Effect of Vitamin C, Alpha Lipoic Acid (ALA), and Pentoxifylline on the Sperm Parameters, Cryosurvival Rate, and MDA Concentration after Thawing in Cryopreservation
Corresponding email: finallysilvia@gmail.com
Published at : 31 Jan 2025
Volume : IJtech
Vol 16, No 1 (2025)
DOI : https://doi.org/10.14716/ijtech.v16i1.6305
Hardianingtyas, L, Lestari, SW, Pratama, G, Kusmardi, K 2025, Effect of Vitamin C, Alpha Lipoic Acid (ALA), and Pentoxifylline on the sperm parameters, cryosurvival rate, and MDA concentration after thawing in cryopreservation, International Journal of Technology, vol. 16 no. 1, pp. 348-358
| Luthfiana Hardianingtyas | Master’s Programme in Biomedical Sciences, Faculty of Medicine, Universitas Indonesia, Jl. Salemba Raya No. 6, Jakarta 10430 Indonesia |
| Silvia W Lestari | Department of Medical Biology, Faculty of Medicine, Universitas Indonesia, Jakarta 10430 Indonesia |
| Gita Pratama | Division of Reproductive Endocrinology and Infertility, Department of Obstetrics and Gynecology, Faculty of Medicine, Universitas Indonesia, Jakarta 10430 Indonesia |
| Kusmardi Kusmardi | Department of Anatomic Pathology, Faculty of Medicine, Universitas Indonesia, Jakarta 10430 Indonesia |
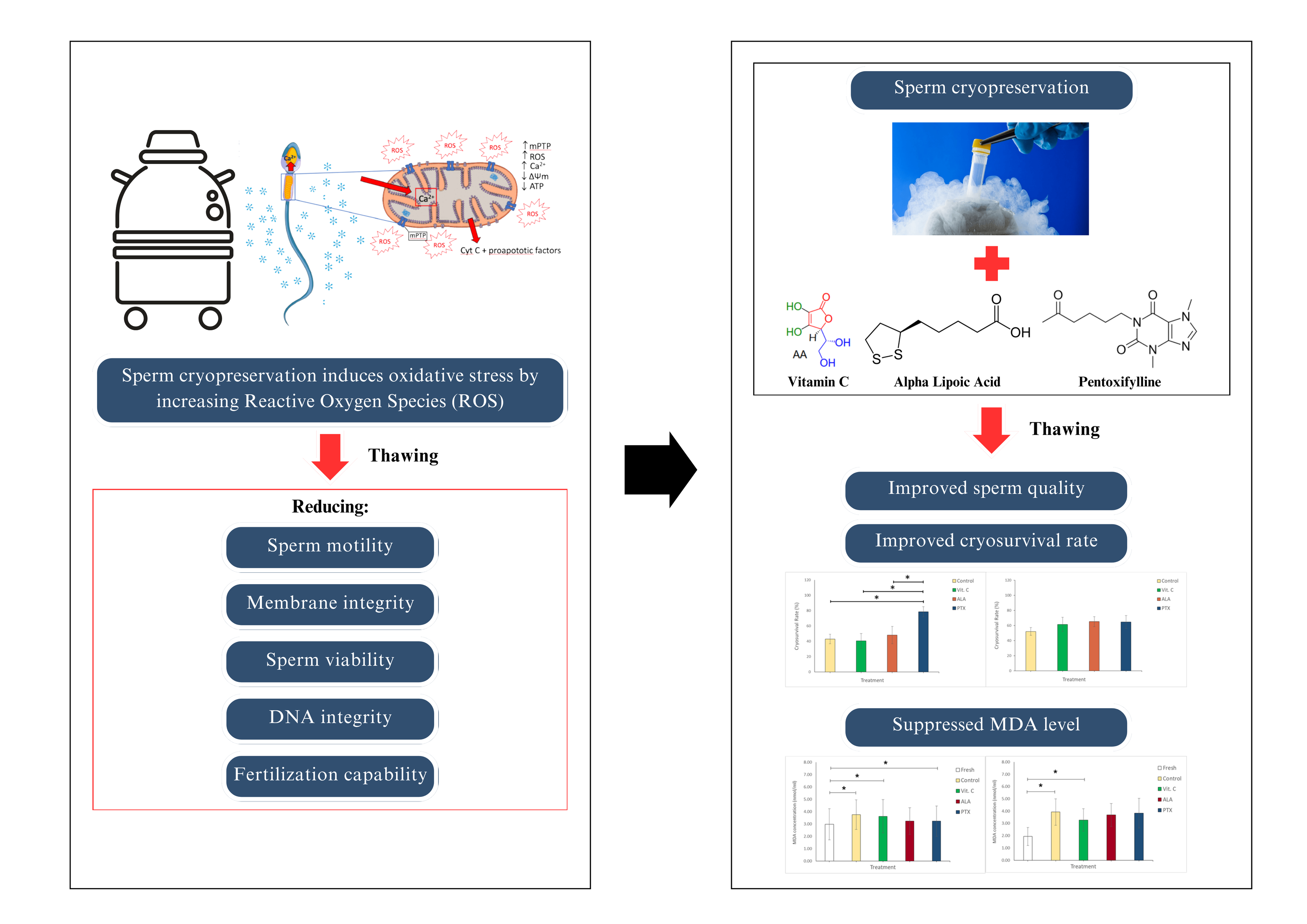
Cryopreservation provides available sperm for subsequent use in Assisted Reproductive Technology (ART), but it still has detrimental effects on sperm quality. Although several investigations of the use of antioxidants in cryopreservation mediums had conducted, the result remains unsatisfactory. Therefore, this study aimed to contribute to the references of the antioxidant role in preserving the quality of sperm in cryopreservation by examining post-thawing sperm quality, cryosurvival rate, and post-thawing malondialdehyde (MDA) concentration. The semen sample of 35 infertile patients was classified as normozoospermic and non-normozoospermic. The semen sample was divided into four aliquots, with the following antioxidants supplemented to cryomedium: vitamin C (Vit C), alpha lipoic acid (ALA), and pentoxifylline (PTX). Subsequently, it was cryopreserved in liquid nitrogen, and the parameters: sperm quality (concentration, motility, and morphology), cryosurvival rate, and MDA concentration were evaluated after thawing. All parameters showed a reduction in post-thawing analysis. PTX group significantly improves sperm progressive motility and cryosurvival rate (CSR) in normozoospermic (42.71 ± 4.30% and 78.54 ± 6.62%) among other treatments. The ALA and PTX groups decreased MDA concentration in normozoospermic, while Vit C showed a decrease in non-normozoospermic as well. We assumed that the antioxidants used in this study differently improved the sperm quality parameter post-thawing in different types of sperm abnormalities, such as PTX optimally increasing sperm motility after thawing, even though all antioxidants are likely to suppress MDA concentration after thawing.. These interventions may be beneficial in improving thawed sperm quality to improve ART program success.
Antioxidant; Cryoprotectant agent; Cryosurvival rate; Sperm cryopreservation; Sperm quality
Assisted reproductive technology (ART) is a method for managing infertility that is carried out by direct collection and in vitro handling of the couples’ gamete outside of the body, including the implantation of the eventual embryo results in the woman’s uterus. The in vitro handling also takes place outside of infertility setting to preserve fertility by cryopreservation (Amjad and Rehman, 2021). Cryopreservation of the gamete can be employed to preserve the fertility of a person, provided as an option for future use of spermatozoa for ART, including in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI). This beneficial procedure has provided couples with an opportunity to obtain embryos from stored sperm for longer than 20 years (Özekici et al., 2020). However, several investigations have identified a decrease in sperm quality and functionality after cryopreservation.
In cryopreservation, the temperature change and osmotic stress were well established to induce structural alteration leading to cell injury and further causing the overproduction of reactive oxygen species (ROS) (Larasati et al., 2022; Peris-Frau et al., 2020). Therefore, it is essential to maintain the original pre-freezing sperm quality. The cryopreservation medium, cryoprotectant (CPA), is one of the factors contributing to the optimum cryosurvival. This idea has driven several studies focused on supplementing CPA with antioxidant species to minimize oxidative damage and improve the recovery of the cryopreserved sperm.
Vitamin C (Vit C) is an antioxidant capable of reducing metals by acting as an electron donor and regenerating Vit E (Torres-Arce et al., 2021). This vitamin type of micronutrient is proven to be a beneficial characteristic of sperm because it is naturally found abundantly in seminal plasma (Ribeiro et al., 2021). In vitro, Vit C administration to human semen could improve motility and viability, reduce H2O2-induced DNA damage, and minimize lipid peroxidation (Amidi et al., 2016; Fanaei et al., 2014; Jenkins et al., 2011; Branco et al., 2010). Meanwhile, in a clinical study, Vit C intake also showed an improvement in sperm parameters (Rafiee et al., 2016; Cyrus et al., 2015; Akmal et al., 2006), a decrease in oxidative stress level (Kowalczyk, 2022), and had a positive relationship with the fertilization rate in ART (Li et al., 2019). The benefits of Vit C for reproduction can be obtained by consuming a variety of citric fruits and vegetables (Henkel et al., 2019; Torres-Arce et al., 2021). Another antioxidant that is described as a potent antioxidant and detoxifying agent in sperm is ALA (Shay et al., 2009). It exerts its antioxidant potential by acting as a ROS scavenger, inhibiting cell death and apoptosis, recycling Vit C and inducing glutathione (GSH) synthesis, contributing to the Krebs cycle, and improving adenosine triphosphate (ATP) production, which is essential for the viability of sperm (Asa et al., 2020; Ibrahim et al., 2008). Furthermore, pentoxifylline (PTX) can increase the rate of sperm motility post-thawing (Xian et al., 2021; Nabi et al., 2017; Stanic et al., 2002). It has been proposed that PTX functions in improving motility by inducing the production of nitric oxide (NO), inhibiting xanthine oxidase from reducing reactive oxygen species (ROS) in abnormal sperm, reducing tumor necrosis factor-alpha (TNF-?), and suppressing lipid peroxidation (Nateghian et al., 2023; Parekattil et al., 2020). A previous report established that normozoospermic contains sperms with ameliorating conditions compared to oligo-/astheno-/terato-/combination (non-normozoospermic) (Shivaprasad et al., 2015). Therefore, this study aims to administer the three antioxidants to the cryopreservation medium/CPA and determine their effectiveness in cryopreserved sperm in two types of sperm abnormalities. This study was the first to analyze the effect of different antioxidants in cryopreservation in two types of sperm abnormalities. The effect of treatment was evaluated by the post-thaw sperm parameters, cryosurvival rate, and MDA concentration in normozoospermic and non-normozoospermic groups.
2.1. Study Population
This experimental study involved 35 infertile male patients who were recruited from Yasmin Infertility Clinic, Cipto Mangunkusumo Hospital, Jakarta, Indonesia, between June and September 2022. These patients had provided informed consent prior to their participation. The experimental procedures were approved by the Ethical Committee of Universitas Indonesia with the ethical number KET-382/UN2.F1/ETIK/PPM.00.02/2022.
2.2. Semen Quality Analysis
Semen samples were collected in a sterile container through masturbation after 3-5 days of sexual abstinence. After the liquefaction process, the semen analysis was performed according to WHO 2010 guidelines, which include sperm concentration (million sperm/ml) and motility rate (%) that are differentiated into three categories as follows: motile progressive (actively moving in linear or in a large circle), non-progressive (motile without progression), and immotile (no movement), and normal morphology rate (%) (World Health, 2010). Meanwhile, the two different semen analysis results included in this study were normozoospermic and non-normozoospermic (oligo-/astheno-/terato-/oligoasthenoterato-zoospermic).
2.3. Sperm Cryopreservation and Thawing
The semen sample was divided into four aliquots (300 µl each) and diluted 1:1 with cryomedium (Kitazato, Biopharma, Japan) in 1.8 ml cryovials: control (diluted with cryomedium only); supplemented with Vit C 300 µM; ALA 0.02 mM; and PTX 25 mmol/ml (Sigma Aldrich, St. Louis, Missouri, USA) (Xian et al., 2021; Asa et al., 2020; Li et al., 2010). Subsequently, cryovials in a cryocane were placed ± 5 cm above the liquid nitrogen level in a coolbox, exposed to the vapor for 15 – 30 min until freezing, and immersed directly into liquid nitrogen. The samples were thawed by incubating them at 37? for a minimum of 24 hours. Following the thawing process, the samples were analyzed to evaluate their post-thawing quality. The cryosurvival rate was determined by dividing the post-thawing total motile percentage by the pre-freezing total motile percentage and multiplying the result by 100 (Saleh et al., 2018).
2.4. Malondialdehyde (MDA) Determination Assay
Altered ROS production and increased oxidative stress levels cause lipid peroxidation (LPO) of the sperm membrane, which is associated with a decrease in motility and fertilizing ability (Agarwal et al., 2014). The thiobarbituric acid reactive substances (TBARS) method was used to determine the oxidative stress level. Two hundred microliters of 20% trichloroacetic acid (TCA) were added to approximately 400 µl of lysed semen samples in 0.8% SDS buffer, followed by centrifugation at 5.000 rpm for 10 min. Afterwards, 400 µl of 0.67% thiobarbituric acid (TBA) was added to the supernatant. The samples were further incubated in a water bath at 96–100? for 10 min. Next, the absorbances were measured at 530 nm in a spectrophotometer. The standard solution used was tetraetoxypropane (TEP), and the calibration equation obtained was y = 0.069x + 0.0031.
Figure 1 A brief methodology scheme in this study. TBARS: Thiobarbituric acid reactive substances
2.5. Statistical Analysis
Statistical analysis was carried out using IBM SPSS Statistics version 26 for Windows. The data for semen quality in each parameter were analyzed between two groups, control vs each treatment, using an independent t-test and Mann-Whitney for the normally and non-normally distributed data, respectively. The cryosurvival rate was analyzed using one-way ANOVA. A paired t-test or 2-independent sample non-parametric test was used to analyze the pre-freezing and post-thawing MDA concentration, and then one-way ANOVA was used to analyze between groups post-thawing. Meanwhile, the statistical analysis is considered significant when the p-value is below 0.05.
3.1. Semen analysis post-thawing
Based on the data in Table 1, Vit C showed the highest sperm concentration, and the PTX group demonstrated the highest progressive motility and morphology parameter. There were significant differences in motility parameters of the PTX group compared to the control cryomedium. In Table 2, ALA had the highest concentration and was likely to improve progressive motility among other groups.
Table 1 Semen analysis result after thawing of normozoospermic group.
The data are shown in mean ± SE. aFresh vs Control; bFresh vs Vit C; cFresh vs ALA; dFresh vs PTX; eControl vs Vit C; fControl vs ALA; dan gControl vs PTX. Vit C: Vitamin C; ALA: Alpha Lipoic Acid; PTX: Pentoxifylline. *: significance level with p<0.05.
Vit C had the highest sperm concentration in the normozoospermic and the sperm's normal morphology in the non-normozoospermic group. At the same time, PTX showed an increase in sperm motility significantly and an insignificant increase in the normal morphology of the normozoospermic group. Meanwhile, in non-normozoospermic, ALA had the highest sperm concentration and progressive motility but was insignificant (Tables 1 and 2).
It is considered bias in sperm concentration to be assessed due to the dilution which was carried out with cryomedium (ratio 1: 1). Although this ratio is the best dilution in producing sperm harvest after cryopreservation (Zidni et al., 2022; Nomura et al., 2018). Concentration and dilution volume both reported altering sperm quality after thawing (Liu et al., 2022). Therefore, in most publications, the sperm parameter concentration is often combined with progressive motility and semen volume, namely motile sperm count.
Based on the sperm motility parameter, the PTX group in the normozoospermic occupied the position with the highest progressive motility and was significantly different compared to the control. Pentoxifylline is widely used in sperm preparation as a motility enhancer. The role of PTX as an activator of sperm motility is by inhibiting the phosphodiesterase (PDE) enzyme as a cyclic adenosine monophosphate (cAMP) degrader. Cyclic AMP stimulates cAMP-dependent kinase that induces protein phosphorylation in flagella for sperm movement (Henkel and Schill, 2003). Supplementation of PTX in cryomedium has been proposed by Xian et al., (2021) on testicular sperm samples. It has been discovered that PTX helps in maintaining the motility of sperm during cryopreservation, leading to an increased rate of motility recovery after thawing (Xian et al., 2021).
Table 2 Semen analysis result after thawing of non-normozoospermic group.
The data are shown in mean ± SE. aFresh vs Control; bFresh vs Vit C; cFresh vs ALA; dFresh vs PTX; eControl vs Vit C; fControl vs ALA; dan gControl vs PTX. Vit C: Vitamin C; ALA: Alpha Lipoic Acid; PTX: Pentoxifylline. *: significance level with p<0.05.
In non-normozoospermic, the ALA group likely showed the highest percentage of progressive motility. The effect of ALA on cryopreserved sperm motility was suggested by Asa et al., (2020) and Shen et al., (2016). ALA stimulates and maintains mitochondrial activity and integrity by its action as a co-enzyme and protects the organelle from the increasing free-radical (Shaygannia et al., 2018). Mitochondrial status is an essential matter in sperm due to its relation to cell energy (ATP), which is essential for sperm motility and fertilization ability. Furthermore, ALA is also involved in the regeneration of Vit C (Asa et al., 2020).
In the morphology parameter, the highest percentage of normal morphology in normozoospermic was shown by the PTX group. This result is in line with the study of infertile men by Nasimi Doost Azgomi et al. (2018) and Safarinejad (2011). The mechanism of PTX in improving normal morphology is through its ability as an antioxidant to inhibit lipid peroxidation. This ability prevents damage to membrane lipids, which can impact the integrity of the sperm membrane, thereby causing morphological changes (Nasimi Doost Azgomi et al., 2018; Safarinejad, 2011). However, Vit C showed the highest normal morphology than other groups in non-normozoospermic. Vit C regenerates oxidized Vit E, thereby reducing lipid peroxidation and maintaining the structural integrity of the cell (Mangoli et al., 2018).
Cryosurvival rate
Based on the data in Figure 2, the highest recovery rate by motility (cryosurvival rate or CSR) was demonstrated significantly by PTX in normozoospermic and insignificantly by ALA in non-normozoospermic.
Figure 2 Cryosurvival rate (%) in (a) normozoospermic and (b) non-normozoospermic groups. Bars represent the mean ± SE. *: significance level at p<0.05. Vit C: Vitamin C; ALA: Alpha Lipoic Acid; and PTX: Pentoxifylline.
Cryosurvival rate (CSR) is one of the success indicators of sperm survival in cryopreservation based on pre-freezing and post-thawing total motility (Saleh et al., 2018). Although motility is indirectly related to fertilization ability, it is considered a crucial factor affecting sperm quality. Sperms need to move actively to achieve the existence of an egg in the female tract from the point of ejaculation or insemination for fertilization, pregnancy, and birth to occur (Kumar and Sharma, 2017). Motility is also used as an indicator of good sperm to be selected in the ICSI procedure (Henkel and Schill, 2003).
Pentoxifylline improved CSR significantly. This result is in line with the higher rate of post-thawing progressive motility than the other groups. The role of PTX in improving motility is to reduce the rate of cAMP degradation by inhibiting the PDE enzyme (Henkel and Schill, 2003). The metabolic status of sperm was found to be lower after thawing compared to the pre-freezing state. In this regard, PTX plays a role in activating immotile sperm by stabilizing cAMP, thereby facilitating their movement. Additionally, PTX has been reported to act as an antioxidant by inhibiting superoxide production, which can otherwise lead to peroxidative damage to cell membranes (Xian et al., 2021; Stanic et al., 2002).
In non-normozoospermic, ALA demonstrated the highest CSR, yet insignificant. ALA preserves motility by a mechanism related to the three factors of sperm motility: regulation, structural integrity, and energy supply. ALA forms an aqueous layer outside the membrane and between the lipid bilayers of the midpiece, resulting in the inhibition of the reaction of ROS to polyunsaturated fatty acid (PUFA). This inhibition maintains the structural integrity of the sperm membrane. This substance also indirectly maintains ATP production, which production in mitochondria depends on the organelle’s internal and external integrity (Ibrahim et al., 2008). As an antioxidant, ALA improves the quality of sperm through its contribution to the regeneration of Vit C (Gürler et al., 2016; Plotnikov et al., 2007).
MDA concentration post-thawing
The evaluation of oxidative stress level is determined by MDA concentration. The result demonstrated an increase after thawing but showed an insignificant reduction in antioxidant groups (Figure 3).
This study demonstrated an increased in MDA concentrations post-thawing compared to the pre-freezing. This result is in line with the study that has been reported to be increased more than fivefold after thawing (Valipour et al., 2021).
The lowest MDA concentration after thawing was obtained in the ALA and PTX groups of the normozoospermic sample. ALA has a role in preserving sperm by suppressing lipid peroxidation events, as Najafi et al. (2021) described. These nucleophiles containing thiol groups generally react with endogenous electrophiles such as free radicals or reactive chemical metabolites. This antioxidant also maintains endogenous antioxidant enzymes, such as superoxide dismutase (SOD), glutathione (GSH), and catalase (CAT). Enzyme glutathione peroxidase (GPx) activity is also known to be increasing during cryopreservation with the addition of ALA (Najafi et al., 2021). Meanwhile, the effect of PTX on reducing lipid peroxidation events is in line with the study conducted by Pal et al., (2005).
In non-normozoospermic, Vit C suppressed the MDA concentration after thawing. In line with the study by Singh et al. (2020), 5 mM of Vit C suppressed MDA production up to two times compared to the control (Singh et al., 2020). The role of Vit C in breaking the chain of lipid peroxidation reactions is by recycling Vit E, which can directly interact with lipid peroxidation, and scavenging ROS as a trigger for lipid peroxidation events. Furthermore, Vit C increases the activity of antioxidant enzymes in cells (Sönmez et al., 2005).
To the best of our knowledge, this is the first study that investigated the modification of CPA with antioxidants (Vit C or ALA or PTX) supplementation into cryomedium that improved sperm analysis parameters, CSR, and decreased MDA concentration after thawing in normo- and non-normozoospermic (oligo-/astheno-/terato-/combination) infertile men (Table 3).
Figure 3 MDA concentration (nmol/ml) in pre-freezing (fresh) and post-thawing of (a) normozoospermic and (b) non-normozoospermic groups. Bars represent mean ± SE. *: significance level at p<0.05. Vit C: Vitamin C; ALA: Alpha Lipoic Acid; and PTX: Pentoxifylline.
Table 3 Summary table of studies with the modification of CPA with antioxidants in human sperm cryopreservation
Abbreviation: MMP: Mitochondrial membrane potential; ALA: alpha lipoic acid; PTX: pentoxifylline.
Antioxidants used in this study of cryopreservation (vitamin C, ALA and pentoxifylline), affected and improved sperm quality in different parameters and types of sperm abnormalities. Pentoxifylline has an optimal effect in the improvement of sperm quality after thawing based on significant increase in progressive motility and cryosurvival rate. Meanwhile, MDA concentration is likely to be suppressed by all antioxidants insignificantly. Moreover, a further study evaluating molecular parameters and embryo quality as the result of fertilization in vitro using thawed sperm intervented with antioxidants is recommended to clarify these overall results.
First author of the manuscript, experimental design, performance of the semen analysis experiment, data collection, statistical analysis of data and supervisory role of the project: LH; Experimental design, performance of the semen analysis, cryosurvival rate and manuscript review: SWL; Experimental design, performance of the MDA concentration and manuscript review: GP and KK.
Conflict of Interest
The authors declare no conflicts of interest.
Agarwal, A, Virk, G, Ong, C & Du Plessis, SS 2014, ‘Effect of oxidative stress on male reproduction’, The World Journal of Men's Health, vol. 32, no. 1, pp.1-17, https://doi.org/10.5534/wjmh.2014.32.1. 1
Akmal, M, Qadri, JQ, Al-Waili, NS, Thangal, S, Haq, A &Saloom, KY 2006, Improvement in human semen quality after oral supplementation of vitamin C, Journal of Medicinal Food, vol. 9, pp. 440-442, https://doi.org/10.1089/jmf.2006.9.440
Amidi, F, Pazhohan, A, Shabani Nashtaei, M, Khodarahmian, M & Nekoonam, S 2016, ‘The role of antioxidants in sperm freezing: a review’, Cell and Tissue Bank, vol. 17, pp. 745-756, https://doi.org/10.1007/s10561-016-9566-5
Amjad, S & Rehman, R 2021, ‘Assisted reproductive techniques’, Subfertility, in: Rehman, R, Sheikh, A, (Eds,), Elsevier, pp. 185-197, https://doi.org/10.1016/C2019-0-02584-8
Asa, E, Ahmadi, R, Mahmoodi, M & Mohammadniya, A 2020, ‘Supplementation of freezing media with alpha lipoic acid preserves the structural and functional characteristics of sperm against cryodamage in infertile men with asthenoteratozoospermia’, Cryobiology, vol. 96, pp. 166-174, https://doi.org/10.1016/j.cryobiol.2020.07.001
Branco, CS, Garcez, MF, Pasqualotto, FF, Erdtman, B & Salvador, M 2010, ‘Resveratrol and ascorbic acid prevent DNA damage induced by cryopreservation in human semen’, Cryobiology, vol. 60, no. 2, pp. 235-237, https://doi.org/10.1016/j.cryobiol.2009.10.012
Cyrus, A, Kabir, A, Goodarzi, D & Moghimi, M 2015, ‘The effect of adjuvant vitamin C after varicocele surgery on sperm quality and quantity in infertile men: a double blind placebo controlled clinical trial’, International Braz J Urol, vol. 41, pp. 230–238
Fanaei, H, Khayat, S, Halvaei, I, Ramezani, V, Azizi, Y, Kasaeian, A, Mardaneh, J, Parvizi, MR & Akrami, M 2014, ‘Effects of ascorbic acid on sperm motility, viability, acrosome reaction and DNA integrity in teratozoospermic samples’, Iranian Journal of Reproduction Medicine, vol. 12, no. 2, pp. 103-110
Gürler, H, Malama, E, Heppelmann, M, Calisici, O & Leiding, C 2016, ‘Effects of cryopreservation on sperm viability, synthesis of reactive oxygen species, and DNA damage of bovine sperm’, Theriogenology, vol. 86, pp. 562–571, https://doi.org/10.1016/j.theriogenology.2016.02.007
Henkel, R, Sandhu, IS & Agarwal, A 2019, ‘The excessive use of antioxidant therapy: A possible cause of male infertility?’ Andrologia, vol. 51, pp. 1–8, https://doi.org/10.1111/and.13162
Henkel, RR & Schill, WB 2003, ‘Sperm preparation for ART’, Reproductive Biology and Endocrinology, vol. 1, pp. 1-22, https://doi.org/10.1186/1477-7827-1-108
Ibrahim, SF, Osman, K, Das, S, Othman, AM, Majid, NA & Rahman, MPA 2008, ‘A study of the antioxidant effect of alpha lipoic acids on sperm quality’, Clinics, vol. 63, no. 4, pp. 545-550, https://doi.org/10.1590/S1807-59322008000400022
Jenkins, TG, Aston, KI & Carrell, DT 2011, ‘Supplementation of cryomedium with ascorbic acid-2-glucoside (AA2G) improves human sperm post-thaw motility’, Fertility and Sterility, vol. 95, pp. 2001-2004, https://doi.org/10.1016/j.fertnstert.2011.02.023
Kowalczyk, A 2022, ‘The role of the natural antioxidant mechanism in sperm cells’, Reproductive Sciences, vol. 29, pp. 1387-1394, https://doi,org/10,1007/s43032-021-00795-w
Kumar, A & Sharma, M 2017, Basics of Human Andrology, In: Basics of Human Andrology, Springer Nature Singapore, Singapore, https://doi.org/10.1007/978-981-10-3695-8_17
Larasati, MD, Lestari, SW, Hestiantoro, A & Pangestu, M 2022, ‘Can cryoprotectant’s modification in spermatozoa cryopreservation be an alternative to improve embryo quality? a review’, International Journal of Technology, vol. 13, no. 8, pp. 1755-1767, https://doi.org/10.14716/ijtech.v13i8.6129
Li, MC, Chiu, YH, Gaskins, AJ, Minguez-Alarcon, L, Nassan, FL, Williams, PL, Petrozza, J, Hauser, R & Chavarro, JE 2019, ‘Men’s intake of vitamin c and b-carotene is positively related to fertilization rate but not to live birth rate on couples undergoing infertility treatment’, The Journal of Nutrition, vol. 149, pp. 1977-1984, https://doi.org/10.1093/jn/nxz149
Li, Z, Lin, Q, Liu, R, Xiao, W & Liu, W 2010, ‘Protective effects of ascorbate and catalase on human spermatozoa during cryopreservation’, Journal of Andrology, vol. 31, pp. 437-444, https://doi.org/10.2164/jandrol.109.007849
Liu, S, Liu, B, Zhao, W, Liu, X, Xian, Y, Cheng, Q, Jiang, M, Yue, H & Li, F 2022, Rapid cryopreservation of small quantities of human spermatozoa by a self-prepared cryoprotectant without animal component, Andrologia, vol. 54, pp. 1-9, https://doi.org/10.1111/and.14318
Mangoli, E, Talebi, AR, Anvari, M, Taheri, F, Vatanparast, M, Rahiminia, T & Hosseini, A 2018, ‘Vitamin C attenuates negative effects of vitrification on sperm parameters, chromatin quality, apoptosis and acrosome reaction in neat and prepared normozoospermic samples’, Taiwanese Journal of Obstetrics and Gynecology, vol. 57, no. 2, pp. 200–204, https://doi.org/10.1016/j.tjog.2018.02.006
Nabi, A, Khalili, MA, Fesahat, F, Talebi, A & Ghasemi-Esmailabad, S 2017, ‘Pentoxifylline increase sperm motility in devitrified spermatozoa from asthenozoospermic patient without damage chromatin and DNA integrity’, Cryobiology, vol. 76, pp. 59-64, https://doi.org/10.1016/j.cryobiol.2017.04.008
Najafi, A, Daghigh Kia, H & Hamishehkar, H 2021, ‘Does alpha-lipoic acid–loaded nanostructured lipid carriers improve post-thawed sperm quality and ameliorate apoptosis-related genes of rooster sperm?’ Poult, Sci, vol. 100, pp. 357-365, https://doi.org/10.1016/j.psj.2020.10.007
Nasimi Doost Azgomi, R, Nazemiyeh, H, Sadeghi Bazargani, H, Fazljou, SMB, Nejatbakhsh, F, Moini Jazani, A, Ahmadi AsrBadr, Y & Zomorrodi, A 2018, ‘Comparative evaluation of the effects of Withania somnifera with pentoxifylline on the sperm parameters in idiopathic male infertility: A triple-blind randomised clinical trial’, Andrologia, vol. 50, pp. 1-9, https://doi.org/10.1111/and.13041
Nateghian, Z, Nasr-Esfahani, M,H, Talaei-Khozani, T, Tavalaee, M & Aliabadi, E 2023, L-carnitine and pentoxifylline supplementation improves sperm viability and motility at low temperature, International Journal of Fertility & Sterility, vol. 17, pp 61-66, https://doi.org/10.22074/IJFS.2022.543872.1232
Nomura, K, Koh, ICC, Iio, R, Okuda, D, Kazeto, Y, Tanaka, H & Ohta, H 2018, ‘Sperm cryopreservation protocols for the large-scale fertilization of Japanese eel using a combination of large-volume straws and low sperm dilution ratio’, Aquaculture, vol. 496, pp. 203-210, https://doi.org/10.1016/j.aquaculture.2018.07.007
Özekici, Ü, C?nc?k, M, Selam, B, Çak?l, YD & Çelik, S 2020, ‘Effects of antioxidants on motility and DNA integrity in frozen-thawed sperm’, Maltepe T?p Derg, vol.12, pp. 41-48, https://doi.org/10.35514/mtd.2020.28
Pal, S, Chakraborty, D, Mandal, MK, Chakraborti, K, Agarwal, A & Bhattacharrya, AK, 2005, ‘Effect of pentoxifylline containing human sperm cryopreservation medium on post-thaw motility of human spermatozoa and lipid peroxidation status of human semen’, ORAL Present, Procedures Techniques-laboratory, vol. 84
Parekattil, SJ, Esteves, SC & Agarwal, A 2020, Male Infertility: Contemporary Clinical Approaches, Andrology, ART and Antioxidants, Second Edition, pp. 1–914, https://doi.org/10.1007/978-3-030-32300-4
Peris-Frau, P, Soler, AJ, Iniesta-Cuerda, M, Martín-Maestro, A, Sánchez-Ajofrín, I, Medina-Chávez, DA, Fernández-Santos, MR, García-álvarez, O, Maroto-Morales, A, Montoro, V & Garde, JJ 2020, ‘Sperm cryodamage in ruminants: Understanding the molecular changes induced by the cryopreservation process to optimize sperm quality’, International Journal of Molecular Sciences, vol. 21(8), p. 2781, https://doi.org/10.3390/ijms21082781
Plotnikov, EY, Kazachenko, AV, Vyssokikh, MY, Vasileva, AK, Tcvirkun, DV, Isaev, NK, Kirpatovsky, VL & Zorov, DB, 2007, ‘The role of mitochondria in oxidative and nitrosative stress during ischemia/reperfusion in the rat kidney’, Kidney International, vol. 72, pp. 1493-1502
Rafiee, B, Morowvat, MH & Rahimi-Ghalati, N 2016, ‘Comparing the effectiveness of dietary vitamin C and exercise interventions on fertility parameters in normal obese men’, Urology Journal, vol. 13, pp. 2635-2639
Ribeiro, JC, Braga, PC, Martins, AD, Silva, BM, Alves, MG & Oliveira, PF, 2021, ‘Antioxidants present in reproductive tract fluids and their relevance for fertility’, Antioxidants, vol. 10, no. 9, p. 1441, https://doi.org/10.3390/antiox10091441
Safarinejad, MR 2011, ‘Effect of pentoxifylline on semen parameters, reproductive hormones, and seminal plasma antioxidant capacity in men with idiopathic infertility: A randomized double-blind placebo-controlled study’, International Urology and Nephrology, vol. 43, pp. 315-328, https://doi.org/10.1007/s11255-010-9826-4
Saleh, R, Assaf, H, El Maged, WMA, Elsuity, M & Fawzy, M 2018, Increased cryo-survival rate in ejaculated human sperm from infertile men following pre-freeze in vitro myo-inositol supplementation, Clin Exp Reprod Med, vol. 45, pp. 177-182, https://doi.org/10.5653/cerm.2018.45.4.177
Shay, KP, Moreau, RF, Smith, EJ, Smith, AR & Hagen, TM 2009, ‘Alpha-lipoc acid as a dietary supplement: molecular mechanisms and therapeutic potential’, Biochimica et Biophysica Acta (BBA) - General Subjects, vol. 1790, no. 10, pp. 1149-1160
Shaygannia, E, Tavalaee, M, Akhavanfarid, GR, Rahimi, M, Dattilo, M & Nasr-Esfahani, MH 2018, ‘Alpha-lipoic acid improves the testicular dysfunction in rats induced by varicocele’, Andrologia, vol. 50, pp. 1-9, https://doi.org/10.1111/and.13085
Shen, T, Jiang, ZL, Li, CJ, Hu, XC & Li, QW 2016, ‘Effect of alpha-lipoic acid on boar spermatozoa quality during freezing-thawing’, Zygote, vol. 24, no. 2, pp. 259–265, https://doi.org/10.1017/S0967199415000155
Shivaprasad, H, Sreenivasa, G, Kavitha, P & Suttur SM 2015, ‘A comprehensive and systematic study of semen quality and sperm functional status in normozoospermic controls and infertile males from south india’, Andrology, vol. 04, no. 137, https://doi.org/10.4172/2167-0250.1000137
Singh, P, Agarwal, S, Singh, H, Verma, PK, Pandey, AK & Kumar, S 2020, ‘Effects of ascorbic acid as antioxidant semen additive in cryopreservation of cross-bred cattle bull semen’, International Journal of Current Microbiology and Applied Sciences, vol. 9, pp. 3089-3099, https://doi.org/10.20546/ijcmas.2020.909.202
Sönmez, M, Türk, G & Yüce, A 2005, ‘The effect of ascorbic acid supplementation on sperm quality, lipid peroxidation and testosterone levels of male Wistar rats’, Theriogenology, vol. 63, pp. 2063-2072, https://doi.org/10.1016/j.theriogenology.2004.10.003
Stanic, P, Sonicki, Z & Suchanek, E 2002, ‘Effect of pentoxifylline on motility and membrane integrity of cryopreserved human spermatozoa’, International Journal of Andrology, vol. 25, pp. 186-190, https://doi.org/10.1046/j.1365-2605.2002.00348.x
Torres-Arce, E, Vizmanos, B, Babio, N, Márquez-Sandoval, F & Salas-Huetos, A 2021, ‘Dietary antioxidants in the treatment of male infertility: counteracting oxidative stress’, Biology, vol. 10, no. 3, p. 241, https://doi.org/10.3390/biology10030241
Valipour, J, Mojaverrostami, S, Abouhamzeh, B & Abdollahi, M 2021, ‘Protective effects of hesperetin on the quality of sperm, apoptosis, lipid peroxidation, and oxidative stress during the process of cryopreservation: An experimental study’, International Journal of Reproductive BioMedicine, vol. 19, no. 1, pp. 35-46, https://doi,org/10,18502/ijrm,v19i1,8178
World Health, 2010, Examination and processing of human semen, World Health Edition, 5th Edn, World Health Organization
Xian, Y, Jiang, M, Liu, B, Zhao, W, Zhou, B, Liu, X, Liu, S & Li, F 2021, A cryoprotectant supplemented with pentoxifylline can improve the effect of freezing on the motility of human testicular sperm, In: Zygote, Cambridge University Press, https://doi.org/10.1017/S0967199421000368
Zidni, I, Lee, H-B, Yoon, J-H, Park, J-Y, Jang, H, Cho, Y-S, Seo, Y-S & Lim, H-K 2022, Cryopreservation of roughscale sole (clidoderma asperrimum) sperm: effects of cryoprotectant, diluent, dilution ratio, and thawing temperature, Animals, vol. 12, no. 19, p. 2553, https://doi.org/10.3390/ani12192553
