Improvement of an Anode-Supported Intermediate Temperature Solid Oxide Fuel Cell with Spray-Coated Calcia-Stabilized Zirconia Electrolytes
Corresponding email: hardev@itb.ac.id
Published at : 24 Dec 2024
Volume : IJtech
Vol 15, No 6 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i6.6308
Yusupandi, F., Ilham, M., Yafi, I.A., Widiatmoko, P., Nurdin, I., Febrianti Khairunnisa, S., Devianto, H., 2024. Improvement of an Anode-Supported Intermediate Temperature Solid Oxide Fuel Cell with Spray-Coated Calcia-Stabilized Zirconia Electrolytes. International Journal of Technology. Volume 15(6), pp. 1971-1981
| Fauzi Yusupandi | 1. Department of Chemical Engineering, Faculty of Industrial Technology, Institut Teknologi Bandung, Jl. Ganesha No. 10, Bandung 40132, Indonesia. 2. Department of Chemical Engineering, Institut Tekno |
| Muhammad Ilham | 1. Department of Chemical Engineering, Faculty of Industrial Technology, Institut Teknologi Bandung, Jl. Ganesha No. 10, Bandung 40132, Indonesia |
| Ilham Ali Yafi | 1. Department of Chemical Engineering, Faculty of Industrial Technology, Institut Teknologi Bandung, Jl. Ganesha No. 10, Bandung 40132, Indonesia |
| Pramujo Widiatmoko | 1. Department of Chemical Engineering, Faculty of Industrial Technology, Institut Teknologi Bandung, Jl. Ganesha No. 10, Bandung 40132, Indonesia |
| Isdiriayani Nurdin | 1. Department of Chemical Engineering, Faculty of Industrial Technology, Institut Teknologi Bandung, Jl. Ganesha No. 10, Bandung 40132, Indonesia |
| Saumi Febrianti Khairunnisa | 1. Department of Chemical Engineering, Faculty of Industrial Technology, Institut Teknologi Bandung, Jl. Ganesha No. 10, Bandung 40132, Indonesia |
| Hary Devianto | 1. Department of Chemical Engineering, Faculty of Industrial Technology, Institut Teknologi Bandung, Jl. Ganesha No. 10, Bandung 40132, Indonesia |
Among the three types of Solid Oxide Fuel Cell (SOFC), intermediate temperature solid oxide fuel cells (IT-SOFC) have been widely developed due to reducing operating costs and materials. In this study, we produced an anode-supported IT-SOFC cell through the dry-pressing method for NiO-CSZ anode production and spray coating technique for Calcia-Stabilized Zirconia (CSZ) electrolyte and calcium cobalt zinc oxide (CCZO)-CSZ cathode fabrication. A single cell was enhanced by changing the anode’s composition, increasing the sintering temperature, using a ball mill as mixing equipment, and multiplying the coating of the electrolyte. The cell with eight times of electrolyte coating had a greater peak power density than that of one time of electrolyte coating even though the curvature of eight times of electrolyte coating was higher. Additionally, the cell performance with eight times of electrolyte achieved the peak power density of 0.24; 0.35; and 1.08 mW/cm2 and the ohmic resistance of 168.3; 90.10; and 26.78 ? at the operating temperature of 600, 700, and 800 °C, respectively.
Anode-supported; Calcia Stabilized Zirconia (CSZ); Curvature; IT-SOFC; Spray coating
Fuel cell directly transforms the chemical energy of various fuels and
an oxidant (often air) into electricity and heat without burning the fuel. The
advantages of fuel cells are high electrical efficiency, silent operation, and
little or no emission (Sazali et al., 2020; Wang and
Jiang, 2017). Solid oxide fuel
cell (SOFC) is one of the highly promising fuel cells in small to large-scale
power plant applications. SOFC is classified as a high-temperature fuel cell
operated between 800 and 1,000 °C. Unlike low-temperature fuel cells (PEMFCs),
which need pure hydrogen as fuel, fossil or renewable fuels such as natural gas
and bioethanol can be used directly in SOFC through internal reforming.
Moreover, noble metals are unnecessary for SOFC’s electrode materials since the
high operating temperature enhances the reaction kinetics of electrodes (Shi et al., 2020; Kaur and Singh, 2020). However, the production and operation cost of
conventional SOFC is too high due to complex materials and high heat
requirements. To overcome these drawbacks, the operating temperature of
The main parts of the SOFC cell
are a porous cermet anode, a dense ceramic electrolyte, and a porous
oxide-based cathode. NiO is frequently used as an anode material which has
great electrical conductivity and oxidation kinetics of hydrogen (Abdalla et al., 2018; Singhal and Kendall, 2003). On the other hand, the common cathode material
is lanthanum-based oxide composites such as lanthanum strontium manganite (LSM)
and lanthanum cobalt ferrite (LSCF). However, the side reaction of
lanthanum-based cathode and zirconia-based electrolyte can occur at high
temperatures to form a high-resistance product (Chen
et al., 2014). Nowadays, calcium-
and cobalt-based materials such as calcium cobalt oxide (CCO) and calcium
cobalt zinc oxide (CCZO) is prospective cathodes in IT-SOFC owing to low cost,
great oxygen reduction activity, suitable thermal expansion with electrolyte
materials and thermoelectric behavior to utilize waste heat to electricity (Yu et al., 2017; Takami and Ikuta, 2005). Additionally, yttria-stabilized zirconia (YSZ)
is widely used as an electrolyte in SOFC systems (Rahmawati
et al., 2017). However, yttria is
costly and has low reserves associated with rare earth materials. Calcia (CaO)
can be an alternative stabilizer to maintain the cubic phase of zirconia at all
temperatures (Kurapova et al., 2017; Muccillo,
Netto, and Muccillo, 2001).
Calcia can be produced from lime which is extremely abundant in the world. In
2018, the world production of lime reached 420 million tons, but the production
of yttria is only 5,000 to 7,000 tons which are entirely centralized in China (USGS, 2019). In industrial
applications, calcia-stabilized zirconia (CSZ) is regularly used to measure
oxygen partial pressure in situ in metal, glass, and refractory processing at
high temperatures (Zhou and Ahmad, 2006).
Generally, there are three types
of SOFC cell design consisting of electrolyte-supported, electrode-supported,
and metal-supported. The electrolyte-supported design offers high mechanical
strength and avoid side reaction between conventional cathodes such as LSM or
LSCF and zirconia-based electrolyte during the sintering process (Stolten and Emonts, 2012). However, the design requires a high operating temperature to decrease
ohmic resistance. Moreover, in electrode configuration, anode-supported design
is more popular than cathode-supported design owing to ease of fabrication,
high electrical conductivity, low operating temperature, and cost-effective
design. Moreover, the disadvantage of the anode-supported design is the large
volume change due to the reduction process during operation, making it prone to
electrolyte cracking (Roehrens et al., 2015; Islam and
Hill, 2013).
Another phenomenon that causes a
crack in the electrolyte is curvature during half-cell sintering in an
anode-supported design. The curvature occurs due to the difference in thermal
expansion and sintering rates of the anode and electrolyte (Cologna et al., 2010). To sort out curvature, the thickness of the electrolyte should be
controlled. In the anode-supported configuration, the thin-film electrolytes
are produced through some techniques such as chemical vapor deposition (CVD) (Gelfond et al., 2009), DC sputtering (Sonderby et al., 2015), and spray coating (Abarzua et al., 2021). Among the methods, spray coating is a low-cost technique for the mass
production of thin electrolytes in anode-supported cells. Previous studies
showed that this method could produce thin dense electrolytes with less than 50
µm of thickness, and the electrolyte film was stable during testing (Yang, Zhang, and Yan, 2022).
Our previous SOFC had greatly
poor electrochemical (ohmic resistance up to 3,624 and 0.001 mW/cm2
of maximum power density) and mechanical performance (0 Mohs of hardness)
since the electrolyte and anode structure are too porous (Widiatmoko et al., 2019). In this work, we exhibited an enhancement to our previous work by the
fabrication and characterization of an anode-supported SOFC single cell using
NiO-CSZ anode, CSZ electrolyte, and CCZO-CSZ cathode with the different
pore-former composition, powder mixing method, sintering condition, and amount
of electrolyte coating from our previous work. These parameters were the key to
producing the robust anode as a support and the dense and thin electrolyte.
2.1. Powder Preparation
Firstly, electrolyte powder was prepared by mixing 3 wt.% of
CaO from Bratachem (technical grade, Bandung, Indonesia) and 97 wt.% of ZrO2
purchased from Pingxiang Ball-Tec New Materials Co., Ltd (technical grade,
Jianxi, China) with 1 wt.% of polyethylene glycol (PEG) as a plasticizer,
polyvinyl alcohol (PVA) as a binder and ethanol from Bratachem (technical
grade, Bandung, Indonesia) as a medium in a ball mill and mixer. Secondly, the
anode powder consisting of 65 wt.% of NiO purchased from Changsha Easchem Co.,
Ltd (technical grade, Changsa, China), 35 wt.% of CSZ, 1 wt.% of PVA and corn
starch (0, 10, and 15 wt.%) was mixed at ball mill and mixer with ethanol.
Lastly, to produce CCZO cathode powder, CaO, Co3O4, and
ZnO from Bratachem (technical grade, Bandung, Indonesia), weighed in a
stoichiometric amount, were blended with 1 wt.% of PVA, PEG, and ethanol in a
ball mill. The drying of all powders is carried out at 100 °C for 24 h.
2.2. Cell Fabrication
To produce an anode-supported IT-SOFC,
the anode powder was molded through dry pressing with a load of 8 tons for 5
minutes. The dimension of the anode was 40 mm in diameter. The NiO-CSZ anode
was sintered at 1,100 °C for 3 h. Electrolyte and cathode powders were mixed
with isopropyl alcohol (IPA) as a dispersant to form a slurry. The electrolyte
slurry was coated onto the anode surface using the spray coating method at a
pressure of 2 bar and continued heating in the oven for 1 h to remove
dispersants. The lining process of the electrolyte was done once and eight
times by coating. The CSZ electrolyte was sintered at 1,100 °C for 3 and 2 h.
Moreover, the cathode slurry was coated onto the anode-electrolyte surface
using a spray coating technique with an effective area of 7 cm2. The
sintering of the CCZO cathode was carried out at 900 ?C for 5 h.
2.3. Physical Characterization
Where b is the height from the flat surface to
the top of the curved substrate, and a is the thickness of the half-cell
sample.
2.4. Electrochemical Characterization
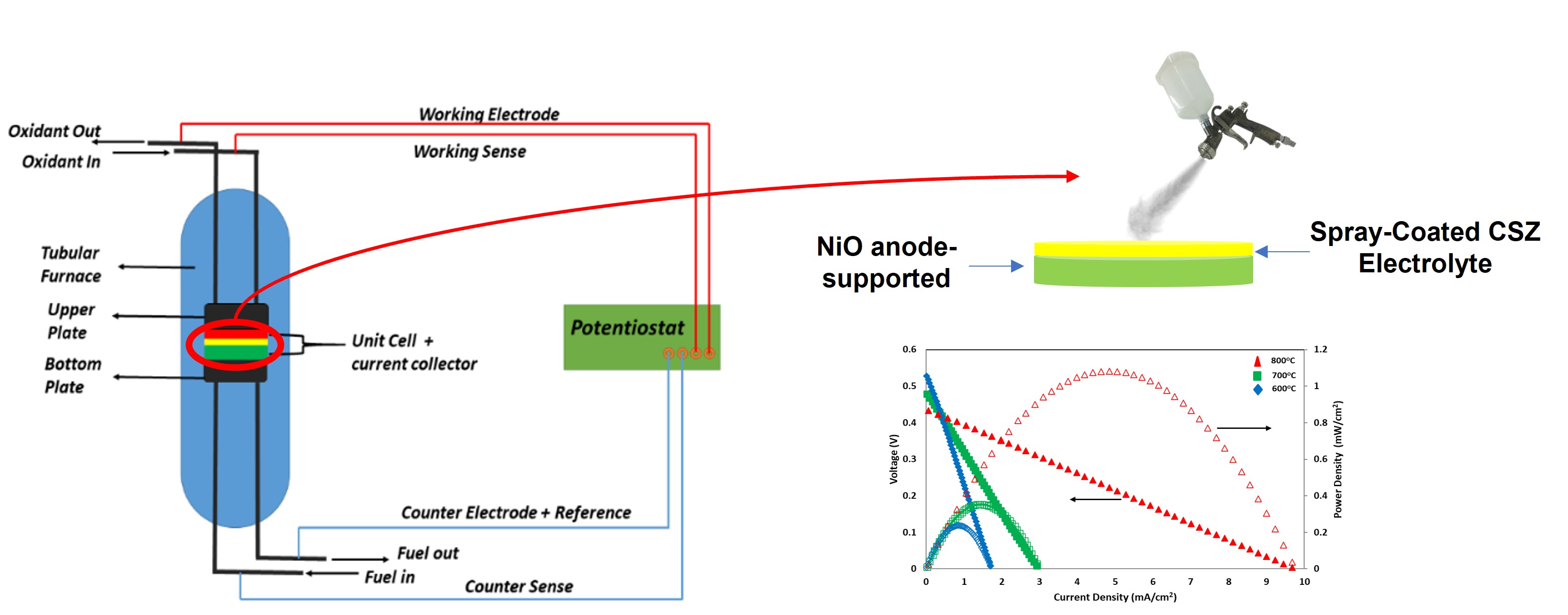
Figure 1 A schematic of SOFC single-cell testing using a tubular furnace
connected to a potentiostat
3.1. Porosity and
Microstructure
Table
1 illustrates the effect of the amount of pore former on the porosity and
hardness of anode sintered at 1,100 °C for 3 h. The porosity of the anode
before reduction without a pore-forming agent was 9.8%, while with the
pore-forming agent was 24.7% for 10 wt.% of corn starch, 1 wt.% of PVA and
40.1% for 15 wt.% of corn starch, 1 wt.% of PVA. Moreover, the hardness of the
cermet without pore former was 7 Mohs, while with pore former was 1 Mohs for 10
wt.% of corn starch, 1 wt.% of PVA, and 0 Mohs for 15 wt.% of corn starch, 1 wt.%
of PVA. The porosity of sintered anode increases with the increasing amount of
corn starch.
Table 1 Effect of amount of pore former on the porosity and hardness of anode sintered at 1,100 °C for 3 h
|
Corn starch (wt%) |
PVA (wt%) |
Porosity before reduction (%) |
Hardness (Mohs) |
|
0 |
0 |
9.8 |
7 |
|
10 |
1 |
24.7 |
1 |
|
15 |
40.1 |
0 |
Figure 2 shows a
cross-sectional and surface view of the anode-supported single cell before
testing. The thickness of the anode and cathode was ~1.1 mm and ~34.8 µm,
respectively. Figure 2a and 2b clearly shows that the morphology of NiO-CSZ
cermet and CCZO-CSZ composite was porous. In addition, the particle size of the
anode was much bigger than that of the cathode since the sintering temperature
of the cathode was lower than that of the anode (900 °C versus 1,100 °C) (Joo and Choi, 2008). The
particle size of the NiO-CSZ anode and CCZO-CSZ cathode was ~1.2 and ~0.5 µm,
respectively. Meanwhile, in the electrolyte part, the microstructure with eight
times of coating was denser than that with one time of coating, as shown in
Figures 2d and 2e. The thickness of electrolyte with one time and eight times
of coating was ~17.0 and ~87.2 respectively. The CSZ electrolyte film will
be thicker with the increasing amount of coating process repetition. However,
the sintered electrolyte with eight times coating still had a porous structure,
and the film thickness of the electrolyte and cathode was not uniform.
Figure 2 SEM micrograph of the (a) cross-section of an electrolyte-supported
IT-SOFC cell before testing; (b) surface view of NiO-CSZ anode; (c) CCZO
cathode; CSZ electrolyte with (d) one time; and (e) eight times of coating
3.2. Curvature of
Anode-Electrolyte Cell
In anode and electrolyte powder preparation, a mixer and ball mill were used as mixing equipment. The sintering process of half-cell (anode-electrolyte), particularly in the anode-supported cell, is the potential for curvature phenomenon. Figure 3 and Table 2 revealed curvature photograph and value in half-cell using a mixer and ball mill in powder preparation with a different dwell time of sintering and amount of electrolyte coating. The half-cell with a mixer in powder preparation shown in Figure 3a has a different angle on both sides. The powder blending with the mixer was not homogenous, so the thermal expansion did not spread uniformly. Meanwhile, the anode-electrolyte cell with ball mill in powder preparation shown in Figure 3b has a similar angle on both sides even though the angle of this half-cell was larger than that of the half-cell with mixer. The curvature value of anode-electrolyte cell sintered at 1,100 °C for 3 h with a mixer and ball mill in powder preparation was 0.89 and 1.18 mm, respectively, as shown in Table 2. This confirmed that the bigger the angle is, the higher the curvature value is. This phenomenon means the anode-electrolyte cell with a ball mill in powder preparation produces homogenous mixtures (Malzbender, Wakui, and Steinbrech, 2006). Additionally, it is reasoned that the sintering rate of the electrolyte was larger than the anode, which could not resist forces applied to the anode by electrolyte sintering (Lankin and Karan, 2009).
Figure
3
Curvature of anode-electrolyte cell sintered at 1,100 °C for 3 h using (a)
mixer; (b) ball mill in preparation; for 2 h using ball mill in preparation
with (c) one time; and (d) eight times of electrolyte coating
The study by (Cologna et al., 2009) reported the curvature could be solved by reducing the dwell time of
half-cell sintering. In Figure 3c, the dwell time of anode-electrolyte
sintering decreased from 3 to 2 h. The picture exhibited the angle on both
sides was lesser than the angle in half-cell with a dwell time of 3 h. The
curvature value reduced by ~62% when the dwell time of sintering decreased. It
is clear that the shorter the anode-electrolyte sample is held at the sintering
temperature, the lesser the curvature occurs. However, when the dwell time of
electrolyte sintering decreases, the electrolyte densification will be worse.
On the other hand, Figure 3d showed that the half-cell with eight times of
electrolyte coating curved sharply on both sides compared with the half-cell
with one time of electrolyte coating, as shown in Figure 3c. The curvature
value of half-cell with eight times of electrolyte coating increased by about
66%. Hence, the thick electrolyte layer enforces greater stress on the anode,
causing an increase in curvature (Ruhma et
al., 2021).
Table 2 Curvature value of four half-cell substrates sintered at 1,100 °C with
different mixing methods, dwell time of sintering, and amount of coating
electrolyte
|
Amount of Electrolyte Coating |
Dwell Time of
Sintering (h) |
b (mm) |
a (mm) |
Curvature (mm) | |
|
Mixer |
Once |
3 |
2.04 |
1.15 |
0.89 |
|
Ball Mill |
3 |
2.33 |
1.15 |
1.18 | |
|
2 |
1.60 |
1.15 |
0.45 | ||
|
Eight times |
2 |
2.54 |
1.20 |
1.34 |
3.3. Electrochemical Performance
Figure
5 Polarization curves of anode-supported cell
with eight times of electrolyte coating at different temperatures.
Impedance measurements of the cell
were performed under OCV, and the curves were fitted with an equivalent circuit
model, as shown in Figure 6. Ohmic resistances (Rohm) of the cell at 600, 700,
and 800 °C were 168.30; 90.10; and 26.78 respectively, as summarized in Table 3. The
thick electrolyte layer leads to high ohmic resistance of the single cell (Park et al., 2018).
On
the other hand, the polarization resistance (Rp) values, including charge and
mass transfer, of 155.10; 103.56; and 11.57 ? were gained at 600, 700, and 800
°C, respectively. Conductivity and gas diffusion in the anode and cathode were
responsible for high polarization resistance (Troskialina, 2015). Moreover, Rohm and Rp values were
significantly lower in this work than in our previous work. Hence, the
improvement in the fabrication of anode-supported IT-SOFC cells enhanced
electrochemical performance. Overall, compared with our previous study, the maximum power density of the cell
at 700 °C was ~350 times higher, while ohmic and polarization resistance at 700
°C was highly reduced at ~40 times and ~500 times, respectively.
Table 3 Summary of resistance values extracted from an equivalent circuit model at 600, 700, and 800 °? under OCV condition
The anode-supported single cell consisting of
NiO-CSZ anode, CSZ electrolyte, and CCZO-CCZO cathode was successfully improved
both in electrochemical and mechanical characteristics. The porosity of the
anode is sufficient to obtain a robust structure and to allow fuel gas
diffusion to TPB. Meanwhile, electrolytes with eight times of coating still had
a porous structure which is highly potential to fuel and oxidant crossover
phenomenon. On the other hand, curvature was overcome by reducing the dwell
time of sintering, but when the electrolyte film was thick, the curvature
increased two times/twice. In electrochemical performance, peak power density,
Rohm, and Rp at 800 °C was 1.08 mW/cm2, 26.78 and 11.57
respectively. However, the spray-coated
CSZ single cell was still lower than commercial YSZ-based SOFC cell. In future studies, the CSZ electrolyte
fabrication technique is key to making denser and thin electrolytes to avoid
fuel crossover.
The authors would
like to thank the financial support provided by Institut Teknologi Bandung
through Research, Community Service, and Innovation ITB 2019 Program (Contract No: 0922b/I1.C06.2/PL/2019) and the
support of laboratory facilities provide by Center for Hydrogen-Fuel Cell
Research, Korea Institute of Science and Technology (KIST).
Abarzua, G., Udayabhaskar, R., Mangalaraja, R.V., Durango-Petro, J., Usuba, J., Flies, H., 2021. A Feasible Strategy for Tailoring Stable Spray-Coated Electrolyte Layer in Micro-Tubular Solid Oxide Fuel Cells. International Journal of Applied Ceramic Technology, Volume 19(3), pp. 1389–1396. doi: 10.1111/ijac.13981
Abdalla, A.M., Hossain, S., Azad A.T., Petra, P.M.I., Begum, F., Eriksson, S.G., Azad, A.K., 2018. Nanomaterials for Solid Oxide Fuel Cells: A Review. Renewable & Sustainable Energy Reviews, Volume 82, pp. 353–368. doi: 10.1016/j.rser.2017.09.046
Amiri, S., Paydar, M.H., 2017. Effect of Pore Formers Characteristics and Melt Infiltration Parameters on Microstructure and Electrical Properties of BaCe0.7Zr0.1Y0.2O3??-Carbonate Composite Electrolyte. Journal of Alloys and Compounds, Volume 735, pp. 172–183. doi: 10.1016/j.jallcom.2017.11.067
Baharuddin, N.A., Muchtara, A., Somalu, M.R., 2017. Short Review on Cobalt-Free Cathodes for Solid Oxide Fuel Cells. International Journal of Hydrogen Energy, Volume 42, pp. 9149–9155. doi: 10.1016/j.ijhydene.2016.04.097
Batool, M., Sattar, M., Barki, U.K., Khan, Z.S., 2018. Synthesis of Ni/YSZ Based Anode and Investigation of Effect of PVA as Pore-Former Upon Porosity, Microstructure and Thermal Behavior for Potential Use In Solid Oxide Fuel Cells (SOFCs). International Journal of Materials Research, Volume 109, pp. 1153–1159. doi: 10.3139/146.111713
Chen, Y., Yang, L., Ren, F., An, K., 2014. Visualizing the Structural Evolution of LSM/xYSZ Composite Cathodes for SOFC by in-Situ Neutron Diffraction. Scientific Reports, Volume 4(1), p. 5179. doi: 10.1038/srep05179
Cologna, M., Contino, A.R., Montinaro, D., Sglavo, V.M., 2009. Effect of Al and Ce doping on the deformation upon sintering in sequential tape cast layers for solid oxide fuel cells. Journal of Power Sources, 193, pp. 80-85. doi: 10.1016/j.jpowsour.2008.12.052
Cologna, M., Contino, A.R., Sglavo, V.M., Modena, S., Ceschini, S., Bertoldi, M., 2010. Curvature Evolution and Control in Anode Supported Solid Oxide Fuel Cells in Advances: Solid Oxide Fuel Cells V. New Jersey: John Wiley & Sons, Inc. doi: 10.1002/9780470584316.ch8
Ding, C., Zhang, J., Liu, Y., Gou, J., Luan, J., 2016. Effects of Pore-Forming Agent on Characterization of NiO/YSZ Porous Anode for SOFC. Materials Science Forum, Volume 848, pp. 389–395. doi: 10.4028/www.scientific.net/MSF.848.389
Gelfond, N.V., Bobrenok, O.F., Predtechensky, M.R., Morozova, N.B., Zherikova, K.V., Igumenov, I.K., 2009. Chemical Vapor Deposition of Electrolyte thin Films Based on Ytrria-Stabilized Zirconia. Inorganic Materials, Volume 45(6), pp. 659–665. doi: 10.1134/S0020168509060144
Horri, B.A., Selomulya, C., Wang, H., 2012. Characteristics of Ni/YSZ Ceramic Anode Prepared using Carbon Microspheres as a Pore Former. International Journal of Hydrogen Energy, Volume 37, pp. 15311–15319. doi: 10.1134/S0020168509060144
Islam, S., Hill, J.M., 2013. Anode Materials Development. In: Solid Oxide Fuel Cells: From Materials to System Modeling. London: RSC Publishing. doi: /10.1039/9781849737777
Joo, J.H., Choi, G.M., 2008. Thick-Film Electrolyte (thickness <20 µm)-Supported Solid Oxide Fuel Cells. Journal of Power Sources, Volume 180, pp. 195–198. doi: 10.1016/j.jpowsour.2008.02.013
Kaur, P., Singh, K., 2020. Review of Perovskite-Structure Related Cathode Materials for Solid Oxide Fuel Cells. Ceramic International, Volume 46, pp. 5521–553. doi: 10.1016/j.ceramint.2019.11.066
Lankin, M.K., Karan, K., 2009. Effect of Processing Conditions on Curvature of Anode/Electrolyte SOFC Half-Cells Fabricated by Electrophoretic Deposition. Journal of Electrochemical Energy Conversion and Storage, Volume 6, p. 021001. doi: 10.1115/1.2971044
Kurapova, O.Y., Glumov, O.V., Pivovarov, M.M., Golubev, S.N., 2017. Structure and Conductivity of Calcia Stabilized Zirconia Ceramics, Manufactured from Freeze-Dried Nanopowder. Reviews on Advanced Material Science, Volume 52, pp. 134–141. doi: 222174886
Malzbender, J., Wakui, T., Steinbrech, W., 2006. Curvature of Planar Solid Oxide Fuel Cells During Sealing and Cooling of Stacks. Fuel Cells, Volume 6, pp. 123–129. doi: 10.1002/fuce.200500109
Muccillo, R., Netto, R.C., Muccillo, E.N., 2001. Synthesis and Characterization of Calcia Fully Stabilized Zirconia Solid Electrolytes. Material Letters, Volume 49, pp. 197–201. doi: 10.1016/S0167-577X(00)00367-0
Nguyen, X-V., Chang, C-T., Jung, G-B., Chan, S-H., Huang, W. C-W., Hsiao, K-J., Lee, W-T., Chang, S-W., Kao, I-C., 2016. Effect of Sintering Temperature and Applied Load On Anode-Supported Electrodes for SOFC Application. Energies, Volume 9, pp. 1–13. doi: 10.3390/en9090701
Park, J.M., Kim, D.Y., Baek, J.D., Yoon, Y.J., Su, P.C., Lee, S.H., 2018. Effect of Electrolyte Thickness on Electrochemical Reactions and Thermo-Fluidic Characteristics Inside A SOFC Unit Cell. Energies, Volume 11(473), pp. 1–25. doi: 10.3390/en11030473
Rahmawati, F., Permadani, I., Syarif, D.G., Soepriyanto, S., 2017. Electrical Properties of Various Composition of Yttrium Doped-Zirconia Prepared from Local Zircon Sand. International Journal of Technology. Volume 8(5), pp. 939–946. doi: 10.14716/ijtech.v8i5.876
Rasmussen, J.F.B., Hendriksen, P.V., Hagen, A., 2008. Study of Internal and External Leaks in Tests of Anode-Supported SOFCs. Fuel Cells, Volume 8, pp. 385–393. doi: 10.1002/fuce.200800019
Roehrens, D., Han, F., Haydn, M., Schafbauer, W., Sebold, D., Menzler, N.H., Buchkremer, H.M., 2015. Advances Beyond Traditional SOFC Cell Designs. International Journal of Hydrogen Energy, Volume 40(35), pp. 11538–11542. doi: 10.1016/j.ijhydene.2015.01.155
Ruhma, Z., Yashiro, K., Oikawa, I., Takamura, H., Kawada, T., 2021. Metal-supported SOFC Fabricated by Tape Casting andIts Characterization: A Study of the Co-sintering Process. Journal of Engineering and Technological Sciences, Volume 53(5), pp. 991–1013. doi: 10.5614/j.eng.technol.sci.2021.53.5.11
Sazali, N., Salleh, W.N.W., Jamaludin, A.S., Razali, M.N.M., 2020. New Perspectives on Fuel Cell Technology: A Brief Review, Membranes, 10, pp. 1–18. doi: 10.3390/membranes10050099
Shi, H., Su, C., Ran, R., Cao, J., Shao, Z., 2020. Electrolyte Materials for Intermediate-Temperature Solid Oxide Fuel Cells. Progress in Natural Science: Materials International, Volume 30(6), pp. 764–774. doi: 10.1016/j.pnsc.2020.09.003
Singhal, S.C., Kendall, K., 2003. High-temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications. Oxford: Elsevier Ltd. doi: 10.1016/B978-1-85617-387-2.X5016-8
Sonderby, S., Christensen, B.H., Almtoft, K.P., Nielsen, L.P., Eklund, P., 2015. Industrial-Scale High Power Impulse Magnetron Sputtering of Yttria-Stabilized Zirconia on Porous NiO/YSZ Fuel Cell Anodes. Surface and Coatings Technology, Volume 281, pp. 150–156. doi: 10.1016/j.surfcoat.2015.09.058
Stolten, D., Emonts, B., 2012. Fuel Cell Science and Engineering: Materials, Processes, Systems and Technology. New Jersey: Wiley. doi: 10.1002/9783527650248
Suzuki, T., Jasinski, P., Petrovsky, V., Anderson, H.U., 2005. Performance of a Porous Electrolyte in Single-Chamber SOFCs. Journal of Electrochemical Society, Volume 152, pp. 27–31. doi: 10.1149/1.1858811
Takami, T., Ikuta, H., 2005. Thermoelectric Properties of One-Dimensional Cobalt Oxide Ca3Co2O6 and The Effect of Zn Doping, In: 24th International Conference on Thermoelectrics, pp. 480–483. doi: 58509459
Troskialina, L., 2015. Improved Performance of Solid Oxide Fuel Cell Operating on Biogas using Tin Anode-infiltration. PhD Dissertation, School of Chemical Engineering., University of Birmingham., Birmingham. Accessed via: https://etheses.bham.ac.uk/id/eprint/6790/1/Troskialina16PhD.pdf
U.S. Geological Survey (USGS), 2019. Mineral Commodity Summaries 2019. U.S. Geological Survey, Virginia. doi: 10.3133/70202434
Wang, S., Jiang, S.P., 2017. Prospects of Fuel Cell Technologies. National Science Review, Volume 4, pp. 163–166. doi: 10.1093/nsr/nww099
Widiatmoko, P., Devianto, H., Nurdin, I., Yusupandi, F., Kevino., Ovani, E.N., 2019. Fabrication and Characterization of Intermediate-Temperature Solid Oxide Fuel Cell (IT-SOFC) Single Cell Using Indonesia’s Resources. IOP Conference Series: Materials Science and Engineering, Volume 550, pp. 1–6. doi: 10.1088/1757-899X/550/1/012001
Yang, Y., Zhang, Y., Yan, M., 2022. A Review on the Preparation of Thin-Film YSZ Electrolyte of SOFCs by Magnetron Sputtering Technology. Separation and Purification Technology, Volume 298, p. 121627. doi: 10.1016/j.seppur.2022.121627
Yu, S., Zhang, G., Chen, H., Guo, L., 2017. A Novel Post-Treatment to Calcium Cobaltite Cathode for Solid Oxide Fuel Cells. International Journal of Hydrogen Energy, Volume 43, pp. 2436–2442. doi: 10.1016/j.ijhydene.2017.12.040
Zhou, M., Ahmad, A., 2006. Synthesis, Processing and Characterization of Calcia-Stabilized Zirconia Solid Electrolytes for Oxygen Sensing Applications. Material Research Bulletin, Volume 41, pp. 690–696. doi: 10.1016/j.materresbull.2005.10.018
