Microwave-assisted Impregnation of Zinc Metal Ions on Surface of Quenched Pulverized Shrimp Shell Waste
Corresponding email: aviantara@rocketmail.com
Published at : 24 Dec 2024
Volume : IJtech
Vol 15, No 6 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i6.6170
Aviantara, D.B., Suciati, F., Hadiko, G., Indrasti, N.S., Yani, M., 2024. Microwave-assisted Impregnation of Zinc Metal Ions on Surface of Quenched Pulverized Shrimp Shell Waste. International Journal of Technology. Volume 15(6), pp. 1946-1958
| Dwindrata Basuki Aviantara | 1. Indonesian National Research and Innovation Agency (BRIN), Geostech Puspiptek Serpong, Banten Province, 15314, Indonesia. 3. Department of Agroindustrial Technology, Faculty of Agricultural Enginee |
| Fuzi Suciati | Indonesian National Research and Innovation Agency (BRIN), Geostech Puspiptek Serpong, Banten Province, 15314, Indonesia |
| Gunawan Hadiko | Ministry of Industry, Machinery Industrial Center, Sangkuriang 12 Bandung, West Java Province, 40135, Indonesia |
| Nastiti Siswi Indrasti | Department of Agroindustrial Technology, Faculty of Agricultural Engineering and Technology, IPB University, Raya Dramaga Street, Bogor, West Java, 16680, Indonesia |
| Mohamad Yani | Department of Agroindustrial Technology, Faculty of Agricultural Engineering and Technology, IPB University, Raya Dramaga Street, Bogor, West Java, 16680, Indonesia |
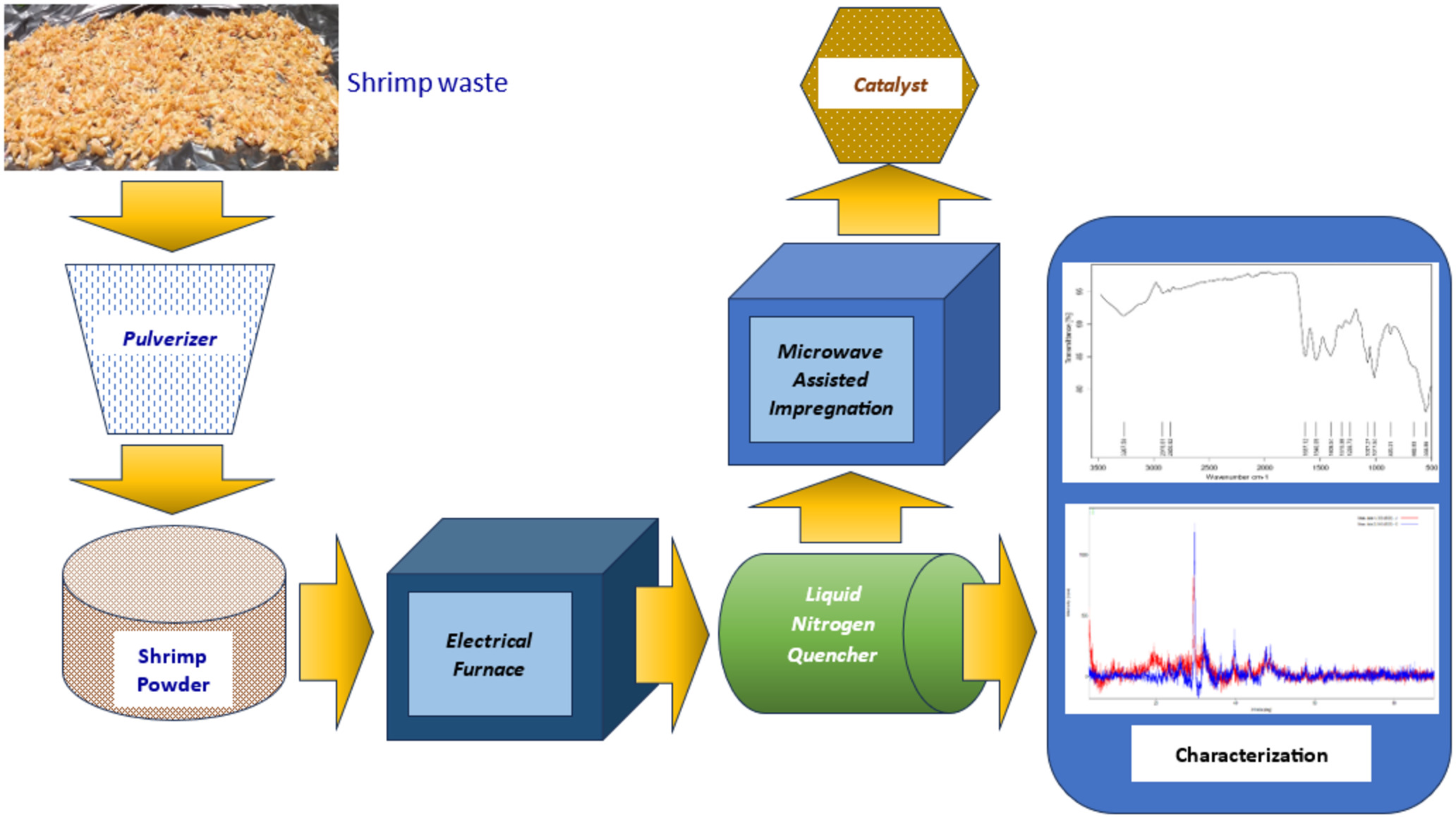
This study evaluated the effects of microwave irradiation on the impregnation efficiency of zinc onto pulverized shrimp shell waste. Prior to impregnation, the pulverized shrimp shell was heat treated at 350, 450, and 550 oC for 1, 2 and 3 hours. After each treatment, the treated pulverized shrimp shell was immediately quenched in liquid nitrogen. Microwave-assisted impregnations were carried out at 80 oC for 10, 20, and 30 minutes and at 50% and 100% of the maximum delivery power rate of the laboratory microwave equipment. The resulting impregnated solids were characterized via TGA, DTA, FTIR, SEM, and XRD. Semi-quantitative measurement of the impregnated zinc was determined based on XRF analysis. The BET analysis of the original pulverized shrimp shell waste provided a surface area of 1.273 m2/g and achieved 30.423 m2/g after 550 oC for 3h heating treatment. An increase in crystallinity index was detected as the pulverized shrimp shell waste was exposed to higher and prolonged heating temperatures. Statistical analysis showed no significant difference (p = 0.05) in impregnated zinc levels among the heat-treated pulverized shrimp shells for the same power output of microwave energy. However, a significant difference was obtained between 50% and 100% power output, of which the latter can impregnate two-fold higher levels of zinc than the former. The results of this study concluded that the microwave-assisted technique might potentially be applied for metal impregnation for the preparation of heterogeneous catalysts, and the power strength of the microwave plays a prominent role in metal impregnation.
Impregnation; Microwave-assisted; Shrimp shell waste; Quenching; Zinc metal
Crustacea are the most diverse groups of aquatic organisms that occupy a wide variety of habitats from the shoreline to the deep ocean and freshwater, where in some cases onto land for part of their life history (Penn, 2019). Crustacean species contribute to approximately 14.5 million tons (about 8%) of the total world supply of fish per annum, of which around half of this production is from the harvesting wild stocks, with major output from extensive and semi-intensive systems of tropical shrimps (Villareal and Juarez, 2022; Penn, 2019). As a result of their economic value, crustacean fisheries, particularly shrimps, are generally heavily exploited worldwide.
Both shrimp and shrimp products are widely consumed all over the world. Thus, it is not surprising that crustacean aquaculture is the largest seafood production sector around the globe. According to IMARC the global shrimp production in 2020 achieved 5.03 million tons (IMARC, 2020) and is expected to arrive at 7.28 million tons by 2025 when the shrimp market turnover value is expected to achieve 67.6 billion US dollars (Nirmal et al., 2020) if compound annual growth rate (CAGR) of 6.1% is assumed. More than 80% of global shrimp production is from Asia (Mao et al., 2017), where Asian countries contributed more than 2.5 million tons of the estimated production of marine shrimp (Suryawanshi and Eswari, 2021; Anderson, Valderrama, and Jory, 2019; FAO, 2017). Shrimp farming and processing plants produce the largest seafood industry worldwide due to their high demand and market value. From this, the shrimp processing industry produced 50 – 60% waste of the catch volume (Nirmal et al., 2020), equal to 6 – 8 million tons of shrimp shell waste per year (Zhao et al., 2021). The process of producing frozen shrimp creates remnants in the form of shrimp waste. Before being put into cold storage, the shrimp are peeled to remove the head, tail, and exoskeleton (Junianto et al., 2021). At present, crustacean-based food industries are creating environmental problems as the leftover crustacean shell wastes are ubiquitous and produce unpleasant odors as such posing environmental and health risks to living organisms (Padida et al., 2021). Traditionally the generated shrimp wastes are used for animal feed (Judhaswati and Damayanti, 2019; Has et al., 2018; Hilkias, Suprijatna, and Ondho, 2017) or are processed for other products such as fermented paste (Latifah and Ria, 2016), shrimp crisp (Asmiarsari et al., 2016) and Asian traditional food (Harmayani et al., 2019). Rather advanced utilization of shrimp shell wastes includes shrimp-based compost (Rusmini, Manullang, and Daryono, 2017) and carotenoid and chitin isolation (Junianto et al., 2021; Zhao et al., 2019). From the latter substance can be derived various products such as glucosamine (Hardoko et al., 2017; Mojarrad et al., 2007), chitosan (Narudin et al., 2022; Pakizeh, Moradi, and Ghassemi, 2021; Islam, Khan, and Alam, 2017), adsorbent (Salawu, Han, and Adeleye, 2022; Zein et al., 2022; Shahrin et al., 2021; Cahyaningrum and Amaria, 2005), bioplastics (Dasumiati, Saridewi, and Malik, 2019) and several products for biomedicals and pharmaceutical purposes (Satitsri and Muanprasat, 2020; Usman et al., 2018; Kusrini et al., 2014; Park and Kim, 2010) including advanced utilization for encapsulation of quantum dots (Lim et al., 2021). Furthermore, wide applications of shrimp shells as biomaterials for catalysis in biodiesel production have been reported (Kishore et al., 2021; Suryawanshi and Eswari, 2021). The usual method for catalyst preparation is incomplete carbonization, followed by metal loading (Yang, Zhang, and Zheng, 2009). On the other hand, the utilization of shrimp shells in the catalysis of environmental pollutants decontamination is still lacking. This paper presented the possibility of synthesizing heterogeneous catalysts from quenched shrimp shell waste for environmental protection purposes using the metal impregnation technique with the assistance of microwave irradiation to accelerate the process. The technique has been reported as well for other purposes such as surfactant production (Qadariyah et al., 2022), synthesis of fatty acids (Maulida et al., 2020), and also alkaline delignification process (Harahap et al., 2019).
2.1. Shrimp Waste Pulverization
The shrimp shell waste was obtained from a local
company, Wirantono Baru Ltd., located in Balaraja Regency, Province of Banten.
The current core business of the company is exporting frozen peeled shrimp from
Indonesia to Japan, European countries, and South American countries.
Initially, the obtained wet shrimp waste was soaked and washed with clean water
and then followed by air-dry. The air-dried shrimp shell waste was then
pulverized into particles of size 170 mesh (approximately particle diameter 90
?m) and stored in a polytetrafluoroethylene box.
2.2. Heating and Quenching
Prior to impregnation with zinc metal
ions, the pulverized shrimp shell waste was heated in an electrical furnace at
temperatures 350, 450, and 550oC for 1, 2, and 3 hours. After that,
the hot powder was immediately quenched in liquid nitrogen and stored in a
polytetrafluoroethylene box until used. The effect of heating treatments on the
physical structure of the pulverized shrimp shell waste at the aforementioned
temperature range was analyzed using XRD Diffractometer Rigaku Miniflex 600.
2.3. Preparation of Zinc Stock Solution
Stock solution for zinc to be
impregnated onto solid shrimp shell waste was prepared by dissolving 41.96 g of
Zn(CH3COO)2.2H2O of pure analytical grade
(purchased from Merck) in aquadest until volume 1,000 mL in a volumetric flask.
This will contain 12.5 g zinc per liter of stock solution. For the impregnation
step, the solid quenched shrimp shell waste (in g) to zinc solution (in mL)
ratio was 1 to 10; as such, the mixture will contain 12.5% of zinc metal ions
loading in bulk solution.
2.4. Impregnation
The process of impregnation was carried
out using a MARS Xpress microwave. The MARS Xpress microwave is capable of
delivering maximum power strength at 1800 W. For the purpose of the
experiments, the power delivered into the impregnation chamber was varied at
50% and 100% of the maximum power, where the impregnation temperature was held
at 80oC. The duration of the impregnation process also varied at 10,
20, and 30 minutes. Two and a half grams of quenched pulverized shrimp shell
waste was put into the MARS Xpress sample holder and then added with 25 mL of
zinc stock solution. Table 1 shows experimental parameters, namely heating
temperature, heating duration, and impregnation time, of which each parameter
is varied at three levels. Responses as levels of impregnated zinc for both 50%
and 100% of microwave power strength were evaluated through the Box-Behnken
design of the experiment.
2.5. Solid Characterization
3.1. Thermogravimetric Profile
Figure 2 Thermogravimetric profile of pulverized shrimp shell
waste.
3.2.
Fourier Transform Infrared Analysis
Figure 3 FTIR peaks of original pulverized shrimp shell waste
(a), quenched pulverized shrimp shell after 350 oC heat treatment
for 1 h (b), and after 550 oC heat treatment for 3 h (c)
3.3. SEM
Characterization
Prior to the heating process surface of the pulverized shrimp shell waste was analyzed using Scanning Electron Microscopy (SEM) Phenom as presented in Figure 4. As can be seen from the figure, a highly irregular morphology of the materials was recognized. Such an irregular morphologic with a size ranging from a few to hundred microns could be correlated with the chitin component as the pattern was similar to the chitin study (Nguyen et al., 2022). The prominent visual colors of pink, red, and yellow demonstrate that the pulverized shrimp shell materials are prominently composed of calcium, carbon, and oxygen elements. This is unsurprising as the major component of shrimp shells is calcium carbonate (Gbnedor et al., 2016).
Figure 4 SEM result of pulverized shrimp shell waste and its
mapping color
3.4. XRD
Characterization
Figure 5 (above) demonstrates XRD peak patterns for pulverized shrimp
shell waste prior to the heating process. As can be seen on the graph, peaks
for 2 appear at 29.6, 39.8, 43.5, 47.7, and 48.7. The diffractogram peaks
resemble calcium carbonate, which is in accordance with SEM findings as
described previously.
Comparing the obtained XRD peak patterns with a diffractogram of
standard calcite mineral (Render
et al., 2016) as well as another study (Gbenebor et al., 2017)
demonstrated that calcite-type calcium carbonate dominates the mineral
content of shrimp shell waste.
Figure 5 (below) is presented superimposed patterns of diffractogram
peaks of the quenched pulverized shrimp shell waste after the heating process
at 350 oC for 1 h (red line) and 550 oC for 3 h (brown
line). Based on Figures 5 and 6, the calculated crystallinity index is
presented in Table 2. As demonstrated by Table 2, increasing the temperature
and prolonging the heating treatment is accompanied by an increase in
crystallinity index as well as an increasing the surface area.
Figure 5 XRD
patterns of original (above) pulverized shrimp shell waste after heating
treatments (below) at 350 oC for 1 h (red line) and 550 oC
for 3 h (blue line).
Table 2 Physical
properties of shrimp shell before and after heating treatment.
|
Treatment |
Crystallinity Index |
Surface Area (m2/g) |
|
Without heating
treatment |
67 |
1.273 |
|
350 oC
for 1 h |
77 |
3.962 |
|
550 oC
for 3 h |
89 |
30.43 |
3.5. XRF
Semi-Quantitative Analysis
Presented in Table 3 are the results of the semi-quantitative elemental
determination of the original pulverized shrimp shell waste using X-ray
Fluorescein (XRF) analysis. As can be seen from the table, the original
pulverized shrimp shell waste contained 12.5% of calcium and a very low level
of zinc at 0.0059%. The big five detected elements in decreasing proportion are
calcium (Ca), phosphorus (P), sodium (Na), silicon (Si), and magnesium (Mg)
consecutively. Table 4 presents the results of microwave-assisted zinc
impregnation onto the pulverized shrimp shell waste's surface. As can be
seen in the table, several other metals commonly found in the shrimp shell are
also reported, in addition to measured zinc levels.
As shown in Table 4, levels of impregnated zinc on the surface of the
pulverized shrimp shell waste are in the range of 3.09 – 3.81% relative to the
solid powder mass if 100% of microwave power strength was applied. This
corresponded to 25 – 30% efficiency in impregnation. This low efficiency might
be attributed to the coarse particle size of the pulverized shrimp shell.
Another study is capable of obtaining a higher porous powder surface area using
a 65 mesh screener pore size instead of 170 mesh, as used in this study. The
impregnation power decreased at a 50% rate of power strength, provided levels
of impregnated zinc were in the range of 0.86 – 2.04%. On average, form the
results, altering the microwave power strength from 100% to 50% resulted in the
latter being capable of providing levels of impregnated zinc half only than the
former.
Results of the Box-Behnken evaluation of the controlled experimental
parameters to response impregnated zinc onto pulverized shrimp shell waste at a
rate of 100% and 50% of microwave energy power strength are presented in Table
5. It can be inferred that the combination of treatments (heating temperature,
heating time, impregnation time) has no significant effects on the response
(levels of impregnated zinc onto the surface of pulverized shrimp shell waste)
based on threshold p = 0.05 significant probability for each rate of microwave
power strength. However, applying paired test using Minitab software (Table 4)
for measured zinc levels for P = 100% and P = 50% resulted in a significant
difference at p = 0.05 probability. The output of the Minitab calculation for
the paired test is presented in Table 6. Thus, from the results, it is
confirmed that the power strength of microwave irradiation plays a prominent
role in controlling levels of impregnated zinc compared to the combination of
heating temperature, heating time, and impregnation time.
Table 3 Elements weight % of original pulverized shrimp shell
|
Element |
Weight
(%) |
Element |
Weight
(%) |
|
Mg |
0.4820 |
Ni |
0.0014 |
|
Al |
0.3330 |
Cu |
0.0031 |
|
Si |
0.5300 |
Br |
0.0680 |
|
P |
1.8500 |
Rb |
0.0014 |
|
S |
0.3680 |
Sr |
0.1470 |
|
Cl |
0.0787 |
Sn |
0.0042 |
|
K |
0.1470 |
I |
0.0022 |
|
Ti |
0.0185 |
Pb |
0.0006 |
|
Cr |
0.0047 |
Na |
0.7040 |
|
Mn |
0.0141 |
Ca |
12.500 |
|
Fe |
0.2230 |
Zn |
0.0059 |
Table 4 XRF semi-quantitative measurement of impregnated zinc
onto the surface of pulverized shrimp shell waste.
|
Treatment |
Mass proportion (%) |
||||||||||||
|
P = 100% |
P = 50% |
||||||||||||
|
TH (oC) |
DH (h) |
DI (min.) |
Zn |
Ca |
Mg |
Na |
P |
Zn |
Ca |
Mg |
Na |
P |
|
|
350 |
1 |
20 |
3.09 |
14.6 |
0.661 |
1.34 |
2.81 |
0.902 |
16.2 |
0.887 |
1.70 |
3.71 |
|
|
350 |
3 |
20 |
3.54 |
17.9 |
0.786 |
1.62 |
3.14 |
0.856 |
18.6 |
0.851 |
1.19 |
3.60 |
|
|
550 |
1 |
20 |
3.81 |
21.6 |
0.926 |
2.53 |
3.72 |
1.43 |
23.2 |
1.08 |
1.07 |
4.55 |
|
|
550 |
3 |
20 |
3.68 |
24.4 |
1.160 |
4.04 |
4.43 |
2.04 |
26.9 |
1.01 |
1.95 |
5.10 |
|
|
350 |
2 |
10 |
3.46 |
18.0 |
0.789 |
1.58 |
3.78 |
1.82 |
19.3 |
0.898 |
1.39 |
3.96 |
|
|
350 |
2 |
30 |
3.31 |
18.2 |
0.859 |
2.12 |
3.36 |
1.70 |
20.1 |
0.931 |
1.32 |
4.07 |
|
|
550 |
2 |
10 |
3.40 |
22.6 |
1.02 |
3.29 |
4.53 |
1.29 |
24.4 |
1.10 |
1.14 |
4.72 |
|
|
550 |
2 |
30 |
3.50 |
21.9 |
1.06 |
2.83 |
4.22 |
1.56 |
23.1 |
1.04 |
1.32 |
4.57 |
|
|
450 |
1 |
10 |
3.64 |
20.8 |
0.877 |
2.07 |
3.99 |
1.24 |
23.3 |
1.02 |
1.36 |
4.47 |
|
|
450 |
1 |
30 |
3.40 |
22.2 |
1.140 |
3.85 |
4.00 |
1.93 |
24.6 |
1.06 |
1.46 |
4.70 |
|
|
450 |
3 |
10 |
3.62 |
21.5 |
0.975 |
2.84 |
4.31 |
1.60 |
23.1 |
1.03 |
1.13 |
4.53 |
|
|
450 |
3 |
30 |
3.40 |
22.6 |
1.190 |
4.42 |
4.33 |
1.87 |
23.5 |
1.08 |
1.38 |
4.84 |
|
|
450 |
2 |
20 |
3.39 |
19.8 |
0.945 |
2.11 |
3.47 |
1.23 |
22.2 |
1.06 |
1.34 |
4.27 |
|
|
450 |
2 |
20 |
3.71 |
19.8 |
0.917 |
2.72 |
3.98 |
1.56 |
21.2 |
1.02 |
1.67 |
4.21 |
|
|
450 |
2 |
20 |
3.70 |
19.6 |
0.968 |
2.70 |
3.67 |
1.87 |
27.4 |
1.01 |
1.91 |
5.16 |
|
Table 5
Box-Behnken analysis for impregnation at P = 100% and P = 50%
|
Term |
p-value at P = 100% |
p-value at P = 50% |
|
Constant |
0.000 |
0.041 |
|
Heating temperature (oC) |
0.952 |
0.921 |
|
Heating time (hour) |
0.879 |
0.524 |
|
Impregnation time (minute) |
0.442 |
0.536 |
|
Heating temperature (oC) *Heating temperature (oC) |
0.421 |
0.531 |
|
Heating time (hour)*Heating time (hour) |
0.891 |
0.717 |
|
Impregnation time (minute) *Impregnation time (minute) |
0.348 |
0.439 |
|
Heating temperature (oC) *Heating time (hour) |
0.175 |
0.497 |
|
Heating temperature (oC) *Impregnation time (minute) |
0.526 |
0.682 |
|
Heating time (hour) *Impregnation time (minute) |
0.959 |
0.659 |
Table 6 Minitab output for a
paired test of P = 100% and P = 50%
|
Descriptive Statistics
Estimation for Paired Difference
µ_difference: population mean of (%Zn (80 oC 100%P) - %Zn
(80 oC 50%P)) Test
|
|||||||||||||||||||||||||||||||||||||||||||
Results of this study showed that no quenching
nor impregnation contact time affects the levels of impregnated zinc. On the
other hand, heating to a temperature up to 550 oC followed by
subsequent quenching resulted in an increase in the degree of crystallinity as
well as surface area. Furthermore, implementing microwave irradiation at full
power strength 100% is capable of impregnating the zinc metal shell two-fold
higher onto the pulverized shrimp's surface than at half power strength 50%.
The microwave-assisted method may potentially be applied for zinc impregnation
in preparing heterogeneous catalysts from shrimp shell waste. The power
strength of microwave irradiation is the key parameter to be controlled to
achieve a better impregnation process.
The authors would like to express our deep, sincerely appreciate to Wirantono Baru Ltd. for allowing us to conduct shrimp waste sampling in the company for the purpose of this study.
Anderson, J.L., Valderrama, D., Jory, D.E., 2019. GOAL 2019: Global Shrimp Production Review. Global Seafood Alliance Available online at: https://www.globalseafood.org/advocate/goal-2019-global-shrimp-production-review/, Accessed on November 4, 2022
Asmiarsari, M.N., Ustadi, Budhiyanti, S.A., Sahubawa, L., 2016. Consumer Acceptance Level of Crispy Shrimp with the Margarine, Corn, Cheese, and Chili Flavour. Journal of Biology, Agriculture and Healthcare, Volume 6(16), pp. 54–64. https://core.ac.uk/download/pdf/234662111.pdf
Biondo, C.E.G., Biondo, P.B.F., Visentainer, J.V., Muñiz, G.I.B., Neves, A.C., Ballester, E.L.C., Wiest, A.M., Alves, H.J., 2015. Charred Shrimp Shells Treated with Potassium Fluoride used as a Catalyst for the Transesterification of Soybean Oil with Methanol. Journal of Renewable and Sustainable Energy, Volume 7, p. 053128. https://doi.org/10.1021/ef900273y
Cahyaningrum, S.E., Amaria, 2005. Utilization of Penaus Monodon Shrimp Shell Waste as Adsorbent of Cadmium (II) in Water Medium. Indonesian Journal of Chemistry, Volume 5(2), pp. 130–134. https://doi.org/10.22146/ijc.21819
Dasumiati, Saridewi, N., Malik, M., 2019. Food Packaging Development of Bioplastic from Basic Waste of Cassava Peel (Manihot uttilisima) and Shrimp Shell. In: IOP Conference Series: Materials Science and Engineering, Volume 602, p. 012053.
DOI 10.1088/1757-899X/602/1/012053
De Rosa, C., de Ballesteros, O.R., Santoro, M., Auriemma, F., 2003. Influence of the Quenching Temperature on The Crystallization of the Transplanar Mesomorphic Form of Syndiotactic Polypropylene. Polymer, Volume 44(20), pp. 6267–6272. http://dx.doi.org/10.1016/S0032-3861(03)00569-X
Food and Agriculture Organization (FAO), 2017. Increased Production of Farmed Shrimp Leads to Improved International Trade. GLOBEFISH - Analysis and Information on World Fish Trade. Available Online at: http://www.fao.org/in-action/globefish/marketreports/resource-detail/en/c/989543/, Accessed on July 10, 2022
Gbenebor, O.P., Adeosun, S.O., Lawal, G.I., Jun, S., Olaleye S.A., 2017. Acetylation, Crystalline and Morphological Properties of Structural Polysaccharide from Shrimp Exoskeleton, Engineering Science and Technology an International Journal, Volume 20, pp. 1155–1165. https://doi.org/10.1016/j.jestch.2017.05.002
Gbenedor, P.O., Adeosun, S.O., Lawal, G.I., Jun, S., 2016. Role of CaCO3 in the Physicochemical Properties of Crustacean-sourced Structural Polysaccharides. Materials Chemistry and Physics, Volume 184, pp. 203–209.
https://doi.org/10.1016/j.matchemphys.2016.09.043
Harahap, A.F.P., Rahman, A.A., Sadrina, I.N., Gozan, M., 2019. Optimization of Pretreatment Conditions for Microwave-assisted Alkaline Delignification of Empty Fruit Bunch by Response Surface Methodology. International Journal of Technology. Volume 10(8), pp. 1479–1487. https://doi.org/10.14716/ijtech.v10i8.3431
Hardoko, Sasmito, B.B., Puspitasari, Y.E., Afandi, H.M., Maulia, N., 2017. Study of Glucosamine Production from Shrimp Shells by Fermentation Using Trichoderma harzianum. The Journal of Experimental Life Science, Volume 7(2), pp. 115–121. DOI:10.21776/UB.JELS.2017.007.02.10
Harmayani, E., Anal, A. K., Wichienchot, S., Bhat, R., Gardjito, M., Santoso, U., Siripongvutikorn, S., Puripaatanavong, J., Payyappallimana, U., 2019. Healthy Food Traditions of Asia: Exploratory Case Studies from Indonesia, Thailand, Malaysia, and Nepal. Journal of Ethnic Foods, Volume 6(1), pp. 1–18. https://doi.org/10.1186/s42779-019-0002-x
Has, H., Napirah, A., Kurniawan, K., Sandiah, H., 2018. Penggunaan Tepung Limbah Udang sebagai Bahan Pakan Sumber Protein Terhadap Performa Produksi Puyuh Fase Layer (Coturnix-Coturnix japonica) (The Use of Shrimp Waste Flour as a Protein Source Feed Material against Layer Phase Quail (Coturnix-Coturnix japonica) Production Performance). Jurnal Ilmu dan Teknologi Peternakan Tropis, Volume 5(3), pp. 31–36. http://dx.doi.org/10.33772/jitro.v5i3.4733
Hilkias, W., Suprijatna, E., Ondho, Y.S., 2017. Pengaruh Penggunaan Tepung Limbah Udang Fermentasi Terhadap Karakteristik Organ Reproduksi Pada Puyuh Petelur (Coturnix coturnix japonica) (Effects of Fermented Shrimp Waste Flour Use on the Reproductive Organs Characteristics of Laying Quail (Coturnix coturnix japonica)). Jurnal Ilmu-Ilmu Peternakan, Volume 27(2), pp. 8–18. DOI:10.21776/UB.JIIP.2017.027.02.02
IMARC, 2020. Shrimp Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2020-2025, IMARC group, USA
Islam, S., Khan, M., Alam, A.N., 2017, Production of Chitin and Chitosan from Shrimp Shell Wastes. Journal of the Bangladesh Agricultural University, Volume 14(2), pp. 253-259. DOI:10.3329/jbau.v14i2.32701
Judhaswati, R.D., Damayanti, H.O., 2019. Potensi Ekonomi Pengolahan Limbah Udang di Kabupaten Pati (Economic Potential of Shrimp Waste Treatment in Pati Regency). Jurnal Litbang, Volume 15(1), pp. 1–12. DOI: https://doi.org/10.33658/jl.v15i1.126
Junianto, Taofani, I.S., Sukmadhani, M.R., Fitriana, I.U., 2021. Chitin Extraction from Shrimp Shells. Global Scientific Journals, Volume 9(5), pp. 2009–2018. https://globalscientificjournal.com/researchpaper/Article_Review_CHITIN_EXTRACTION_FROM_SHRIMP_SHELLS.pdf
Kishore, S.C., Perumal, S., Atchudan, R., Sundramoorthy, A.K., Alagan, M., Sangaraju, S., Lee, R.Y., 2022. A Review of Biomass-Derived Heterogeneous Catalysts for Biodiesel Production. Catalyst, Volume 12(1501), pp. 1–12. DOI: 10.3390/catal12121501
Kusrini, E., Arbianti, R., Sofyan, N., Abdullah, M.A.A., Andriani, F., 2014. Modification of Chitosan by Using Samarium for Potential Use in Drug Delivery System. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, Volume 120, pp. 77–83. http://dx.doi.org/10.1016/j.saa.2013.09.132
Latifah, U.S., Ria, D.S., 2016. Pemanfaatan Limbah Kepala Udang Windu (Penaeus monodon) untuk Pembuatan Terasi dengan Kajian Penambahan Garam dan Lama Fermentasi (Utilization of Windu Shrimp Head Waste (Penaeus monodon) for Making Shrimp Paste with a Study of Salt Addition and Fermentation Duration). Jurnal Rekapangan, Volume 10(1), pp. 67–72. http://ejournal.upnjatim.ac.id/index.php/teknologi-pangan/article/view/700/579
Li, D., 2008. Mesomorphic State. In: Li, D., (ed.), Encyclopedia of Microfluidics and Nanofluidics. Springer, Boston, MA.
Lim, M.J., Shahri, N.N.M., Taha, H., Mahadi, A.H., Kusrini, E., Lim, J.W., Usman, A., 2021. Biocompatible Chitin-Encapsulated CdS Quantum Dots: Fabrication and Antibacterial Screening. Carbohydrate Polymers, Volume 260, p. 117806. https://doi.org/10.1016/j.carbpol.2021.117806
Mao, X., Guo, N., Sun, J., Xue, C., 2017. Comprehensive Utilization of Shrimp Waste Based on Biotechnological Methods: A Review. Journal of Cleaner Production, Volume 143, pp. 814–823. https://doi.org/10.1016/j.jclepro.2016.12.042
Mark, J., Ngai, K., Graessley, W., Mandelkern, L., Samulski, E., Koenig, J., Wignall, G., 2004. The Mesomorphic State. In: Physical Properties of Polymers. Cambridge: Cambridge University Press, pp. 316–380. https://doi.org/10.1017/CBO9781139165167
Maulida, A., Zahrati, Z., Kamila, H., Mukhriza, T., Gani, A., Supardan, M.D., 2020. Intensification of Synthesis of Fatty Acid Isopropyl Ester using Microwave. International Journal of Technology. Volume 11(3), pp. 492–500. https://doi.org/10.14716/ijtech.v11i3.3513
Mojarrad, J.S., Nemati, M., Valizadeh, H., Ansarin, M., Bourbour, S., 2007. Preparation of Glucosamine from Exoskeleton of Shrimp and Predicting Production Yield by Response Surface Methodology. Journal of Agricultural and Food Chemistry, Volume 55(6), pp. 2246–2250. DOI: 10.1021/jf062983a
Narudin, N.A.H., Rosman, N.A., Shahrin E.W.E., Sofyan, N., Mahadi, A.H., Kusrini, E., Hobley, J., Usman, A., 2022. Extraction, Characterization, and Kinetics of N-deacetylation of Chitin Obtained from Mud Crab Shells. Polymers and Polymer Composites, Volume 30, pp. 1–11. https://doi.org/10.1177/09673911221109611
Nguyen, H.D.C., de Vries, R., Stoyanov, S.D., 2022. Chitin Nanowhiskers with Improved Properties Obtained using Natural Deep Eutectic Solvent and Mild Mechanical Processing. Green Chemistry, Volume 24, pp. 3834–3844.
https://doi.org/10.1039/D2GC00305H
Nirmal, N.P., Santivarangkna, C., Rajput, M.S., Benjakul, S., 2020. Trends in Shrimp Processing Waste Utilization: An industrial prospective. Trends in Food Science & Technology, Volume 103, pp. 20–35. https://doi.org/10.1016/j.tifs.2020.07.001
Padida, Q.P., Maningasb, R.V., dela Cruz, C.P.P., Lapie, L.P., Alforja, N.S., 2021. Crustacean Waste Shells as Adsorption Materials for Heavy Metals in an Aqueous Solution. Environmental Contaminants Reviews, Volume 4(2), pp. 33–35. DOI:10.26480/ecr.02.2021.33.25
Pakizeh, M., Moradi, A., Ghassemi, T., 2021, Chemical Extraction and Modification of Chitin and Chitosan from Shrimp Shells. European Polymer Journal, Volume 159, p. 110709. https://doi.org/10.1016/j.eurpolymj.2021.110709
Park, B.K., Kim, M.M., 2010. Applications of Chitin and its Derivatives in Biological Medicine. International Journal of Molecular Sciences, Volume 11, pp. 5152–5164. DOI: 10.3390/ijms11125152
Penn, J.W., Caputi, N., de Lestang, S., Johnston, D., Kangas, M., Bopp, J., 2019. Crustacean Fisheries. In: Encyclopedia of Ocean Sciences. 3rd Edition, Volume 2, pp. 324–337. DOI:10.1016/B978-0-12-409548-9.09577-4
Polaskova, M., Peer, P., Cermak, R., Ponizil, P., 2019. Effect of Thermal Treatment on Crystallinity of Poly(ethylene oxide) Electrospun Fibers. Polymers, Volume 11(9), pp. 1384–1392. https://doi.org/10.3390/polym11091384
Qadariyah, L., Sahila, S., Sirait, C., Purba, C.P.E., Bhuana, D.S., Mahfud, M., 2022. Surfactant Production of Methyl Ester Sulfonate from Virgin Coconut Oil using Aluminum Oxide with Microwave Assistance. International Journal of Technology. Volume 13(2), pp. 378–388. https://doi.org/10.14716/ijtech.v13i2.4449
Render, D., Samuel, T., King, H., Vig, M., Jeelani, S., Babu, R.J., Rangari, V., 2016. Biomaterial-derived Calcium Carbonate Nanoparticles for Enteric Drug Delivery. Journal of Nanomaterials, Volume 2016, pp. 1–8 . DOI: 10.1155/2016/3170248
Rusmini, Manullang R.R., Daryono, 2017. Development of Shrimp Shells-based Compost and Plant-based PesticideUusing Bio-activators from Golden Apple Snails and Their Effects on the Kenaf Plant Growth and Pest Population. Nusantara Bioscience, Volume 9(3), pp. 260–267. https://doi.org/10.13057/nusbiosci/n090304
Salawu, O.A., Han, Z., Adeleye, A.S., 2022. Shrimp Waste-derived Porous Carbon Adsorbent: Performance, Mechanism, and Application of Machine Learning. Journal of Hazardous Materials, Volume 437, pp. 1–16. https://doi.org/10.1016/j.jhazmat.2022.129266
Satitsri, S., Muanprasat, C., 2020. Chitin and Chitosan Derivatives as Biomaterial Resources for Biological and Biomedical Applications, Molecules, Volume 25, pp. 5961–5985.
DOI: 10.3390/molecules25245961
Shahrin, E.W.E.S., Narudin, N.A.H., Shahri, N.N.M., Nur, M., Lim, J.-W., Bilad, M.R., Mahadi, A.H. Hobley, J., Usman, A., 2023. A Comparative Study of Adsorption Behavior of Rifampicin, Streptomycin, and Ibuprofen Contaminants from Aqueous Solutions onto Chitosan: Dynamic Interactions, Kinetics, Diffusions, and Mechanisms. Emerging Contaminants, Volume 9 (1), p. 100199. https://doi.org/10.1016/j.emcon.2022.100199
Shi, F., Ma, J., Wang, P., Ma, Y. 2012. Effect of Quenching Temperatures on the Morphological and Crystalline Properties of PVDF and PVDF–TiO2 Hybrid Membranes. Journal of the Taiwan Institute of Chemical Engineers, Volume 43(6), pp. 980–988. https://doi.org/10.1016/j.jtice.2012.06.003
Suryawanshi, N., Eswari, J.S., 2021. Shrimp Shell Waste as a Potential Raw Material for Biorefinery—A revisit. Biomass Conversion and Biorefinery, Volume 12(5), pp. 1977–1984. https://doi.org/10.1007/s13399-020-01271-2
Usman, A., Kusrini, E., Widiantoro, A.B., Hardiya, E., Abdullah, N.A., Yulizar, Y., 2018. Fabrication of Chitosan Nanoparticles Containing Samarium Ion Potentially Applicable for Fluorescence Detection and Energy Transfer. International Journal of Technology, Volume 9(6), pp. 1112–1120. https://doi.org/10.14716/ijtech.v9i6.2576
Villareal, H., Juarez, L., 2022. Super-intensive Shrimp Culture: Analysis and future challenges. Journal of World Aquaculture Society, Volume 53, pp. 928–932. https://doi.org/10.1111/jwas.12929
Vlaev, L.T., Georgieva, V.G., Tavlieva, M.P., 2015, On the Kinetic Mechanism of Non-isothermal Degradation of Solids, In: Tiwari, A., Raj, B., (ed.), Scrivener Publishing LLC, Canada, pp. 547–578. DOI:10.1002/9781119117711.ch22
Yang, L., Zhang, A., Zheng, X., 2009. Shrimp Shell Catalyst for Biodiesel Production. Energy & Fuels, Volume 23(8), pp. 3859–3865. https://doi.org/10.1021/ef900273y
Yun, S.H., Dibos, A., Wu, J.Z., Kim, D.K., 2004. Effect of Quench on Crystallinity and Alignment of Boron Nanowires. Applied Physics Letters, Volume 84(15), pp. 2892–2894. https://doi.org/10.1063/1.1705720
Yusuf, M.T.O., Masahid, A.D., Ratnawati, L., Indrianti, N., Ekafitri, R., Sholichah, E., Afifah, N., Sarifudin, A., Hikal, D.M., Sami, R., Khojah, E, Aljahani, Al-Moalem, M.H., Fikry, M., 2022. Impact of Heating Temperature on the Crystallization, Structural, Pasting, and Hydration Properties of Pre-gelatinized Adlay Flour and Its Implementation in Instant Porridge Product. Crystals, Volume 12(5), pp. 689–698. DOI:10.3390/cryst12050689
Zaid, M.H.M., Matori, K.A., Aziz, S.H.A., Kamari, H.M., Fen, Y.W., Yaakob, Y., Sa'at, N.K., Gürol, A., ?akar, E., 2019. Effect of Heat Treatment Temperature to the Crystal Growth and Optical Performance of Mn3O4 Doped ?-Zn2SiO4 Based Glass-ceramics. Results in Physics, Volume 15, p. 102569. https://doi.org/10.1016/j.rinp.2019.102569
Zein, R., Chaidir, Z., Zilfa, Z., Fauzia, S., Ramadhani, P., 2022, Isotherm and Kinetic Studies on the Adsorption Behavior of Metanil Yellow Dyes onto Modified Shrimp Shell-Polyethylenimine (SS-PEI). Jurnal Kimia Valensi, Volume 8(1), pp. 10–22. DOI:10.15408/jkv.v8i1.22566
Zhao, D., Huang, W.C., Guo, N., Zhang, S., Xue, C., Mao, X., 2019. Two-Step Separation of Chitin from Shrimp Shells using Citric Acid and Deep Eutectic Solvents with the Assistance of Microwave. Polymers, Volume 11(3), pp. 409–4019. https://doi.org/10.3390/polym11030409
Zhao, W., Gu, J., Wang, X., Hu, T., Wang, J., Yu, J., Dai, X., Lei, L., 2021. Effects of Shrimp Shell Powder on Antibiotic Resistance Genes and the Bacterial Community During Swine Manure Composting. Science of The Total Environment, Volume 752, p. 142162. https://doi.org/10.1016/j.scitotenv.2020.142162
