Development of Auto-PIVOT: Automated Platform In Vitro for Cell Tissue Culture
Corresponding email: yudan.whulanza@ui.ac.id
Published at : 30 Dec 2022
Volume : IJtech
Vol 13, No 8 (2022)
DOI : https://doi.org/10.14716/ijtech.v13i8.6176
Irsyad, M., Whulanza, Y., Katili, P.A., Antarianto, R.D., Jasirwan, C.O.M., Bugtai, N., 2022. Development of Auto-PIVOT: Automated Platform In Vitro for Cell Tissue Culture. International Journal of Technology. Volume 13(8), pp. 1651-1662
| Muhammad Irsyad | Department of Mechanical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, West Java 16424 Indonesia |
| Yudan Whulanza | 1. Department of Mechanical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, West Java 16424 Indonesia, 2. Research Center for Biomedical Engineering, Faculty of Engineerin |
| Puspita Anggraini Katili | 1. Research Center for Biomedical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, West Java 16424 Indonesia, 2. Biomedical Engineering Study Program, Department of Electri |
| Radiana Dhewayani Antarianto | Department of Histology, Faculty of Medicine, Universitas Indonesia, Jakarta 10430, Indonesia |
| Chyntia Olivia Maurine Jasirwan | Division of Hepatobilliary, Department of Internal Medicine, Faculty of Medicine, Universitas Indonesia, Jakarta 10430, Indonesia |
| Nilo Bugtai | Department of Manufacturing Engineering and Management, Gokongwei College of Engineering, De La Salle University, 1004 Manila, Philippines |
Bioreactors are growing in popularity among network
engineers. Bioreactors are generally chambers used for cell culture processes
with predetermined parameters. Pivot is a bioreactor system for cell culture
that is controlled automatically and remotely using multi-chambers that allow
inflow of fresh liquid or output for sample collection. This system facilitates
gas exchange between the culture medium and ambient gas. The system provides a
storage bag for fresh media and a peristaltic pump is used to move the media in
a closed loop during perfusion and stirring in the cell culture chamber. The
flow rates used is 2 mL/min. The system has a container for direct sample
measurement to obtain pH, oxygen, and carbon dioxide parameters. The bioreactor
system is also equipped with a mixing vessel which allows the addition of
desired nutrients or additives to the system. The results of a computational
fluid dynamics (CFD) and shear stress-based flow analysis have been conducted.
The simulation results demonstrate that the applied Pivot parameters correspond
to an ideal environment to hepatocyte cell viability and growth. This research
is expected to increase the number of cells produced without reducing the
quality of each chamber and be carried out simultaneously, automatically, and
remotely controlled.
Automated system; Bioreactor; Cell culture; PIVOT; Remotely controlled
Tissue
engineering triggers the rapid development of various technology in medical
applications (Hinman et al., 2020;
Birla, 2014). Currently, engineered tissues realized in a
laboratory have been used at the clinical level (Mason et al., 2011). This advancement was supported by the
establishment of the triad of tissue engineering: cells, scaffolding, and signals (Jain & Bansal, 2015). Bioreactor represents a
device that transmits signals in tissue engineering. A bioreactor carries out
biological and/or biochemical processes, including microbial and mammalian cell
culture processes in a monitored and controlled environment. Moreover, bioreactors
also enable us to realize an operating condition by intervening in the culture
environment (Birla, 2014).
Compared to petri dishes,
bioreactors have been proven to have better performance in the sense that
bioreactors are able to facilitate tissue culture in three dimensions (3D) with
the help of biomaterial scaffolds (Costa et al.,
2020; Paez?Mayorga et al., 2019; Nadhif et al., 2017). In this 3D environment, the cultured
tissues produce properties that resemble the targeted mimetic tissue (Valls-Margarit
et al., 2019; Villa-Diaz et al., 2013). In addition, tissue culture in
bioreactors can be carried out dynamically (Nadhif et al.,
2017), allowing the perfusion process
of growth factors, nutrients, medicines, metabolites, and other culture media (Petrenko et al.,
2017). The perfusion process can also
be carried out to flow oxygen (O2) (Schmid et al.,
2018) and carbon dioxide (Schuerlein et al.,
2017) in a controlled manner that
affects cell respiration and acidity (pH) of the environment (Be?karde? et al.,
2018; Petrenko et al., 2017). Some bioreactors include electrical
electrodes for stimulation and signal recording (Whulanza et al.,
2022). The cells given by this
electrical stimulant are cells that can be excited, such as nerve and muscle
cells (Khodabukus et al.,
2019; Huang et al., 2012). Meanwhile, recording electrodes
were used to identify cell activity, such as nerve cells and cardiac wall cells
in bioreactors (Li et al., 2018;
Sagita et al., 2018).
During the operation, a bioreactor is
often put into incubators to maintain the culture temperature (Bilgen et al.,
2013), considering that there are
still few bioreactors that integrate thermal modules (Liu et al., 2019). In fact, the placement of bioreactors that
consist of several components in the incubators often makes it difficult for
researchers to run experiments. Nadhif et al. developed a thermal control
module that can be integrated with bioreactors (Nadhif et al.,
2019). Therefore, the development of a
bioreactor with integrated temperature control IoT system became our research
of interest in this phase. One of the examples also found is from Rahmat et
al., who monitored microalgae cultivation inside a photobioreactor using an IoT
system (Rahmat et al.,
2020). Long-term exposure to high
temperatures may have detrimental effects on cells, such as inhibiting cell
proliferation and increasing the number of necrotic cells (Zhu et al., 2015; Reissis
et al., 2013). An automated system is required
to be implemented in a bioreactor to maintain a suitable temperature for the
cells over time (Wang et al., 2020). As is well known, the incubation period for
cell culture may range from 24 hours to 7 days (Schmid et al.,
2018; Meinert et al., 2017). However, research on cell culture bioreactors
that can be observed remotely in real-time is still few and with limited
parameters.
The aseptic requirements of the cell
culture procedure necessitate a closed system; therefore, clean room access is
necessary for manual operations. In biopharmaceutical production facilities,
the high costs and demand for cleanroom space are obstacles to reserving
certain room sections exclusively for the bioreactor system. Research using
mini cleanrooms so far is one of the implementations used on
micro-electromechanical systems (MEMS). Vutla et.al reported the results of an
analysis based on airflow simulation in a MEMS clean room aimed at predicting
the distribution of 0.5 um airborne particles according to ISO-5 standards (Rao Vutla et al.,
2019). ISO-5 or Classification 100
Cleanrooms are a stricter classification of cleanrooms across a smaller
cross-section of industries and applications. ISO 5 Cleanrooms are used in
biotechnology, pharmaceutical, nanotechnology, and precision manufacturing
industries. The installment of cleanroom is costly due to its operation with
normally large area that contain 5-100 working people.
Regarding the constraints mentioned, our
approach here is minimizing the space by applying the automatic and remote
controlled system in the mini cleanroom to maintain the sterility of all
bioreactor components. In this study, experiments using thermal, pressure and
pH modules as case studies to be connected with the Internet of Things (IoT)
system and using the finite element method to determine airflow and temperature
in a clean room.
2.1. Pivot System Configuration
In this system component, cell culture chamber is the bioreactor's
heart: It contains the monolayer of cells or the scaffold upon which cells are
seeded. Polydimethylsiloxane (PDMS), a biocompatible silicone polymer, is used
to construct the entire chamber (Sylgard 184, Dow Corning). The bioreactor
system includes the following components: 1) bioreactor cell chamber; 2) mixing
chamber; 3) peristaltic pump; 4) air supply device with filter and 5)
electrical system.
2.2. Electrical
System Configuration
The
backbone of the structure mainly consisted of five components: a DC power
supply with emergency battery as optional, a heating system included the
driver, environment sensing included: temperature, pH, humidity sensors, and
microcontroller altogether with IoT platform as previously developed by our
group (Assyarify et al., 2022). This is accomplished using an Arduino microcontroller and
custom-designed software to maintain the required cell environment parameter
values. Multiple input ports on the Arduino microcontroller receive feedback
regarding all controlled parameters. Through a series of lines of code, the
software compares the feedback input to a predetermined value deemed optimal
for the growth of the cells. By sending a signal through the output port to the
water heater, pressure regulator, solenoid valve and others, any action
required to maintain system balance and correct any parameter changes can be
performed.
In
this study, Wi-Fi microcontrollers were employed: ESP-Arduino Mega; Arduino
microcontroller, which have been utilized in various studies. The temperature
sensor transmitted the measured chamber temperature to the microcontroller. In
this setup, a 60 W (12V, 5A) DC power supply was selected as the heater consumed
50 W power. This circuit forms a logic to control the temperature input with
occurred disturbance, resulting in required values. Thus, the heater conveyed
the heat to the water and transferred it to the bioreactor chamber.
2.3.
Internet-of-Things (IoT) Platform
The
thermal module, pH module, and pH module were remotely monitored and presented
in a control panel with a commercial user interface Blynk. Blynk provides data
communication between the bioreactor system and the internet mainframe.
Built-in equipment and IoT implementation enable real-time automatic parameter
control and monitoring of real-time processes. The sensor's output will be
transmitted to the Blynk server through a Wi-Fi connection and displayed on a
web browser platform and smartphone application. Users are able to specify
download parameters for retrieving data from the database.
2.4. Clean Room
Installation
A
clean room is specifically designed and controlled to minimize particulate
matter levels complied with the standard of the International Organization for
Standardization (ISO) 14644-1. The clean room was designed equipped with
ducting (HEPA H13 filter). The clean room will contain the PIVOT system to
ensure the air quality and sterility with positive pressure environment.
Important assumed data that needed in order realizing the cleanroom is good air
exchange standards for rooms; the amount of air flow intake corresponds to the
volume of the room; estimated cooling load from indoors and outside air
temperature and good airflow input and output location
2.5. Modelling and Simulation of PIVOT system
There
were two stages of model and simulation that were conducted in this study. The
first one is the study of mini cleanroom as the outer containment of the
system. The second one was the simulation of cell culture chamber due to the
fluid dynamic in the chamber. The 3D model of the mini cleanroom was drawn
using an Autodesk Inventor 2022 software and exported to Ansys Fluent Student
License 2022 R2 in STEP file format.
Furthermore, finite element modeling (FEM) model of cell culture chamber
was simulated to observe the shear stress reacted on the surface of the
bioreactor that directly affect to the living cell cultured on it.
2.6. Trial Test
Auto-PIVOT
trials were conducted to characterize temperature and monitor air pressure and
pH for 30 minutes (1,800 seconds). In order to characterize the temperature,
four temperature sensors are placed in each corner of the water heating
chamber. Using a Digital 4-Channel K-Type Thermocouple Thermometer,
measurements were taken. The measurement results from the bioreactor's built-in
thermistor will be compared and analyzed. The controlled temperature was set to
37°C following the reference temperature for liver cell culture. Injecting a mixture of air from the
compressor and CO2 gas into the mixing chamber allows for pH
regulation. Air from the compressor is used in place of oxygen to avoid
potential fire hazards. The pH control's actuator is a solenoid valve connected
to the CO2 gas hose. When the pH of the mixing chamber falls below
7, the CO2 gas hose is shut off by a solenoid valve. If the value is
greater than seven, the solenoid valve will open and CO2 gas will be
mixed with air from the compressor.
3.1. The
PIVOT system
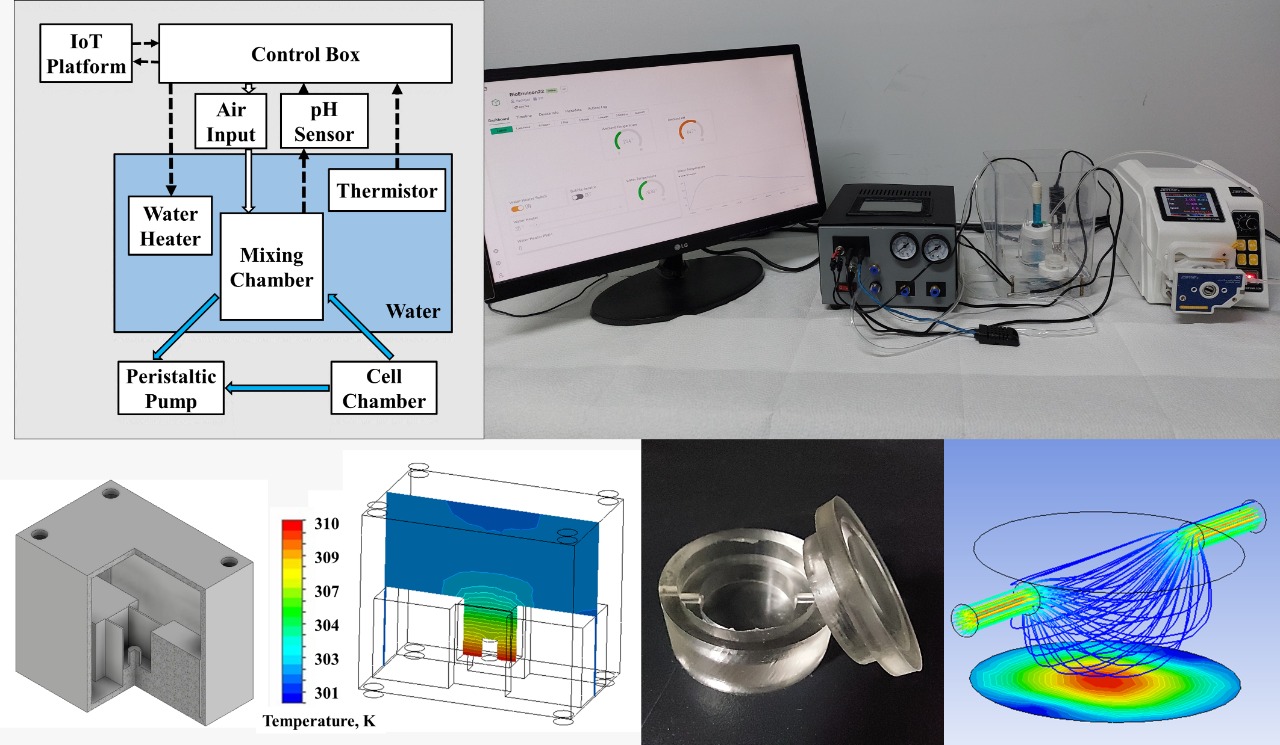
The PIVOT platform consists of these main components: 1) cell culture chamber; 2) reservoir/ mixing chamber; 3) heating medium; peristaltic pump; 4) electrical system and 5) mini cleanroom box with HEPA filter and air supply. The electrical system provides the power supply, controller and sensors temperature, pH, humidity and the IoT platform. All of this component was presented in schematic diagram as in Figure 1a and realized in Figure 1b and 1c.
Figure 1 a)
Schematic of Auto-Pivot Platform; b) bioreactor cell culture configuration and
c) complete setup of the platform
The cell culture chamber
made of polydimethylsiloxane elastomeric polymer (PDMS) serves as bioreactors
(inset Figure 1c). An extra chamber serves as reservoir that allows the oxygen
and temperature sensors tapped into the liquid. Another chamber can be
connected to this reservoir chamber to flow important nutrients as required.
This reservoir acted as a conditioning environment that finally flowed to the
cell cultured bioreactor.
Culture chambers were heated
using a water bath heating system that utilized the distilled water's heat
capacity to keep the culture chamber in desired temperature. The water bath was
heated by resistive heaters placed directly in the heating pod. An electronic unit
controls the resistive heaters through a power regulator.
The pressure is controlled
through the serial to PWM board that generates the analog 0-10V control signal
for the pressure regulator. Typically, the pressure regulators are designed to
be used in a closed system and the imposed pressure is guaranteed for a static
environment. The pressure in the mixing chamber is obtained with a low
resistance imposed by a sensor. An IoT system was successfully implemented by
transmitting the measured temperature data from the culture chamber sensor.
Since external disturbances to a bioreactor system could be detrimental to the
cultured tissue, the measurement data was sent to a web server every second to
allow for the early detection and correction of any errors. A real-time
sequential graph of the measured temperature is displayed at a dashboard to
provide an easily comprehensible data visualization (as featured in Supplementary
Material 1).
Simultaneously with the data
collected during the experiment, the web server received and stored the data in
its database. In addition, the data could be retrieved using the history panel,
as depicted in Supplementary Material 1. In this regard, a user could set a
specific time frame for the result to be displayed. Unfortunately, the data
could only be displayed within a single frame for a maximum of 15 minutes. As
presented in Supplementary Material 1, this web-based application offered a
history management function for downloading the desired timeframe in a
spreadsheet file format. Consequently, the user could view all measured data
for further analysis.
3.2. Clean Room Installation and Simulation
This phase realized an installment
parameter for the clean room system of the bioreactor environment. This
parameter shall formulate good and sterilized air exchange standards for the
workspace of the cell culture. The amount of airflow intake corresponds to the
volume of the room that needs to be conditioned. Estimation of cooling load
from indoor and outside air temperatures was estimated.
Table 1 Simulation Parameters of Mini Cleanroom
|
Physical Features |
Value |
Unit |
|
Heat
conduction in solid |
Off |
-- |
|
Time
dependent |
On |
-- |
|
Gravitational
effect |
Off |
-- |
|
Rotation
|
Off |
-- |
|
Flow
type |
Laminar
and turbulent |
-- |
|
Relative
humidity |
50.0 |
% |
|
Default
roughness |
0 |
Micrometer |
|
Default
wall condition |
Adiabatic
wall |
-- |
|
Static
pressure |
101325 |
Pa |
|
Temperature
|
300.0 |
K |
The
mini cleanroom roof has four fan filter units (FFUs) in each corner and
parallel to the four air vents on the floor. In this study, we used clean rooms
that refer to ISO 5 which can be implemented in biotechnology, pharmaceuticals,
nanotechnology, and various clean technology manufacturing applications. In a
typical ISO-5 cleanroom, airflow occurs at v=0.5 ms-1 from the top vent to the
bottom vent. Since particles with a diameter of 0.5 m have a negligible mass,
it is assumed that their flow is governed by the fluid flow, making it possible
to predict the path and presence of particles in the domain. A detail input
parameter for the simulation environment was summarized in table 1.
According to the simulation results was sufficient to ensure the clean air transmission within the minichamber. With a constant of 6 Air Changes per Hour (ACH), subjects shall get fresh air by adding a 25 x 25 cm 450 cfm inlet fan to the room. This could be realized using a 400 cfm exhaust fan. The simulation was realized successfully and found that the humidity of the room was relatively constant at 50-60% RH. Hypothetically, room temperature reaches the targeted temperature at an average of 33oC. The visualization results were presented at Figure 2.
Figure 2 Clean room air transmission simulation
visualization result a) velocity profile in perspective view and b) pressure
profile in the side view
The cleanroom was
successfully designed with floor dimensions of 40x60 cm, a height of 50 cm, and
an estimated volume of 38.5 cm2 of free space after deducting the
volume of the Pivot device. The four vents at each top and bottom corner (as
inlet and outlet holes) of the cleanroom have a diameter of 5 cm, and a HEPA
filter is installed at each vent to maintain cleanroom sterility.
The results of the
simulation utilizing the mini cleanroom model demonstrate laminar airflow from
the inlet to the outlet, as presented in Figure 2a and 2b. Maintaining the
temperature equilibrium on the water chamber's surface is dependent on laminar
airflow. Supplementary material 2 depics the velocity of airflow in the mini
cleanroom. Similarly, the simulation visualization shows that the temperature
at the inlet with 300K (26°C) to the water surface can be maintained at 310K.
(37°C). This is also due to the fact that the outlet is designed to be
perpendicular to the inlet at every corner, preventing turbulence that can
lower the water's room temperature and affect the bioreactor's flow
temperature.
3.3. Finite Element Analysis of Bioreactor
The simulation in the second phase is
conducted to observe the temperature profile, and fluid dynamic in the
bioreactor. Note that the bioreactor was seated on the heating source in this
system which affect the temperature profile in the mini cleanroom. The simulation
environment employed the parameter shown in Table 2.
Here, the actual shear stress on the wall depends linearly on the density and viscosity of the culture medium employed. The default element size used for meshing the model is 4.16 x 10-3 m, resulting in 11081 nodes and 53064 elements. The resulting meshing model is imported into the Fluent module and analyzed using a three-dimensional double-precision model.
Table 2 Finite element parameters of bioreactor chamber simulation
|
Boundary Condition |
Value |
Unit |
|
Viscosity |
103 |
Pa.s |
|
Fluid
density |
1,000 |
kg/m3 |
|
Flow
rate |
2-3 |
mL/min |
|
Pressure
|
1 (760)
|
Atm
(mmHg) |
|
Temperature
|
37 |
°C |
|
Slip
condition |
no-slip
boundary |
-- |
|
Reference
fluid |
water |
-- |
A
model of the bioreactor chamber's within the cleanroom is also represented in
Figure 3a. The realization of the bioreactor in the cleanroom was represented
in the figure 3c. Furthermore, the
placement of supporting equipment such peristaltic pump and electrical system.
These two supporting systems was also inserted in the simulation since its
connected directly to the cell culture chamber trough a plastic hose. The
dynamic of fluid shall contribute to the connection system of this fluid
connection. It is assumed that that the heat generated by peristaltic and the
electrical system is neglected at this moment. Therefore, figure 3b shows that
the heat source is limited to the heating system of the bioreactor.
The numerical study also highlights the shear stress that involved in the cell culture chamber. Our previous study indicated that cell viability corresponds with the shear generated on the bioreactor’s substrate. The standard transport equation is utilized to determine the flow rate ensuring shear stress in our bioreactor chamber, particularly in the surface area where the cell culture mainly occured (Kehtari et al., 2018).
Figure 3 a) CAD
model Isometric view of an auto-PIVOT system within the mini cleanroom; b). the
profile temperature in the chamber that used the heating system in the culture
chamber; and c) realization of the PIVOT within the mini cleanroom
The simulation results indicate that the shear stress experienced by hepatocytes in the cell chamber at a flow rate parameter of 2 mL/min is well above the minimum limit. As the simulation and visualization results in Figure 4, the resulting shear stress is only 4.6x10-7 Pa. In order to prevent cell death caused by stress and inadequate nutrient input due to a too-slow flow rate. According to previous research, the shear stress in normal hepatic sinusoids does not exceed 0.2 Pa. Using a shear stress of 0.05 Pa on iPSCs-derived hepatocytes (iPSCs-Heps) cultured in a bioreactor device, Kehtari et al. observed a higher level of hepatic markers than in static conditions. Flow cytometry and immunocytochemistry analysis revealed that iPSCs cultured in the device successively gained the features of definitive endodermal cells, hepatoblasts, and mature hepatocytes. During the experiment, albumin and urea secretion were significantly greater in the micro-bioreactor device than in the culture plates (Kehtari et al., 2018). In addition to the shear stress and flow velocity parameters, paying attention to the surface roughness and wettability parameters on the cell chamber's surface is an additional way to ensure high viability. The currently utilized cell chamber is molded from PDMS, which can be enhanced by the biomachining procedure (Whulanza et al., 2016).
Figure 4 a) Cell
culture chamber; b) CAD model of PDMS cell chamber and c) Velocity and wall
share on the chamber
3.4. Trial Test
Temperature measurements at
the four corners of the water-heating chamber using a 4-Channel K-Type thermocouple
and in the center of the water-heating chamber using a thermistor Pivot show a
similar trend. The rise times of thermistor, ch1, ch2, ch3, and ch4 are 855,
763, 825, 772, and 782 seconds, respectively. The average rise time required to
reach the target temperature is about 13 minutes. According to the graph and
rise time, it is known that ch1 has a higher temperature. It is due to its
proximity to the water heater.
The monitoring outcomes
showed no change in the pH value, which averaged approximately 6.78. It is
presumably because the air supply from the compressor contains little dissolved
O2, which is insufficient to alter the pH considerably. To
counteract this, the regulated addition of huge volumes of O2 is
required, hence the usage of O2 tanks cannot be avoided. Due to the
possible fire hazards posed by the use of O2, security protocols
must also be enhanced. The monitoring results are shown in Figure 5b
Furthermore, developing the pivot parameter also requires precise PID control of the heater. The highest temperature at the end of the measurement session reached 38.6°C at ch1, ch3, and ch4. Due to a temperature deviation of 1.6°C, some consideration may be required regarding the type of cell used. The measurement duration also needs to be increased to ensure the steady-state value of the temperature PID control, where bioreactors are usually used for days. As a result, some evaluation and development of the algorithms used for PID control are required.
Figure 5 a) measurement of temperature profile during the
testing of Auto-PIVOT and b) view window in the Blynk software during the
operation
The
3D cell culture which is the main feature of this bioreactor has become new
standard (Khafaga et al., 2022; Pichler et al.,
2022). Moreover, the substrate
and cell interaction are being optimized in current setting such as using the
perfusion of fludic movement for benefit of cell viability (Yu et al 2022; Nadhif et al., 2020). The observation of single cell has also put
important milestone in the trend of bioreactor (Czosseck et al., 2022; Clement et al 2022; Whulanza
et al., 2014). The progress of microfluidic device fabrication enables
this observation nowadays (Charmet et al., 2020; Suwandi
et al., 2014). This micro-scale environmental control method has
the potential to be applied to a broader range of cell and tissue types in the
future. Though since different cells and tissues have different environmental
parameter needs. This trend in micronization technology is not restricted to
the cellular level; instead, it is slowly moving towards the molecular level (Utomo et al., 2021; Whulanza
et al., 2016). Moreover, the
development of PIVOT is also one of the steps required to advance tissue
engineering into technology 4.0 and a method for preventing the spread of COVID-19
within the laboratory (Berawi et al., 2020a; 2020b).
The Internet of Things system has been successfully integrated
with PIVOT's thermal control, pH, and pressure monitoring to enable remote
monitoring. The server also stores measurement results data so that the data
can be reviewed for further analysis. The simulation results indicate that the
design parameters of shear stress less than 0.2 Pa and fluid velocity of 2
mL/min are compatible with and reliable for developing hepatocyte cells.
Adjusting the thermal control to achieve a steady state at a predetermined
temperature is necessary for further research. Designing and testing a pressure
control module for remote air and CO2 input using hepatocyte cells
is required.
Authors thanks to the Grant of RIIM
(Riset dan Inovasi untuk Indonesia Maju) 2022.
Assyarify, H., Nadhif, M.H., Gates, W.,
Whulanza, Y., 2022. IoT-Enabled Tissue-Engineering Bioreactors for Real-Time
Remote Monitoring: A Trial Using a Thermal Module. AIP Conference
Proceedings, Volume 2537(1), p. 050011
Berawi, M.A., Suwartha, N., Kusrini, E.,
Yuwono, A.H., Harwahyu, R., Setiawan, E.A., Yatmo, Y.A., Atmodiwirjo, P.,
Zagloel, Y.T., Suryanegara, M., Putra, N., Budiyanto, M.A., Whulanza, Y., 2020.
Tackling the COVID-19 Pandemic: Managing the Cause, Spread, and Impact. International
Journal of Technology, Volume 11(2), p. 209
Berawi, M.A., Suwartha, N., Surjandari,
I., Zagloel, T.Y.M., Asvial, M., Harwahyu, R., Suryanegara, M., Setiawan, E.A.,
Maknun, I.J., Kusrini, E., Kartohardjono, S., Sofyan, N., Yuwono, A.H., Harjanto,
S., Putra, N., Budiyanto, M.A., Whulanza, Y., 2020. Accelerating Sustainable
Energy Development through Industry 4.0 Technologies. International Journal
of Technology, Volume 11(8), p. 1463
Be?karde?, I.G., Ayd?n, G., Bekta?, ?.,
Cengiz, A., Gümü?derelio?lu, M., 2018. A Systematic Study for Optimal Cell
Seeding and Culture Conditions in a Perfusion Mode Bone-Tissue Bioreactor. Biochemical
Engineering Journal, Volume 132, pp. 100–111
Bilgen, B., Chu, D., Stefani, R., Aaron,
R.K., 2013. Design of a Biaxial Mechanical Loading Bioreactor for Tissue
Engineering. Journal of Visualized Experiments: JoVE, Volume 74, p.
50387
Birla, R., 2014. Introduction to
Tissue Engineering: Applications and Challenges. IEEE Press
Costa, J., Ghilardi, M., Mamone, V., Ferrari,
V., Busfield, J.J.C., Ahluwalia, A., Carpi, F., 2020. Bioreactor with
Electrically Deformable Curved Membranes for Mechanical Stimulation of Cell
Cultures. Frontiers in Bioengineering and Biotechnology, Volume 8, p. 22
Charmet, J., Rodrigues, R., Yildirim, E.,
Dallmann, R., Whulanza, Y., 2020. Low-Cost Microfabrication Toolbox. Micromachines, Volume 11(2), p. 135
Clément, V., Roy,
V., Paré, B., Goulet, C.R., Deschênes, L.T., Berthod, F., Bolduc, S. and
Gros-Louis, F., 2022. Tridimensional Cell Culture of Dermal
Fibroblasts Promotes Exosome-Mediated Secretion of Extracellular Matrix
Proteins. Scientific Reports, Volume 12(1), p. 19786
Czosseck, A., Chen,
M.M., Nguyen, H., Meeson, A., Hsu, C.C., Chen, C.C., George, T.A., Ruan, S.C.,
Cheng, Y.Y., Lin, P.J. and Hsieh, P.C., 2022. Porous Scaffold for Mesenchymal Cell Encapsulation and Exosome-Based
Therapy of Ischemic Diseases. Journal
of Controlled Release, Volume 352,
pp. 879–892
Hinman, S.S., Kim, R., Wang, Y.,
Phillips, K.S., Attayek, P.J., Allbritton, N.L., 2020. Microphysiological
System Design: Simplicity Is Elegance. Current Opinion in Biomedical
Engineering, Volume 13, pp. 94–102
Huang, J., Lu, L., Zhang, J., Hu, X.,
Zhang, Y., Liang, W., Wu, S., Luo, Z., 2012. Electrical Stimulation to
Conductive Scaffold Promotes Axonal Regeneration and Remyelination in a Rat
Model of Large Nerve Defect. PLOS ONE, Volume 7(6), p. e39526
Jain, A., Bansal, R., 2015. Applications
of Regenerative Medicine in Organ Transplantation. Journal of Pharmacy and
Bioallied Sciences, Volume 7(3), p. 188
Kehtari, M., Zeynali, B., Soleimani, M.,
Kabiri, M., Seyedjafari, E., 2018. Fabrication of a Co-Culture Micro-Bioreactor
Device for Efficient Hepatic Differentiation of Human Induced Pluripotent Stem
Cells (HiPSCs). Artificial Cells, Nanomedicine, and Biotechnology,
Volume 46(sup2), pp. 161–170
Khafaga, A.F.,
Mousa, S.A., Aleya, L., Abdel-Daim, M.M., 2022. Three-dimensional (3D) Cell
Culture: A Valuable Step In Advancing Treatments for Human Hepatocellular
Carcinoma. Cancer Cell International, Volume 22(1), p. 243
Khodabukus, A., Madden, L., Prabhu,
N.K., Koves, T.R., Jackman, C.P., Muoio, D.M., Bursac, N., 2019. Electrical
Stimulation Increases Hypertrophy and Metabolic Flux in Tissue-Engineered Human
Skeletal Muscle. Biomaterials, Volume 198, pp. 259–269
Li, R.A., Keung, W., Cashman, T.J.,
Backeris, P.C., Johnson, B.V., Bardot, E.S., Wong, A.O.T., Chan, P.K.W., Chan,
C.W.Y., Costa, K.D., 2018. Bioengineering an Electro-Mechanically Functional
Miniature Ventricular Heart Chamber from Human Pluripotent Stem Cells. Biomaterials,
Volume 163, pp. 116–127
Liu, C., Sun, Y., Li, N., Zhang, B.,
Zhen, F., 2019. Impact of Temperature Fluctuation on Anaerobic Fermentation
Process of Upgrading Bioreactor under Solar Radiant Heating. Applied Thermal
Engineering, Volume 156, pp. 382–391
Mason, C., Brindley, D.A.,
Culme-Seymour, E.J., Davie, N.L., 2011. Cell Therapy Industry: Billion Dollar
Global Business with Unlimited Potential. Regenerative Medicine, Volume
6(3), pp. 265–272
Meinert, C., Schrobback, K., Hutmacher,
D.W., Klein, T.J., 2017. A Novel Bioreactor System for Biaxial Mechanical
Loading Enhances the Properties of Tissue-Engineered Human Cartilage. Scientific
Reports, Volume 7(1), p. 16997
Nadhif, M.H., Assyarify, H., Waafi,
A.K., Whulanza, Y., 2020. Reflecting on Mechanical Functionalities in
Bioreactors for Tissue Engineering Purposes. International Journal of
Technology, Volume 11(5), p. 1066
Nadhif, M.H., Hadiputra, A.P., Utomo,
M.S., Whulanza, Y., 2019. Fabrication and Characterization of an Affordable
Conditioned Bio-Specimen Transporter (Conbiport) for Urban Areas. International
Journal of Technology, Volume 10(8), p. 1626
Nadhif, M.H., Whulanza, Y., Istiyanto,
J., Bachtiar, B.M., 2017. Delivery of Amphotericin B to Candida Albicans
by Using Biomachined Lab-on-a-Chip. Journal of Biomimetics, Biomaterials and
Biomedical Engineering, Volume 30, pp. 24–30
Paez?Mayorga, J., Hernández?Vargas, G.,
Ruiz?Esparza, G.U., Iqbal, H.M.N., Wang, X., Zhang, Y.S., Parra?Saldivar, R.,
Khademhosseini, A., 2019. Bioreactors for Cardiac Tissue Engineering. Advanced
Healthcare Materials, Volume 8(7), p. 1701504
Petrenko, Y.A., Petrenko, A.Y., Martin,
I., Wendt, D., 2017. Perfusion Bioreactor-Based Cryopreservation of 3D Human
Mesenchymal Stromal Cell Tissue Grafts. Cryobiology, Volume 76, pp.
150–153
Pichler, R., Rizzo,
L., Tröndle, K., Bühler, M., Brucker, H., Müller, A.L., Grand, K., Farè, S.,
Viau, A., Kaminski, M.M. and Kuehn, E.W., 2022. Tuning The 3D Microenvironment of Reprogrammed Tubule Cells
Enhances Biomimetic Modeling of Polycystic Kidney Disease. Biomaterials, Volume 291, p.
121910
Rahmat, A., Jaya, I., Hestirianoto, T.,
Jusadi, D., Kawaroe, M., 2020. Design a Photobioreactor for Microalgae
Cultivation with the IOTs (Internet of Things) System. Omni-Akuatika,
Volume 16(1), p. 53
Rao Vutla, S., Prakash Regalla, S.,
Ramaswamy, K., 2019. A Laminar Fluid Flow Model for Study of Ventilation
Systems in Micro-Electromechanical Systems (MEMS) Clean Room. Journal of
Physics: Conference Series, Volume 1276(1), p. 012057
Reissis, Y., García-Gareta, E., Korda,
M., Blunn, G.W., Hua, J., 2013. The Effect of Temperature on the Viability of
Human Mesenchymal Stem Cells. Stem Cell Research & Therapy, Volume
4(6), p. 139
Sagita, I.D., Whulanza, Y., Dhelika, R.,
Nurhadi, I., 2018. Designing Electrical Stimulated Bioreactors for Nerve Tissue
Engineering. AIP Conference Proceedings, Volume 1933(1), p. 040019
Schmid, J., Schwarz, S., Meier-Staude,
R., Sudhop, S., Clausen-Schaumann, H., Schieker, M., Huber, R., 2018. A
Perfusion Bioreactor System for Cell Seeding and Oxygen-Controlled Cultivation
of Three-Dimensional Cell Cultures. Tissue Engineering Part C: Methods,
Volume 24(10), pp. 585–595
Schuerlein, S., Schwarz, T., Krziminski,
S., Gätzner, S., Hoppensack, A., Schwedhelm, I., Schweinlin, M., Walles, H.,
Hansmann, J., 2017. A Versatile Modular Bioreactor Platform for Tissue
Engineering. Biotechnology Journal, Volume 12(2), p. 1600326
Suwandi, D., Whulanza, Y., Istiyanto, J.,
2014. Visible Light Maskless Photolithography for Biomachining Application. Applied Mechanics and Materials, Volume 493, pp.
552–557
Utomo, M.S., Nadhif, M., Bayani, G.F.E.,
Whulanza, Y., 2021. A Low Cost, off-the-Shelf Bioreactor as Enabling Technology
for Physiological Modeling. In: The 5th Biomedical Engineering's Recent
Progress in Biomaterials, Drugs Development, and Medical Devices: Proceedings
of the 5th International Symposium of Biomedical Engineering (ISBE) 2020.
Depok, Indonesia, p. 050017
Valls-Margarit, M., Iglesias-García, O.,
Di Guglielmo, C., Sarlabous, L., Tadevosyan, K., Paoli, R., Comelles, J.,
Blanco-Almazán, D., Jiménez-Delgado, S., Castillo-Fernández, O., Samitier, J.,
Jané, R., Martínez, E., Raya, Á., 2019. Engineered Macroscale Cardiac
Constructs Elicit Human Myocardial Tissue-like Functionality. Stem Cell
Reports, Volume 13(1), pp. 207–220
Villa-Diaz, L.G., Ross, A.M., Lahann,
J., Krebsbach, P.H., 2013. Concise Review: The Evolution of Human Pluripotent
Stem Cell Culture: From Feeder Cells to Synthetic Coatings. STEM CELLS,
Volume 31(1), pp. 1–7
Wang, B., Wang, Z., Chen, T., Zhao, X.,
2020. Development of Novel Bioreactor Control Systems Based on Smart Sensors
and Actuators. Frontiers in Bioengineering and Biotechnology, Volume 8,
p. 7
Whulanza, Y., Aditya, R., Arvialido, R.,
Utomo, M.S., Bachtiar, B.M., 2017. Ease Fabrication of PCR Modular Chip for
Portable DNA Detection Kit. In: Biomedical Engineering’s Recent Progress in
Biomaterials, Drugs Development, and Medical Devices: Proceedings of the First
International Symposium of Biomedical Engineering (ISBE 2016). Depok City,
Indonesia, p. 040006
Whulanza, Y., Arafat, Y.B., Rahman,
S.F., Utomo, M.S., Kassegne, S., 2022. On-Chip Testing of a Carbon-Based
Platform for Electro-Adsorption of Glutamate. Heliyon, Volume 8(5), p.
e09445
Whulanza, Y., Nadhif, H., Istiyanto, J.,
Supriadi, S., Bachtiar, B., 2016. PDMS Surface Modification Using Biomachining
Method for Biomedical Application, Journal of Biomimetics, Biomaterials and
Biomedical Engineering, Volume 26, pp. 66–72
Whulanza, Y., Widyaratih, D.S.,
Istiyanto, J., Kiswanto, G., 2014. Realization and Testing of Lab-On-Chip for
Human Lung Replication. ARPN Journal of Engineering and Applied Sciences,
Volume 9(11), pp. 2064–2067
Zhu, S., Wang, J., Xie,
B., Luo, Z., Lin, X., Liao, D.J., 2015. Culture at a Higher Temperature Mildly
Inhibits Cancer Cell Growth but Enhances Chemotherapeutic Effects by Inhibiting
Cell-Cell Collaboration. PLOS ONE, Volume 10(10), p. e0137042
