiomac.2016.01.032
Hejazi, M, Behzad, T, Heidarian, P & Nasri-Nasrabadi, B 2018, ‘A Study of the effects of acid, plasticizer, cross-linker, and extracted chitin nanofibers on the properties of chitosan biofilm’, Composites Part A: Applied Science and Manufacturing, vol. 109, pp. 221-231, https://doi.org/10.1016/j.compositesa.2018.02.038
Imani, NAC, Kusumastuti, Y, Petrus, HTBM, Timotius, D, Putri, NRE & Kobayashi, M 2022, ‘Preparation, characterization, and release study of nanosilica/chitosan composite films’, International Journal of Technology, vol. 13, no. 2, pp. 444-453, https://doi.org/10.14716/ijtech.v13i2.4733
Jiang, SJ, Zhang, T, Song, Y, Qian, F, Tuo, Y & Mu G 2019, ‘Mechanical properties of whey protein concentrate based film improved by the coexistence of nanocrystalline cellulose and transglutaminase’, International Journal of Biological Macromolecules, vol. 126, pp. 1266-1272, https://doi.org/10.1016/j.ijbiomac.2018.12.254
Jing, M, Sui, G, Zhao, J, Zhang, Q & Fu, Q 2019, ‘Enhancing crystallization and mechanical properties of poly(lactic acid)/milled glass fiber composites via self-assembled nanoscale interfacial structures’, Composites Part A: Applied Science and Manufacturing, vol. 117, pp. 219-229, https://doi.org/10.1016/j.compositesa.2018.11.020
Kaya, M, Baublys, V, Satkauskiene I, Akyus, B, Bulut, E & Tubelye, V 2015, ‘First chitin extraction from plumatella repens (bryozoa) with comparison to chitins of insect and fungal origin’, International Journal of Biological Macromolecules, vol. 79, pp. 126-132, https://doi.org/10.1016/j.ijbiomac.2015.04.066
Khan, A, Khan, RA, Salmieri, S, Tien, CL, Riedl, B, Bouchard, J, Chauve, G, Tan, V, Kamal, MR & Lacroix, M 2012, ‘Mechanical and barrier properties of nanocrystalline cellulose reinforced chitosan based nanocomposite films’, Carbohydrate Polymers, vol. 90, no. 4, pp. 1601-1608, https://doi.org/10.1016/j.carbpol.2012.07.037
Kurek, M, Galus, S & Debeaufort, F 2014, ‘Surface, mechanical and barrier properties of bio-based composite films based on chitosan and whey protein’, Food Packaging and Shelf Life, vol. 1, no. 1, pp. 56-67, https://doi.org/10.1016/j.fpsl.2014.01.001
Kusmono & Abdurrahim, I 2019, ‘Water sorption, antimicrobial activity, and thermal and mechanical properties of chitosan/clay/glycerol nanocomposite films’, Heliyon, vol. 5 no. 8, pp. e02342
Li, Y, Jiang, Y, Liu, F, Ren, F, Zhao, G & Leng, X 2011, ‘Fabrication and characterization of TiO2/whey protein isolate nanocomposite film’, Food Hydrocolloids, vol. 25, no. 5, pp. 1098-1104, https://doi.org/10.1016/j.foodhyd.2010.10.006
Marichelvam, MK, Jawaid, M & Asim, M 2019, ‘Corn and rice starch-based bio-plastics as alternative packaging materials’, Fibers, vol. 7, no. 4, p. 32, https://doi.org/10.3390/fib7040032
Mohammadian, M, Moghaddam, AD, Sharifan, A, Dabaghi, P & Hadi, S 2021, Structural, physico-mechanical, and bio-functional properties of whey protein isolate-based edible films as affected by enriching with nettle (Urtica Dioica L) leaf extract, Journal of Food Measurement and Characterization, vol. 15, no. 5, pp. 4051–4060, https://doi.org/10.1007/s11694-021-00988-6
Narudin, N,A,H, Mahadi, A,H, Kusrini, E & Usman, A 2020, ‘Chitin, chitosan, and submicron-sized chitosan particles prepared from scylla serrata shells’, Materials International, vol. 2, no. 2, pp. 139-149, https://doi.org/10.33263/Materials22.139149
Narudin, NAH, Rosman, NA, Shahrin, EWE, Sofyan, N, Mahadi, AH, Kusrini, E, Hobley, J & Usman, A, 2022, Extraction, characterization, and kinetics of N-Deacetylation of chitin obtained from mud crab shells, Polymers and Polymer Composites, vol. 30, pp. 1-11, https://doi.org/10.1177/09673911221109611
Pakizeh, M, Moradi, A & Ghassemi, T 2021, ‘Chemical extraction and modification of chitin and chitosan from shrimp shells’, European Polymer Journal, vol. 159, p. 110709, https://doi.org/10.1016/j.eurpolymj.2021.110709
Pereda, M, Ponce, AG, Marcovich, NE, Ruseckaite, RA & Martucci, JF 2011, ‘Chitosan-gelatin composites and bi-layer films with potential antimicrobial activity’, Food Hydrocolloids, vol. 25, no. 5, pp. 1372–1381, https://doi.org/10.1016/j.foodhyd.2011.01.001
Poerwadi, B, Katikowati, CW, Oktavian, R & Novaresa, O 2020, Manufacture of a hydrophobic silica nanoparticle composite membrane for oil-water emulsion separation, International Journal of Technology, Volume 11(2), pp. 364-373, https://doi.org/10.14716/ijtech.v11i2.3279
Sari, NH, Wardana, ING, Irawan, YS & Siswanto, E 2018, Characterization of the chemical, physical, and mechanical properties of naoh-treated natural cellulosic fibers from corn husks, Journal of Natural Fibers, vol. 15 no. 4, pp. 545–558, https://doi.org/10.1080/15440478.2017.1349707
Sothornvit, R, Rhim, JW & Hong, SI 2009, ‘Effect of nano-clay type on the physical and antimicrobial properties of whey protein isolate/clay composite films’, Journal of Food Engineering, vol. 91, no. 3, pp. 468–473, https://doi.org/10.1016/j.jfoodeng.2008.09.026
Talebian, N & Zare, E 2014, ‘Structure and antibacterial property of nano-SiO2 supported oxide ceramic’, Ceramics International, vol. 40, no. 1, pp. 281-287, https://doi.org/10.1016/j.ceramint.2013.05.135
Wang, JL, Cheng, F & Zhu, PX 2014, ‘Structure and properties of urea-plasticized starch films with different urea contents’, Carbohydrate Polymers, vol. 101, no. 1, pp. 1109-1115, https://doi.org/10.1016/j.carbpol.2013.10.050
Yusoff, NH, Pal, K, Narayanan, T & Souza, FGD 2021, ‘Recent trends on bioplastics synthesis and characterizations: polylactic acid (PLA) incorporated with tapioca starch for packaging applications’, Journal of Molecular Structure, vol. 1232, p. 129954, https://doi.org/10.1016/j.molstruc.2021.129954
Zhai, X, Zhang, X, Ao, H, Yin, Y, Li, X & Ren, D 2021, ‘Preparation and characterization of whey protein isolate/chitosan/microcrystalline cellulose composite films’, Packaging Technology and Science, vol. 34, no. 9, pp. 589–599, https://doi.org/10.1002/pts.2597
Zhang, R, Wang, X & Cheng, M 2018, ‘Preparation and characterization of potato starch film with various size of nano-SiO2’, Polymers, vol. 10, no. 10, p. 1172, https://doi.org/10.1002/pts.2597
Zhang, W, Chen, J, Chen, Y, Xia, W, Xiong, YL & Wang, H 2016, ‘Enhanched physicochemical properties of chitosan/whey protein isolate composite film by sodium laurate-modified TiO2 nanoparticles’, Carbohydrate Polymers, vol. 138, pp. 59-65, https://doi.org/10.1016/j.carbpol.2015.11.031
Zuwanna, I, Riza, M & Aprilia, S. 2021, ‘The impact of solvent concentration on the characteristic of silica from rice husk ash using sol gel method’, IOP Conference Series: Materials Science and Engineering, vol. 1087(1), p. 012060, DOI: 10.1088/1757-899X/1087/1/012060
