The Effect of Gas Flow Rate, Solid-To-Solvent Ratio, and Temperature on Micro-Carbon Synthesis from Pine Resin using Spray Pyrolysis and The Application as Masks Coating Material
Corresponding email: yessie.sari@apps.ipb.ac.id
Published at : 28 May 2025
Volume : IJtech
Vol 16, No 3 (2025)
DOI : https://doi.org/10.14716/ijtech.v16i3.6252
Jayadi, J, Widayatno, WB, Wismogroho, AS, Mulya, MAJ, Maddu, A & Sari, YW 2025, ‘The effect of gas flow rate, solid-to-solvent ratio, and temperature on micro-carbon synthesis from pine resin using spray pyrolysis and the application as masks coating material’, International Journal of Technology, vol. 16, no. 3, pp. 949-959
| Jayadi Jayadi | 1. Department of Physics, Faculty of Mathematics and Natural Sciences, IPB University, Bogor, 16680, Indonesia. 2. National Research and Innovation Agency, Tangerang Selatan, 15310, Indonesia |
| Wahyu B. Widayatno | National Research and Innovation Agency, Tangerang Selatan, 15310, Indonesia |
| Agus S. Wismogroho | National Research and Innovation Agency, Tangerang Selatan, 15310, Indonesia |
| Marga A. J. Mulya | National Research and Innovation Agency, Tangerang Selatan, 15310, Indonesia |
| Akhiruddin Maddu | Department of Physics, Faculty of Mathematics and Natural Sciences, IPB University, Bogor, 16680, Indonesia |
| Yessie W. Sari | Department of Physics, Faculty of Mathematics and Natural Sciences, IPB University, Bogor, 16680, Indonesia |
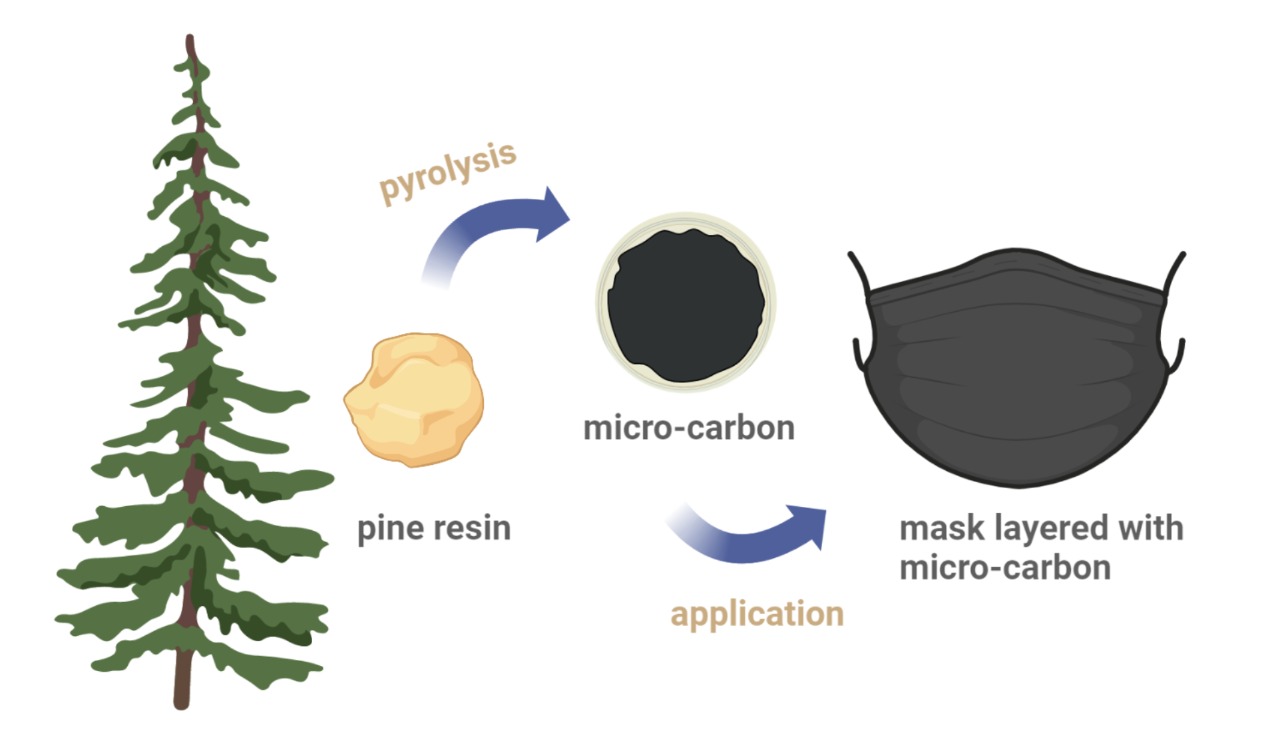
This study aims to develop
an additional layer in masks using micro-carbon to enhance protection against
inhalation of droplets. Carbon microstructures were obtained using spray
pyrolysis method, with the precursor being resina colophonium dissolved in acetone
and ethyl acetate. Subsequently, the effect of varying the precursor-solvent
ratio, heating temperature, and nitrogen flow rate on the shape, size, and
content of the products was assessed. The results showed that the highest
carbon content achieved was approximately 96%, with the smallest size measuring
139 nm. The penetration test results after applying the product on masks fabric
revealed a significant reduction in penetration up to level 2. In addition, the
contact angle test showed that the addition of carbon led to a lower reduction
(6.7°) compared to regular masks fabric with a 20.4° decrease. This improvement
could contribute to reducing the risk of droplet inhalation during masks
application.
Carbon; Mask; Penetration level; Resina colophonium; Spray pyrolysis
Severe acute
respiratory syndrome coronavirus 2 (SARS-CoV2) is primarily transmitted through
the inhalation of aerosol particles or droplets containing the virus, which are
emitted during breathing and speaking
According to
previous studies, carbon-based precursor materials are traditionally
synthesized using a solution of methanol (Fang et al., 2018), benzene (Melati and
Hidayati, 2016), xylene (Singhal et al., 2019), or toluene (Khabushev et al.,
2022) derived from fossil fuels. However, the limited nature of these
resources
In line with
these results, Liu et al. conducted a study on the ultrasonic spray pyrolysis
method for synthesizing nitrogen-doping carbon nanotubes. The procedures were
carried out at injection rates of 0.25, 0.5, and 0.75 ml/minute, while the gas
flow rate at each inlet was maintained at 150 sccm
2.1. Materials
Pine resin (resina colophonium), which was essential for the
experiments, was obtained from the local market. The solvents used were acetone
(pro analysis 99.75%, Mallinckrodt Chemicals) and ethyl acetate (pro analysis
99.8%, Merck KGaA).
2.2. Micro-Carbon Preparation
Micro-carbon
materials, which were crucial for the study, were obtained through spray
pyrolysis of pine resin. In this process, pine resin was dissolved in either
acetone (GAC) or ethyl acetate (GEA). Pine resin was firstly crushed by a Hamer
mill (5000 rpm German) until it reached a size of 60 mesh to facilitate the
dissolving process.
The
in-house spray pyrolysis was utilized for the experiment. This instrument was
configured to allow the precursor (GAC or GEA) into the inlet, atomized, and
subsequently conveyed into a heating reactor with the aid of nitrogen gas.
During the heating process, pine resin underwent decomposition, which yielded
carbon granules that were deposited onto a 1000-wire mesh positioned at the
outlet of the heating reactor. Spray pyrolysis was set for 20 minutes for each
variation. This study involved varying the ratio of pine resin to
solvent and adjusting the nitrogen gas flow rate during micro-carbon synthesis.
Specifically, the ratios of pine resin to solvent were set at 1:4, 1:8, and
1:16 (g/ml). The heating temperature was varied at 800, 1000, and 1200°C, while
the nitrogen gas flow rates were adjusted to 0.5, 1, and 1.5 liter/minute.
These parameters were selected to achieve the highest percentage of carbon
content while maintaining a smooth carbon surface to repel droplets.
2.3. Application of Micro-Carbon as Mask Layer
Polypropylene
sheet, which was a common medical mask, (80 x 80 cm), was used as masks.
Micro-carbon was deposited on this sheet using vacuum chambers with a volume of
1 liter (VC1621SG, VacuumChambers.ue., Poland).
Before deposition, the product was first dissolved in acetone with a
ratio of 0.3 g/ml. The dissolved micro-carbon was placed in the vacuum chamber
at around 25 ± 2°C and under a pressure of 50 kPa for 30 minutes. Micro-carbon
coated masks were further dried using the conventional oven at 60°C for 24
hours
2.4. Sample Characterization
2.4.1. Morphological Analysis
The synthesized micro-carbon
powder was separated from the surface of wire mesh 1000 for morphological
analysis using Field Emission Scanning Electron Microscopy (FESEM, JIB-4610F,
Jeol Ltd Japan). Images obtained from FESEM were further analyzed using ImageJ
to obtain the average particle size. As many as 20 particles were evaluated to
obtain this parameter, FESEM analysis was also combined with energy-dispersive
X-ray spectroscopy to evaluate the carbon content.
2.4.2. Penetration
Test
Penetration test was performed according to
ISO 16603:2004 about measuring the penetration resistance of clothing materials
to blood and body fluids. There were 6 pressure levels involved in this test
(Table 1), and to imitate saliva penetration on a surface, chicken egg white
was utilized as it was considered to have a surface tension close to saliva.
The surface tension of egg white without yolk contamination was 50 mN/m
The penetration test was conducted by applying varying pressure levels
after egg white was applied to the test samples. The test was terminated once
egg white penetrated through the test sample. When penetration did not occur,
the test proceeded to the next level by increasing the pressure. Table 2 listed
samples involved in this study.
Table 1
Test level in penetration test
and waiting time for each level
|
Level |
Pressure (kPa) |
Time (Minutes) |
|
1 |
0 |
5 |
|
2 |
1.75 |
|
|
3 |
3.5 |
|
|
4 |
7 |
|
|
5 |
14 |
|
|
6 |
20 |
2.4.3. Contact Angle
Test
Assessing the absorption property of masks coated with micro-carbon
involved conducting contact angle test on the samples. This test entailed
dropping a liquid onto the test samples, capturing an image of the sample, and
observing the angle formed between the liquid and the sample
Table 2 Sheet samples code used
|
Code |
Sample |
|
PS sample |
polypropylene sheet
without any coating |
|
PAC sheet |
commercial polypropylene
sheet coated with activated carbon |
|
GAC sheet |
polypropylene sheet coated
with carbon dissolved in GAC |
|
GEA sheet |
polypropylene sheet coated
with carbon dissolved in GEA |
3.1. Micro-Carbon
Synthesis: Effect of Solid-To-Solvent Ratio
The
morphologies of micro-carbon resulting from pyrolysis at 1000°C with various
solid-to-solvent ratios (1:4, 1:8, and 1:16) were shown in Figure 1. FESEM results
indicated that micro-carbon particles had round and smooth surfaces. In this
test, the value of carbon content in the sample could also be known. As shown
in Table 3, all samples were rich in carbon content with a value of more than
90%. The highest carbon content was obtained in samples from GAC precursors
with a composition ratio of 1:8, which reached 96.8% wt.
Figure 1 FESEM images of micro-carbon particles size
obtained with pine resin solvent ratio of (a) 1:4 (b) 1:8 (c) 1:16 from GAC
samples and (d) 1:4 (e) 1:8 (f) 1:16 from GEA samples
Particle size could
be determined quantitatively based on the measuring scale performed on FE-SEM
test results. Micro-carbon of GAC samples had a smaller particle size than
micro-carbon from GEA samples (Figure 2). Micro-carbon from precursor GAC with
pine resin to acetone ratio of 1:8 had the smallest average particle size,
which was 0.12 µm. Figure 2 showed the normal
distribution, the average value (µ), and the standard deviation (?) of all samples. The
distribution of carbon diameter of GAC samples indicated less distribution
compared to GEA counterpart. This could be due to the agglomeration of crushed
pine resin in ethyl acetate. Previous studies indicated that agglomeration in
ethyl acetate was quicker to form rather than in ethanol
Table 3 Carbon content in samples with pine resin to solvent ratio
|
Pine resin to solvent
ratio |
Relative carbon content (%
wt) | |
|
GAC |
1:4 |
95.625 |
|
1:8 |
96.862 | |
|
1:16 |
96.525 | |
|
GEA |
1:4 |
96.275 |
|
1:8 |
96.037 | |
|
1:16 |
96.337 |
Figure 2
Particle size distribution of GAC and GEA from 20 particles of each sample
3.2. Micro-Carbon
Synthesis: Effect of Pyrolysis Temperature
Figure 3, the
measurement scale of FE-SEM test resulting from carbon samples synthesized at
various heating temperatures with GAC at pine resin to solvent ratio of 1:16
and a nitrogen gas flow rate of 1 liter/minute. Additionally, it was observed
that synthesis at 800°C resulted in micro-carbon with the lowest particle size,
0124 ± 0.008 µm. However, the particle sizes in these samples were observed to
have a heterogeneous morphology. An improved morphology heterogeneity was
observed at micro-carbon obtained from pyrolysis at 1000 and 1200°C (Figures 3b
and c, respectively). Micro-carbon synthesized at 1200°C had the highest
particle size homogeneity, and the particle surface had an imperfection. Figure
3c showed that the surface of these particles was not smooth, and the
distribution of particle size was obtained from various pyrolysis temperatures.
Pyrolysis
temperature also influenced the content of the resulting carbon, and the sample
at a synthesis temperature of 1000°C had the highest carbon content, reaching
96.5% wt (Table 4). The increase in carbon content at 1000oC and
1200oC compared to 800oc reflected the increasing degree
of carbonization
3.3. Micro-Carbon
Synthesis: Effect of Nitrogen Flow
Table
5 showed that samples synthesized at 1200°C had similar carbon content to the
sample at 1000°C but had bigger particle sizes (Figure 6) and rougher surfaces
(Figure 5). Exploring whether adjusting the nitrogen gas flow rate could
improve either carbon content, particle size, or surface characteristics, the
nitrogen flow rate variation test was conducted on GAC 1:16 sample heated at
1200°C.
Figure 3 FESEM images of GAC samples obtained from pine resin, a solvent ratio of 1:16, nitrogen gas flow rate of 1 liter/minute, and synthesis temperature, (a) 800°C, (b) 1000°C, and (c) 1200°C
Figure
4 Particle size distribution of GAC precursor
1:16 with nitrogen gas flow rate of 1 liter/minute at
a varied synthesis temperature
Table
4 Carbon content in samples of GAC 1:16 precursors
with variations in synthesis temperature
|
Synthesis
temperature (°C) |
Relative
carbon content (% wt) |
|
800 |
92.475 |
|
1000 |
96.525 |
|
1200 |
96.275 |
Based on the image
processing of FESEM images, the smallest particle size was formed at a flow
rate of 0.5 liter/minute, which was 0.167 ± 0.008 µm. All carbon materials were
synthesized at a pyrolysis temperature of 1200°C with varied nitrogen flow
rates showing rough surfaces as shown in Figure 5.
Table 5 Carbon content in samples from GAC 1:16
precursors with a synthesis temperature of 1200°C and variations in nitrogen
flow rate
|
Nitrogen flow rate (liter/ minute) |
Relative carbon content
(at%) |
|
0.5 |
14.48 |
|
1 |
96.28 |
|
1.5 |
97.15 |
An
interesting observation was made regarding the significant influence of
nitrogen flow rate on the carbon content of the samples. As shown in Table 5,
at a nitrogen flow rate of 0.5 l/minute, micro-carbon with a content of 14.48%
wt was obtained, suggesting the presence of volatiles that acted as catalysts
for secondary reactions, potentially reducing carbon yield
Figure
5 Carbon particle size of GAC precursor 1:16 with
synthesis temperature 1200°C and nitrogen gas flow rate (a) 0.5 liter/minute
(b) 1 liter/minute (c) 1.5 liter/minute
3.4. Application of
Micro-Carbon as Masks Layer
Both GAC and GEA micro-carbon obtained at pine resin to solvent ratio of 1:8, 1000°C pyrolysis temperature, and 1 liter/minute of nitrogen flow rate were tested for their application as masks coating materials. The selection was based on the data discussed in sections 3.1, 3.2, and 3.3, indicating that at this condition, the product had the smallest particle size, the smoothest surface, and the highest carbon content. The small particle size was preferred as micro-carbon particles were expected to be able to close the gap in polypropylene sheet.
Figure
6 Particle size distribution of GAC precursor 1:16
with synthesis temperature 1200°C and varied nitrogen gas flow rate
Figures 7 a, b, c, and d showed the dropped
water on polypropylene sheet coated with the product, and the yellow line on
these figures indicated the contact angle. Observations were conducted for 1
hour and contact angle data were recorded every 10 minutes. Table 6 indicated
the data from the contact angle observations. Based on the data in Figure 8a,
all samples had initial contact angles above 90°, indicating that all paper
masks were hydrophobic
 The
contact angle was observed to decrease along with the observation time.
Comparison between all samples in this test at minute 0 and minute 60 could be
seen in Figure 8b. A significant decrease was experienced by PS sample, from an
initial contact angle of 118.221° to 97.802° (20.4° difference). The least
decrease in contact angle was experienced in GAC sheet from 121.530° to
114.744° (6.786° difference). This indicated that the presence of a carbon
layer on polypropylene sheet could reduce or slow down the absorption process.
GEA sheet had less performance in this test (10.8° drop from 120.4°) compared
to GAC sheet. This result designated that the difference in size affected the
results of this test. PAC sheet, which was polypropylene coated with commercial
activated carbon, had a higher contact angle drop from 118.852° to 101.162°
(17.69° difference) compared to polypropylene coated with GAC and GEA
micro-carbon. Activated carbon was generally used as an adsorbent which relied
on the porous surface
The
contact angle was observed to decrease along with the observation time.
Comparison between all samples in this test at minute 0 and minute 60 could be
seen in Figure 8b. A significant decrease was experienced by PS sample, from an
initial contact angle of 118.221° to 97.802° (20.4° difference). The least
decrease in contact angle was experienced in GAC sheet from 121.530° to
114.744° (6.786° difference). This indicated that the presence of a carbon
layer on polypropylene sheet could reduce or slow down the absorption process.
GEA sheet had less performance in this test (10.8° drop from 120.4°) compared
to GAC sheet. This result designated that the difference in size affected the
results of this test. PAC sheet, which was polypropylene coated with commercial
activated carbon, had a higher contact angle drop from 118.852° to 101.162°
(17.69° difference) compared to polypropylene coated with GAC and GEA
micro-carbon. Activated carbon was generally used as an adsorbent which relied
on the porous surface
Figure 7 Initial contact angle for (a) PS sample, (b)
PAC sheet, (c) GEA sheet, (d) GAC sheet
In Table 6, presented below, the result of the
penetration test showed that both PS sample and PAC sheet were effectively
penetrated by egg white at level 1 or without any pressure applied. Activated
carbon coated on polypropylene sheet proved unable to withstand the penetration
of egg white in the test. This could occur because the pores in the activated
carbon could serve as a pathway for egg white to penetrate the sheet.
Table 6 Penetration test results for all samples and carbon weight applied
|
Test
Sample |
Carbon
weight (g) |
Penetration
level |
Pressure
(kPa) |
|
PS
sample |
- |
Level
1 |
0 |
|
PAC
sheet |
n.d |
Level
1 |
0 |
|
GAC
sheet |
0.0043 |
Level
1 |
0 |
|
0.0150 |
Level
1 |
0 | |
|
0.0167 |
Level
2 |
1.75 | |
|
GEA
sheet |
0.0044 |
Level
1 |
0 |
|
0.0166 |
Level
2 |
1.75 | |
|
0.0206 |
Level
3 |
3.5 |
Figure 8 Contact angle test data (a) taken every 10 minutes for 1 hour, (b) test data distribution after 20 measurements at minutes 0 and 60
Penetration was also experienced by both GAC and GEA
sheets. However, it was observed that a pressure of min 1.75 kPa was required
to let egg white penetrate these sheets. This result showed the potential of
micro-carbon synthesized from pine resin as masks coating materials. At GEA
sheet, it was observed that an increase in the mass of the product resulted in
an increase in required pressure for the egg white penetration. This indicated
that the amount of micro-carbon had an effect on masks adsorption properties.
Therefore, further studies were needed to evaluate the optimum micro-carbon
mass required for the optimum adsorption.
In conclusion, the study analysis indicated that
micro-carbon was successfully synthesized using a mixture of resina colophonium
mixed with acetone and ethyl acetate as solvents. The precursor composition,
synthesis temperature, and nitrogen flow rate played significant roles in
shaping the size, and carbon content during spray pyrolysis method.
Specifically, a 1:8 ratio of resina colophonium to acetone resulted in
particles measuring 139 nm with a carbon content of 96.682%, while the same
ratio with ethyl acetate produced larger particles (301 nm) with a slightly
lower content of 96.037%. Furthermore, coating polypropylene sheets with the
product led to improved performance. This was evidenced by superior contact
angle and penetration test results compared to non-coated and commercially
coated sheets. The addition of micro-carbon effectively reduced the risk of
droplet inhalation.
The authors are grateful to the National Research and Innovation Agency for the facilities and funding provided with contract number B-392/III/HK.01.00/2/2022.
Author
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Jayadi, Wahyu B. Widayatno and Agus S. Wismogroho. The first draft of the manuscript was written by Jayadi and Marga A. J. Mulya. Akhiruddin Maddu and Yessie W. Sari carried out thorough data analysis and interpretation. All authors commented on previous versions of the manuscript.
Conflict of Interest
The authors declare that they have no known competing financial interests
or personal relationships
that could have appeared to influence the work reported in this paper.
Afre, RA, Soga,
T, Jimbo, T, Kumar, M, Ando, Y, Sharon, M, Somani, PR & Umeno, M 2006,
'Carbon nanotubes by spray pyrolysis of turpentine oil at different
temperatures and their studies', Microporous and Mesoporous Materials,
vol. 96, no. 1–3, pp. 184–190, https://doi.org/10.1016/j.micromeso.2006.06.036
Anfinrud, P,
Stadnytskyi, V, Bax, CE & Bax, A 2020, 'Visualizing speech-generated oral
fluid droplets with laser light scattering', New England Journal of Medicine,
vol. 382, no. 21, pp. 2061–2063, https://doi.org/10.1056/NEJMc1913036
Aristri, MA,
Lubis, MAR, Laksana, RPB, Falah, F, Fatriasari, W, Ismayati, M, Wulandari, AP,
Nurindah, N & Ridho, MR 2021, 'Bio-polyurethane resins derived from liquid
fractions of lignin for the modification of ramie fibers', Jurnal Sylva
Lestari, vol. 9, no. 2, article 223, http://dx.doi.org/10.23960/jsl29223-238
Chatterjee, R,
Sajjadi, B, Chen, WY, Mattern, DL, Hammer, N, Raman, V & Dorris, A 2020,
'Effect of pyrolysis temperature on physicochemical properties and
acoustic-based amination of biochar for efficient CO2 adsorption', Frontiers
in Energy Research, vol. 8, article 85, https://doi.org/10.3389/fenrg.2020.00085
Cheng, Y, Ma, N,
Witt, C, Rapp, S & Wild, PS, Andreae, MO, Pöschl, U, & Su, H 2021,
'Face masks effectively limit the probability of SARS-CoV-2 transmission', Science,
vol. 372, no. 6549, pp. 1439–1443, https://doi.org/10.1126/science.abg6296
Dizbay-Onat,
M 2023, 'Evaluation of physical adsorption properties of the activated carbon
layers used in the commercial face mask inserts', Eng, vol. 4, no. 1,
pp. 434–443, https://doi.org/10.3390/eng4010026
Efiyanti,
L, Rifa'i, FA, Maslahat, M, Indrawan, DA, Wibowo, S, Darmawan, S, Pari, R,
Pasaribu, G, Rahmila, YI, Agustarini, R, Aswandi, A, Kholibrina, CR, Santoso,
A, Djarwanto, Komarayati, S, Gusmalina, Pari, G & Hendra, D 2023,
'CO/activated carbon from Paraserianthes falcataria as a green catalysts for
plastic waste pyrolysis', RASAYAN Journal of Chemistry, vol. 16, no. 3,
pp. 1149–1162, https://doi.org/10.31788/RJC.2023.1638347
Fang,
R, Huang, H, Ji, J, He, M, Feng, Q, Zhan, Y & Leung, DY 2018, 'Efficient
MnOx supported on coconut shell activated carbon for catalytic oxidation of
indoor formaldehyde at room temperature', Chemical Engineering Journal,
vol. 334, pp. 2050–2057, https://doi.org/10.1016/j.cej.2017.11.176
Gittings,
S, Turnbull, N, Henry, B, Roberts, CJ & Gershkovich, P 2015,
'Characterisation of human saliva as a platform for oral dissolution medium
development', European Journal of Pharmaceutics and Biopharmaceutics,
vol. 91, pp. 16–24, https://doi.org/10.1016/j.ejpb.2015.01.007
Harahap,
M, Daulay, N, Zebua, D & Gea, S 2023, 'Nanofiber cellulose/lignin from oil
palm empty fruit bunches and the potential for carbon fiber precursor prepared
by wet-spinning', International Journal of Technology, vol. 14, no. 1,
pp. 152–161, https://doi.org/10.14716/ijtech.v14i1.5082
Ionescu,
MI, Zhang, Y, Li, R, Sun, X, Abou-Rachid, H, & Lussier, LS 2011,
'Hydrogen-free spray pyrolysis chemical vapor deposition method for the carbon
nanotube growth: Parametric studies', Applied Surface Science, vol. 257,
no. 15, pp. 6843–6849, https://doi.org/10.1016/j.apsusc.2011.03.011
Jayadi,
Widayatno WB, Wismogroho AS, Firdharin C, Maddu, A, Alatas, H & Sari, YW
2024, 'Effect of precursor solvent on the carbon micro-structures derived from
spray pyrolysis of pine resin (gondorukem): Preliminary study', Jurnal Sains
Materi Indonesia, vol. 25, no. 2, pp. 67–76, https://doi.org/10.55981/jsmi.2024.893
Khabushev,
EM, Krasnikov, DV, Goldt, AE, Fedorovskaya, EO, Tsapenko, AP, Zhang, Q,
Kauppinen, EI, Kallio, T & Nasibulin, AG 2022, 'Joint effect of ethylene
and toluene on carbon nanotube growth', Carbon, vol. 189, pp. 474–483, https://doi.org/10.1016/j.carbon.2021.12.052
Li, X, Wang, Y, Lv, J & Yang, Y 2022,
'Investigations of foaming, interfacial and structural properties of
dispersions, batters and cakes formed by industrial yolk-contaminated egg white
protein', LWT, vol. 154, pp. 1–9, https://doi.org/10.1016/j.lwt.2021.112776
Liu,
J, Zhang, Y, Ionescu, MI, Li, R & Sun, X, 2011, 'Nitrogen-doped carbon
nanotubes with tunable structure and high yield produced by ultrasonic spray
pyrolysis', Applied Surface Science, vol. 257, no. 17, pp. 7837–7844, https://doi.org/10.1016/j.apsusc.2011.04.041
Melati,
A, & Hidayati, E 2016, 'Synthesis and characterization of carbon nanotube
from coconut shells activated carbon', Journal of Physics: Conference Series,
vol. 694, article 012073, https://doi.org/10.1088/1742-6596/694/1/012073
Miranda, J, Partal, P, Cordobés, F & Guerrero,
A, 2002, ‘Rheological characterization of egg yolk processed by spray-drying
and lipid-cholesterol extraction with carbon dioxide’, Journal of the
American Oil Chemists Society, vol. 79, no. 2, pp. 183-190
Papaioannou,
N, Marinovic, A, Yoshizawa, N, Goode, AE, Fay, M, Khlobystov, A, Titirici, MM
& Sapelkin, A 2018, 'Structure and solvents effects on the optical
properties of sugar-derived carbon nanodots', Scientific Reports, vol.
8, no. 1, article 6559, https://doi.org/10.1038/s41598-018-25012-8
Park,
J-S, Kim, JK, Hong, JH, Cho, JS, Park, S-K & Kang, YC 2019, 'Advances in
the synthesis and design of nanostructured materials by aerosol spray processes
for efficient energy storage', Nanoscale, vol. 11, no. 41, pp.
19012–19057, https://doi.org/10.1039/C9NR05575D
Politaeva,
N, Taranovskaya, E, Mukhametova, L, Ilyashenko, S, Atamanyuk, I, Al Afif, R,
& Pfeifer, C 2020, 'Cotton fiber and carbon materials filters for efficient
wastewater purification', International Journal of Technology, vol. 11,
no. 8, pp. 1608-1617, https://doi.org/10.14716/ijtech.v11i8.4538
Rashidi,
NA, & Yusup, S 2019, 'Production of palm kernel shell-based activated
carbon by direct physical activation for carbon dioxide adsorption', Environmental
Science and Pollution Research, vol. 26, no. 33, pp. 33732–33746, https://doi.org/10.1007/s11356-018-1903-8
Reza,
MS, Hasan, ABMK, Ahmed, AS, Afroze, S, Bakar, MSA, Islam, SN & Azad, AK
2021, 'COVID-19 prevention: Role of activated carbon', Journal of
Engineering and Technological Sciences, vol. 53, no. 4, pp. 628–638, https://doi.org/10.5614/j.eng.technol.sci.2021.53.4.4
Rucitra,
AL & Amna, AUF 2021, 'Integration of statistical quality control (SQC) and
fault tree analysis (FTA) in the quality control of resina colophonium
production in company X', In: IOP Conference Series: Earth and
Environmental Science, vol. 924, no. 1, article 012062, https://doi.org/10.1088/1755-1315/924/1/012062
Shukla,
S, Bhattacharjee, S, Weber, AZ & Secanell, M 2017, 'Experimental and
theoretical analysis of ink dispersion stability for polymer electrolyte fuel
cell applications', Journal of The Electrochemical Society, vol. 164,
no. 6, pp. F600–F609, https://doi.org/10.1149/2.0961706jes
Singhal,
S, Dixit, S & Shukla, AK 2019, 'Structural analysis of carbon nanospheres
synthesized by CVD: An investigation of surface charges and its effect on the
stability of carbon nanostructures', Applied Physics A, vol. 125,
article 80, https://doi.org/10.1007/s00339-018-2372-0
Song,
L 2019, The overview of today’s pine chemical industry in China, Pine
Chemicals Association, Vancouver
Suhas,
Gupta, VK, Carrott, PJM, Singh, R, Chaudhary, M & Kushwaha, S 2016,
'Cellulose: A review as natural, modified and activated carbon adsorbent', Bioresource
Technology, vol. 216, pp. 1066–1076, https://doi.org/10.1016/j.biortech.2016.05.106
Sujiono,
EH, Zabrian, D, Zharvan, V & Humairah, NA 2022, 'Fabrication and
characterization of coconut shell activated carbon using variation chemical
activation for wastewater treatment application', Results in Chemistry,
vol. 4, article 100291, https://doi.org/10.1016/j.rechem.2022.100291
Sukumar,
V, Manieniyan, V, Senthilkumar, R & Sivaprakasam, S 2020, 'Production of
bio oil from sweet lime empty fruit bunch by pyrolysis', Renewable Energy,
vol. 146, pp. 309–315, https://doi.org/10.1016/j.renene.2019.06.156
Sulistya,
EH, Hui-Hui, L, Attenborough, NK, Pourshahrestani, S, Kadri, NA, Zeimaran, E,
Razak, NAbA, Horri, BA & Salamatinia, B 2020, 'Hydrothermal synthesis of
carbon microspheres from sucrose with citric acid as a catalyst:
Physicochemical and structural properties', Journal of Taibah University for
Science, vol. 14, no. 1, pp. 1042–1050, https://doi.org/10.1080/16583655.2020.1794566
Ueki,
H, Furusawa, Y, Iwatsuki-Horimoto, K, Imai, M, Kabata, H, Nishimura, H &
Kawaoka, Y, 2020, 'Effectiveness of face masks in preventing airborne
transmission of SARS-CoV-2', mSphere, vol. 5, no. 5, article e00637-20, https://doi.org/10.1128/msphere.00637-20
Vishwanathan,
LG, Bhowmik, S & Sharon, M 2018, 'Natural precursors for synthesis of
carbon nano materials by chemical vapor deposition process: A review', International
Journal of Science and Research, vol. 7, no. 2, pp. 1475–1485, https://doi.org/10.21275/ART2018338
Wang,
M, Wang, Q, Li, T, Kong, J, Shen, Y., Chang, L, Xie, W & Bao, W, 2021,
‘Catalytic upgrading of coal pyrolysis volatiles by porous carbon materials
derived from the blend of biochar and coal’, ACS Omega, vol. 6, no. 5, pp.
3800–3808
Whulanza,
Y, Supriadi, S, Chalid, M, Kreshanti, P, Agus, AA, Napitupulu, P, Supriyanto,
JW, Rivai, E & Purnomo, A 2020, 'Setting acceptance criteria for a national
flocked swab for biological specimens during the COVID-19 pandemic', International
Journal of Technology, vol. 11, no. 5, pp. 888–899, https://doi.org/10.14716/ijtech.v11i5.4335
Yan,
Q, Toghiani, H, Yu, F, Cai, Z & Zhang, J 2011, 'Effects of pyrolysis
conditions on yield of bio-chars from pine chips', Forest Products Journal,
vol. 61, no. 5, pp. 367–371, https://www.doi.org/10.13073/0015-7473-61.5.367
Yuan,
Y & Lee, TR, 2013, 'Contact angle and wetting properties', Springer
Series in Surface Sciences, vol. 51, no. 1, pp. 3–34, https://doi.org/10.1007/978-3-642-34243-1_1
