Optimization Concentration of Irgacure® 2959 as Photo-initiator on Chitosan-Kappa-Carrageenan Based Hydrogel for Tissue Sealant
Corresponding email: nugrahenips@ugm.ac.id
Published at : 30 Dec 2022
Volume : IJtech
Vol 13, No 8 (2022)
DOI : https://doi.org/10.14716/ijtech.v13i8.6166
Kamala, F.Z., Budhijanto, W., Purnomo, E., Nugraheni, P.S., 2022. Optimization Concentration of Irgacure® 2959 as Photo-initiator on Chitosan-Kappa-Carrageenan Based Hydrogel for Tissue Sealant. International Journal of Technology. Volume 13(8), pp. 1715-1725
| Fiskina Zulfa Kamala | Department of Fisheries, Faculty of Agriculture, Universitas Gadjah Mada, Sleman, Yogyakarta, 55281, Indonesia |
| Wiratni Budhijanto | Department of Chemical Engineering, Faculty of Engineering, Universitas Gadjah Mada, Sleman, Yogyakarta, 55281, Indonesia |
| Eko Purnomo | Division of Pediatric Surgery, Department of Surgery, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Yogyakarta, 55281, Indonesia |
| Prihati Sih Nugraheni | Department of Fisheries, Faculty of Agriculture, Universitas Gadjah Mada, Sleman, Yogyakarta, 55281, Indonesia |
Hydrogel
is a three-dimensional network of hydrophilic polymers widely used in the
biomedical field, one of which is a tissue sealant. The material that can be
used in the manufacture of hydrogels is chitosan. The ability of chitosan in
the formation of hydrogel is still limited by its swelling and adhesive
properties, so it requires other materials such as crosslinkers, one of which
is kappa-carrageenan which can stabilize and increase the viscosity of the
hydrogel. The addition of Irgacure® 2959 as a photo-initiator to the
hydrogel can produce free radicals that can bind to the active group of the
polymer. This study aims to determine the effect of adding Irgacure®
2959 in the manufacture of hydrogel based on a chitosan-kappa-carrageenan
solution formulated as a colon tissue adhesive. The treatments given were the
addition of Irgacure® 2959 as much as 0%, 1%, 2%, 3%, and 4%. The
characteristics of the hydrogel tested qualitatively (simulation adhesion
test), degree of swelling, resistance to water, degree of crystallinity, and
ability to inhibit bacterial growth. The addition of Irgacure® 2959
did not show significantly different results from the control that indicating
it cannot increase the crosslinking between chitosan and kappa-carrageenan.
This is predicted due to the presence of steric hindrance from
kappa-carrageenan, causing very limited crosslinking due to the molecular size
difference between chitosan and kappa-carrageenan. Further, it is necessary to
optimize the ratio of the chitosan and kappa-carrageenan to get the balance
ratio that supports the occurrence of cross-linking.
Carrageenan; Chitosan; Hydrogel; Irgacure® 2959; Photo-initiator
Hydrogels
are three-dimensional networks of hydrophilic polymers with 90-99% moisture
content that facilitate efficient mass and oxygen transfer and endogenous
regeneration, drug delivery, and wound healing (Yegappan
et al., 2018). One of the materials that can be used as raw material for
making hydrogels is chitosan which has flexible, non-toxic, hydrophilic,
anti-bacterial properties and provides wound healing effects, so it is widely
used in biomedical and tissue engineering (Zhang et
al., 2018). The properties of chitosan make it potential to be used as a
raw material for making tissue sealant.
Tissue
sealant is a substance with the ability to bind two tissue substrates together.
One of these tissue sealants can be applied to glue the tissue in the wound
that is produced after the operation process on the colon tissue. Materials
that are usually used as tissue sealants are macromolecules (Nayeb et al., 2011) which have biocompatible and
biodegradable properties (Bao et al, 2020), one of which is chitosan (Asadpour et al., 2020). Chitosan was chosen as an
alternative to reduce the shortcomings that arise from tissue sealants with
previously used materials, namely cyanoacrylate, fibrin, and gelatin.
Chitosan-based hydrogels are still limited by their swelling and adhesive
properties and are weak in charge interactions without forming bonds between
related chains (Phuong et al, 2019), thus
requiring the addition of other materials as crosslinkers. Crosslinker material
is added to form crosslinks between the polymers used which can affect the
shape of the cavity in the hydrogel and affect the hydrogel's ability. The use
of this crosslinker can help increase swelling ability and more stable bond
strength (Ding et al., 2020). One of the
materials that can be used as a crosslinker in chitosan hydrogel is carrageenan
with good mechanical properties, biocompatible and non-toxic (Derkach et al., 2018) and can stabilize and form
viscosity that it can be used as a gelling agent by forming a helical network
with double molecule resulting from the sulfate group (Wang
et al, 2018). There are various carrageenan types, including iota-,
kappa-, and lambda-carrageenan (Darmayanti et al.,
2016). In this study, we used kappa carrageenan as the basis for the
hydrogel.
The use of chitosan and carrageenan in
the manufacture of hydrogels is based on the properties of each polymer needed
in medical applications. Chitosan-carrageenan film hydrogel has been made to be
applied to fibroblast cells (Yu et al., 2018),
showing that chitosan-carrageenan can heal cells because it is non-toxic, which
causes cells to grow well. Chitosan-carrageenan-based hydrogel has the
potential to produce a more flexible hydrogel so that it can be an alternative
to tissue sealant that has previously been used but have brittle and less
flexible properties. According to intestinal surgeons, 80% of cases of
postoperative failure of the colon occur due to the use of adhesive tissue that
is brittle and inflexible so that it is not compatible with peristalsis in the
intestines so that chitosan-carrageenan hydrogel can be used as a tissue
sealant, especially to be applied to the colon. Preparation hydrogels as tissue
sealant by using crosslinking will produce better mechanical strength and
hydrophilicity. The hydrogel produced by the crosslinking method produces a
cross-linked structure that is not easily soluble in water, and the gel form
can be maintained (Yu et al., 2018). The
crosslinking method used in the manufacture of hydrogels, namely the
photo-crosslinking method, refers to the research that used Irgacure®
2959 which has benzoyl and ketone active ingredients as photo-initiators in the
manufacture of hydrogels (Qi et al., 2013).
The addition of Irgacure® 2959 will change the crosslink strength,
which can affect the results of the hydrogel characteristics test. Therefore,
the chitosan-carrageenan-based hydrogel formulation was carried out with the
optimization of Irgacure® 2959 as a photo-initiator with the aim of
knowing the characteristics of the hydrogel as a colonic tissue adhesive.
The research
was started by optimizing the concentration of chitosan and carrageenan
solutions to find the concentrations to be used as the basis for making
hydrogels which were selected based on the level of viscosity produced. Next,
optimization of the solution mixing method was carried out to find the right
method to use in the manufacture of hydrogels based on the homogeneity of the
resulting hydrogels. The next step is to optimize the concentration of Irgacure®
2959 to determine the concentration range to be selected for further
characterization of the hydrogel.
2.1. Optimization of chitosan
and carrageenan solutions
Optimization
of chitosan and carrageenan solutions needs to be done to determine the right
concentration of chitosan and carrageenan for hydrogel manufacture.
Optimization of the concentration of chitosan solution was carried out by
dissolving chitosan powder (DD. 98 from Bio Chitosan Indonesia) with 0.5%
acetic acid (Merck) at a concentration of 1%, 2%, and 3%. The optimization of
the carrageenan solution was carried out by dissolving the carrageenan powder
(CV Karagen Indonesia) using distilled water at a temperature of 50oC
with a concentration of 1%, 2%, and 3%.
2.2. Optimization of the mixing
method
Optimization of the mixing method was
carried out by several methods, such as mixing the chitosan solution with the
carrageenan solution directly, done by adding a solution of carrageenan (2%)
into a solution of chitosan (2%) with a ratio of 1:1 then stirring using a
magnetic stirrer. Mixing the chitosan and carrageenan solutions dropwise, done
by adding a solution of carrageenan (2%) into a solution of chitosan (2%)
dropwise while stirring continuously at a temperature of 60oC. Also,
mixing the chitosan solution with carrageenan powder by using chitosan solution
(2%) and carrageenan powder (2%, 3%, 4%, and 5%) was carried out by adding
carrageenan powder into the chitosan solution slowly at a temperature of 50oC
and stirring continuously. The observed results from the optimization of the
mixing method were observed qualitatively, namely the homogeneity and viscosity
of the hydrogel by tilting the hydrogel at an angle of approximately 45oC.
The selected results based on hydrogel will be used to optimize the
concentration of Irgacure® 2959.
2.3. Optimization concentration
of Irgacure® 2959
The manufacture of hydrogels with the
addition of Irgacure® 2959 refers to the earlier research (Qi et al., 2013) by dissolving chitosan (2%),
which was stirred at 45-50oC for 1 hour. Then in a warm state,
carrageenan powder (4%) was added little by a little while stirring for 15
minutes at 45-50oC. Irgacure® 2959 powder (Jinan
Huijinchuan Chemical CO., Ltd) was added in the dark at a concentration of 1%,
2%, 3%, 4%, and 5% and continuously stirred at 40oC for 15 minutes.
The hydrogel was irradiated with 8-watt UV light with a wavelength of 235nm for
15 minutes. The resulting hydrogel was observed for homogeneity, and then the
concentration was chosen to be used in the chitosan-carrageenan hydrogel
characteristics test. Based on the hydrogel produced, the concentration of
Irgacure® 2959 was chosen to be used in the study with the following
research design below (Table 1).
Table
1 Research design of chitosan-carrageenan hydrogel
|
Treatment |
Chitosan (%) (solution) |
Carrageenan (%) (powder) |
Irgacure® 2959 (%) |
|
A |
2% |
4% |
0% |
|
B |
2% |
4% |
1% |
|
C |
2% |
4% |
2% |
|
D |
2% |
4% |
3% |
|
E |
2% |
4% |
4% |
2.4. Chitosan-carrageenan
hydrogel analysis and characterization
2.4.1. Swelling
ratio
Swelling ratio testing was
carried out using a hydrogel film that was cut to a size of 1cmx1cm and weighed
(wo). The hydrogel film was put into a phosphate buffer saline (PBS) solution
and soaked for 24 hours in a water bath shaker at 37oC. After 24 h,
the films were lifted, dried, and weighed (w). The swelling ratio is calculated
by the formula:
Where w is
hydrogel film weight after soaked (g) and wo is hydrogel film before soaked
(g).
2.4.2. Water
resistance
Water resistance testing
is done by forming a hydrogel into a layer that is printed on silicone. The
water resistance was tested by cutting the hydrogel film (1cmx1cm) and weighing
it (W0), then putting it in aquadest at room temperature. The hydrogel is
lifted, dried, and weighed (W) per one minute, then repeated until the weight
is constant. Water resistance is calculated by the formula:
Where w is the
hydrogel film’s constant weight after soaked (g) and wo is dried hydrogel film
(g).
2.4.3. Adhesion
simulation on smoked beef
Simulation of adhesion was
carried out by applying chitosan-carrageenan hydrogel between two pieces of
smoked beef measuring 2x5 cm and then allowed to stick (Ono
et al., 2000). Smoked beef is pulled on both sides to measure its
adhesive qualitatively.
2.4.4. Degree
of crystallinity (X-Ray Diffraction)
Testing the degree of
crystallinity with X-Ray Diffraction (XRD) was carried out using a sample with
a size of 1.9 x 1.4 cm with a thickness of 1 mm according to the size of the
sample holder. The sample was placed in a holder and then analyzed with an
X-ray diffraction tool for 6 minutes. The results will appear on the monitor
screen. Furthermore, the degree of crystallinity is calculated using the
formula:
2.4.5.
Antibacterial activity
Antibacterial activity
testing was carried out using the paper disk diffusion method using Escherichia coli bacteria. The bacteria to be used were first
rejuvenated in a solution of Nutrient broth (NB) and incubated for 24 hours at
37°C. The bacteria that had grown on the NB were then taken to be poured and
spread over the Nutrient Agar (NA). Furthermore, to test the antibacterial
ability, paper discs with a size of 0.5 cm were immersed in a hydrogel sample
aseptically, then placed on NA and incubated with an incubator (Isuzu Sesakusho
Co., LTD) for 24 hours at 37°C. Antibacterial activity can be calculated by
measuring the clear zone (inhibition zone) that appears around the paper disc.
3.1. Optimization of chitosan
and carrageenan solutions
Based on the characteristics of the hydrogel, the concentration of the chitosan and carrageenan solution selected was 2%. This concentration was chosen because it has a viscosity that is neither too liquid nor too thick, so it can be suitable for its use as a tissue adhesive in wounds in the intestine. The gel used as a wound dressing should have a viscosity that is neither too liquid nor thick so that it can be easily applied (Sularsih, 2013). In addition, the selection of 2% chitosan concentration is expected to provide a good antibacterial effect (Damayanti et al., 2016); 2% chitosan has an optimal ability to form gels and has good antibacterial ability.
3.2. Optimization of the mixing method
The
method chosen was based on the viscosity and homogeneity of the hydrogel.
Hydrogel with a viscosity that is not too liquid and homogeneous is predicted
to improve the mechanical characteristics of the hydrogel. Based on the
resulting hydrogel, mixing chitosan and carrageenan in the form of a solution,
either mixed directly or dropwise, will produce an inhomogeneous hydrogel
characterized by the presence of lumps, and the resulting hydrogel is too
liquid. The formation of lumps in the mixture was predicted because, in the
form of a solution, the carrageenan sulfate group with a negative charge had
bound to cationic water, thus triggering the formation of lumps when added to
the chitosan solution. Therefore, optimization was carried out with another
method, namely by adding carrageenan in powder form to the chitosan solution.
Optimization
of the mixing method by adding carrageenan in powder using a solution
concentration of 2% chitosan and 2% carrageenan produces a liquid solution so
that optimization is carried out by increasing the concentration of carrageenan
to 3%, 4%, and 5%. The addition of carrageenan in powder form to the chitosan
solution can form a homogeneous hydrogel because it is predicted that the
sulfate group of carrageenan can directly bind to the amine group of chitosan
to form a crosslink. Based on the characteristics of the hydrogel produced, the
use of a concentration of 4% carrageenan powder was chosen to be mixed in a 2%
chitosan solution to add Irgacure® 2959 and test its
characteristics.
3.3. Optimization concentration of
Irgacure® 2959
The
addition of Irgacure® 2959 was carried out to strengthen the
crosslinking of the hydrogel (Qi et al., 2013) so
that the characteristics of the hydrogel produced would be better. Also, the
use of Irgacure® 2959 in hydrogels applied in the medical field is
due to its good ability to tolerate fibroblasts. Then Irgacure® 2959
was also successfully used in an in vitro condrocytes encapsulation experiment.
Photopolymerization reactions are becoming a system that is increasingly being
used in medical applications as tissue engineering. it was stated that Irgacure®
2959 is a material that has a tolerance with a wide susceptibility to various
cell types and chemical concentrations (Williams et
al., 2005). So, the addition of Irgacure® 2959 is appropriate
for the manufacture of the hydrogel as a tissue adhesive. The addition of
Irgacure® 2959 to the hydrogel causes a change in the viscosity of
the hydrogel. The more Irgacure® 2959 is used, would make the more
hydrogel become thicker. Based on the characteristics of the viscosity and
homogeneity of the hydrogel. The selected concentration of Irgacure®
2959 is 1%, 2%, 3%, and 4%. Hydrogels with good homogeneity or well-mixed are
predicted to form more regular bonds in hydrogels so that they can strengthen
bonds and produce better characteristic values. Irgacure® 2959 which
is active and exposed to UV light will abstract protons and produce free
radicals, namely benzoyl and ketyl to form intermolecular covalent bonds. These
bonds will form a hydrogel network which is a growth mechanism for the
photopolymerization chain (Pei et al., 2018).
The hydrogel, with the addition of the selected Irgacure® 2959
concentration, was then tested for its characteristics, including swelling
ratio, water resistance, degree of crystallinity (XRD), simulation of adhesion,
and antibacterial ability.
3.4. Chitosan-carrageenan
hydrogel analysis and characterization
3.4.1. Swelling
ratio
The swelling ratio test is presented in Figure 1. The hydrogel forms a three-dimensional macromolecular network able to absorb the water beyond its own volume (Barleany et al., 2020). Based on the results of statistical analysis, the addition of Irgacure® 2959 in the manufacture of hydrogels does not have a significant effect on the swelling ratio value. An increase in the concentration of Irgacure® 2959 will cause the swelling ratio value to decrease due to an increase in intermolecular forces that strengthen crosslink (Qi et al., 2013). The higher the crosslinking that occurs, the denser the intermolecular walls and the lower the swelling ratio value (Cui et al., 2014). The higher the crosslinking, the denser the network and the smaller the pores formed so that the volume of solvent that can occupy the cavities and hydrogel can be used to swell less so that the swelling ratio value is small. However, in this study, the swelling ratio value did not decrease as the concentration of Irgacure® 2959 was added. This is because it is predicted that there will be a steric hindrance that occurs due to the size of the carrageenan molecule that is too large (Sedayu et al, 2019) so that it inhibits the formation of crosslinks in the hydrogel and does not provide a significant difference to the swelling ratio value.
Figure 1 Swelling ratio test
3.4.2. Water
resistance
The water resistance test is presented in Figure 2. Based on the results that have been statistically analyzed, the addition of Irgacure® 2959 to the manufacture of chitosan-carrageenan hydrogel did not give a significant difference to water resistance values. The water resistance of hydrogels is influenced by the bonds formed on the hydrogels. The addition of Irgacure® 2959 can cause the formation of crosslinks in the hydrogel (Qi et al., 2013). The crosslinks formed will make the intermolecular cavities smaller so that the volume of the cavities that can be occupied by the solvent will be smaller and cause the value of water resistance to increase. However, in this study, the increase of Irgacure® 2959 did not increase the water resistance value, which was predicted to occur due to the presence of steric hindrance in the hydrogel due to the large difference in the molecular size of the polymer used. The steric hindrance causes no increase in crosslinking that occurs in the hydrogel as the concentration of Irgacure® 2959 is added so that the water resistance value of the hydrogel produced is not significantly different.
Figure 2 Water resistance test
3.4.3. Adhesion
simulation on smoked beef
Hydrogel adhesion simulation
was carried out by testing using smoked beef (Ono
et al., 2000). The results showed that the increase of concentration of
Irgacure® 2959 added to the manufacture of hydrogels did not give a
significant difference when used to glue smoked beef. The hydrogel used to glue
smoked beef produces a weak adhesion. The simulation shows that the resulting
hydrogel has not shown the desired results because, qualitatively, the hydrogel
is considered not strong enough to bind two tissues together. Qualitatively,
the smoked beef that has been applied with hydrogel already feels attached to
each other but is not too strong and is still easy to separate. This is in line
with the results of the swelling and water resistance of the
chitosan-carrageenan hydrogel, which showed no significant difference with the
increase in the concentration of Irgacure® 2959 added.
3.4.4. Degree of
crystallinity (X-Ray Diffraction)
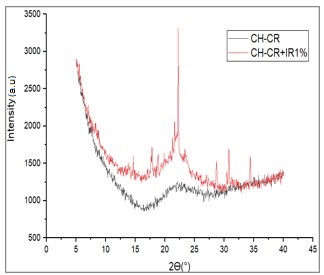
Degree of crystallinity test of the hydrogel was carried out using X-Ray Diffraction (XRD), which aims to determine the crystalline phase formed and changes in the crystal structure that occur in the chitosan-carrageenan hydrogel. The test is carried out with X-Ray Diffraction and then connected to an interface on a computer to find out the results obtained (Kurniawan & Setiyorini, 2014). The results of the XRD test are in the form of crystallinity and amorphous value data that compose the test sample (Warsiki et al., 2020). Based on the results of the degree of crystallinity test, which showed changes in the crystal structure, it could be seen that the bonding occurred in the chitosan-carrageenan hydrogel. XRD test results can be seen in Figure 3.
Figure 3 Hydrogels X-Ray Diffraction result
Based on the XRD results, chitosan does
not have a diffraction peak or can be referred to as amorphous according to the
earlier research (Nugroho et al., 2011),
which shows that there is no diffraction peak in chitosan because it is
predicted that the chitosan has dissolved well. The XRD results of carrageenan
also show that there is no diffraction peak as in the study because the
carrageenan polymer is non-crystalline material, so it does not produce a
diffraction peak (Ulfah & Nugraha, 2014).
The diffractogram of the chitosan-carrageenan hydrogel sample also showed no
peak appearance because both materials were non-crystalline materials. XRD test
results on Irgacure® 2959 showed a diffraction peak with a degree of
crystallinity of 63.39%. Then the chitosan-carrageenan hydrogel with the
addition of 1% Irgacure® 2959 was chosen because it has the lowest
swelling and the highest water resistance value showing diffraction peaks with
a degree of crystallinity of 25.84%. The appearance of diffraction peaks on XRD
results of chitosan-carrageenan hydrogel with the addition of 1% Irgacure®
2959 indicates that there are crosslinks formed but in small amounts. These
peaks can be formed, indicating the level of crystallinity due to changes in
the bonds between polymers due to the addition of Irgacure® 2959.
Based on the data, it shows a change in the bond that occurs so that the
resulting test value changes even though it is not significantly different.
3.4.5.
Antibacterial activity
Antibacterial test was carried out to
determine the ability of chitosan-carrageenan hydrogel to inhibit bacterial
growth. Wounds on the body are susceptible to infection by bacteria that can
hinder the wound-healing process, so tissue adhesive is needed that can inhibit
bacterial growth (Kurniawaty & Putranta, 2019).
The ability to inhibit bacteria can be seen from the resulting clear zone,
which is formed to show the effectiveness of chitosan in inhibiting bacterial
growth (Amanda et al., 2020). The results of
the ability chitosan-carrageenan hydrogel test with the addition of Irgacure®
2959 to inhibit bacterial growth can be seen in Figure 4. In the test, the
negative control used nutrient broth solution, and the positive control used 2%
chitosan solution.
Table 2 Inhibition diameter zone
|
Concentration of Hydrogel |
Control - |
Control + |
0% |
1% |
2% |
3% |
4% |
|
Clear Zone (cm) |
0.00 |
1.33 |
1.33 |
1.17 |
1.00 |
1.00 |
0.83 |
Figure 4 Antibacterial activity test
The clear zone formed in NA
is included in the weak category in inhibiting bacterial growth (Surjowardojo et al., 2015). Based on the results
of statistical analysis, the addition of Irgacure® 2959 to the
hydrogel was not significantly different from the hydrogel without the addition
of Irgacure® 2959. Although not significantly different, increasing
the concentration of Irgacure® 2959 used is known to decrease the
hydrogel's ability to inhibit bacteria. The ability to inhibit bacteria in
hydrogels comes from the polycationic amine group of chitosan, which will
interact with the bacterial cell wall so it can disrupt the metabolism of
bacteria and inhibit their growth (Nurainy et al.,
2008). The non-formation of these bonds causes the number of amine
groups to remain unchanged so that the chitosan-carrageenan hydrogel has the
same ability as the chitosan solution to inhibit bacterial growth. While the
chitosan-carrageenan hydrogel with the addition of Irgacure® 2959
decreased the ability to inhibit bacteria due to the amine group in chitosan
partially bound to Irgacure® 2959.
The absence of significant
differences in the test results for each parameter is thought to be due to the
presence of steric hindrance formed due to the large difference in molecular
size between chitosan and carrageenan, making it difficult for interactions to
occur (Chang et al., 2018). The use of a
higher ratio of carrageenan resulted in more molecules of carrageenan sulfate
groups, thus inhibiting the formation of bonds in the hydrogel, and resulting
in less crosslinking, so that the hydrogel density distance did not change and
resulted in the degree of swelling, which was not significantly different from
the control. The steric hindrance in the hydrogel causes a cavity to form due
to the bond does not change so the water resistance of the hydrogel and the
ability of the cavity in the hydrogel to store the solvent to be used for the
development of the hydrogel was not significantly different for each hydrogel
with the addition of Irgacure® 2959.
The changes in test values in
the test of the characteristics of the chitosan-carrageenan hydrogel with the
addition of Irgacure® 2959 can occur due to changes in the bonds that
occur in chitosan and Irgacure® 2959. This change in bonding is
indicated by the XRD results of the chitosan-carrageenan hydrogel with the
addition of Irgacure® 2959 as much as 1%, which changes by showing
the presence of a distraction peak or the presence of crystalline in the
hydrogel is different from the chitosan-carrageenan hydrogel. This change was
supported by the antibacterial test results on the hydrogel, which showed a
decrease in the value of the chitosan-carrageenan hydrogel with the addition of
Irgacure® 2959. This decrease in antibacterial ability was due to
the free amine group in chitosan, which played a role in attacking cell
membranes in bacteria due to its binding to Irgacure® 2959. The
amine groups that attack the protein on the bacterial cell membrane are the
same, causing the antibacterial properties of the chitosan solution and the
chitosan-carrageenan hydrogel to have the same value. The glueing ability which
was assessed qualitatively from the chitosan-carrageenan hydrogel with the
addition of Irgacure® 2959, showed the same results in each
treatment, which means that the results of the quantitative test on the
hydrogel showed no significant difference. It is necessary to optimize the
chitosan-carrageenan ratio with a higher amount of chitosan or reduce the
amount of carrageenan used so that the sulfate group molecules no longer
inhibit the formation of bonds so that cross-links can occur and form stronger
bonds.
Based on the research that
has been done, it is known that the addition of Irgacure® 2959 to
the manufacture of chitosan-carrageenan-based hydrogel with a ratio of 1:2
using the photo-crosslinking method has not shown good potential as a tissue
adhesive based on the results of characteristic tests. It is necessary to
optimize the ratio to eliminate steric barriers in the hydrogel so that it can
produce cross-links that will improve the water resistance properties and
degree of swelling of the hydrogel. The application of tissue adhesive hydrogel
to be used in the intestine must have good water resistance because the
location of the intestine is in an environment that is always wet to minimize
damage and failure during the process of glueing the tissue. The degree of
swelling is an important indicator to determine the success of the treatment
procedure. Hydrogels are designed to help reconstruct damaged tissues, so the
increased volume will contribute to hemostasis without disturbing surrounding
tissues. Further, it is necessary to optimize the ratio of the chitosan and
kappa-carrageenan to get the balance ratio that supports the occurrence of
cross-linking so that it can help determine which hydrogel is good to use as a
tissue adhesive, especially to be applied to the colon.
The Irgacure® 2959 concentration that added to the
chitosan-carrageenan-based hydrogel did not significantly affect the values for
swelling ratio, water resistance, simulation of adhesion, and antibacterial
activity. This is predicted because there is steric hindrance due to
differences in the size of the chitosan and carrageenan molecules, thus
inhibiting the formation of crosslinks in the hydrogel. Further research is
needed to obtain hydrogel results that can be used as tissue sealants.
This paper was
funded by the Lecturer Research Grant of Agriculture Faculty 2021, Universitas
Gadjah Mada so this research that became part of the first author's thesis
could be carried out properly.
Amanda, E.,
Khoirun, N., Yulianto, A.,
2020. Development of Antibacterial Morganella morganii Bioplastic for Food
Packaging. Jurnal
Kimia dan Kemasan
(Journal of Chemistry and Packaging), Volume 42(1), pp. 29?36
Asadpour, S.,
Kargozar,
S., Moradi,
L., Ai,
A., Nosrati,
H., Ai,
J.,
2020. Natural Biomacromolecule Based Composite Scaffolds from Silk Fibroin,
Gelatin And Chitosan Toward Tissue Engineering Applications. International Journal of Biological
Macromolecules, Volume 154, pp. 1285?1294
Bao, Z., Gao,
M., Sun, Y., Nian, R., Xian, M.,
2020. The Recent Progress of
Tissue Adhesive in
Design Strategies, Adhesive Mechanism and Applications. Materials Science & Engineering: C, Volume 111, p. 110796
Barleany, D.R., Ananta, C.V., Maulina,
F., Rochmat, A., Alwan, H., Erizal, 2020. Controlled Realease of Metformin
Hydrogen Chloride from Stimuli-responsive Hydrogel Based on
Poly(N-Isopropylacrylamide)/Chitosan/Polyvinyl Alcohol Composite. International Journal of Technology, Volume 11(3), pp.
511?521
Chang, S.H., Wu, C.H., Tsai, G.J., 2018.
Effects of Chitosan Molecular Weight on Its Antioxidant and Antimutagenic
Properties. Carbohydrate polymers, Volume 181, pp.1026 ? 1032
Cui, L., Jia, J., Guo, Y., Liu, Y., Zhu,
P., 2014. Preparation and Characterization of IPN Hydrogels
Composed of
Chitosan and
Gelatin Crosslinked by
Genipin. Carbohydrate Polymers, Volume 99, pp. 31?38
Damayanti, W.,
Rochima,
E., Hasan,
Z.,
2016. Application of Chitosan as Antibacterial for Pangasius Fillet at Low Temperature Storage. Jurnal Pengolahan Hasil Perikanan Indonesia (Journal of Processing of Indonesian Fishery Products), Volume 19(3), pp. 321?328
Darmayanti,
M.G., Radiman, C.L., Sudarma, I.M., 2016. Kappa-Carrageenan as an Attractive
Green Substitute for Polyacrylamide in Enhanced Oil Recovery Applications. International Journal of
Technology, Volume 7(3), pp. 431?437
Derkach, S.R.,
Voronko, N.G., Kuchina, Y.A, Kolotova, D.S., Gordeeva, A.M., Faizullin, D.A., Gusev, Y.A., Zuev, Y.F., Makshakova, O.N., 2018. Molecular
Structure and
Properties of
KAPPA-Carrageenan-Gelatin Gels. Carbohydrate Polymers, Volume 197, pp. 66?74
Ding, H., Geng, Y., Lu
J., Zhao,
Y., Bai,
B.,
2020. Impacts of
Crosslinker Concentration on
Nanogel Properties And Enhanced Oil Recovery Capability. Fuel, Volume
267, p. 117098
Kurniawan, B.E.,
Setiyorini,
Y.,
2014. Effect of Holding Time Variation on Quench Annealing Heat Treatment on
Mechanical and Microstructural Properties of AISI 3401 Manganese Steel. Jurnal Teknik Pomits (Journal of Engineering Pomits), Volume 3(1)
Kurniawaty, E., Putranta, N.R., 2019. Potency
of Chitosan Biopolymer for Wound Treatment. Medula, Volume 9(3), pp. 459?464
Nayeb, H.F., Salahshoor, K.S., Afshar, T.F., Shariatinia, Z., 2011. A Novel
Topical Biocompatible Tissue Adhesive Based on Chitosan-Modified Urethane
Pre-Polymer. Iranian Polymer Journal, Volume 20, pp.671?680
Nugroho, A.,
Nurhayati,
N.D.,
Utami,
B., 2011.
Synthesis and Characterization of Chitosan
Membranes for Application as A Heavy Metal Detection Sensor.
Molekul, Volume
6(2), pp. 123?136
Nurainy, F., Rizal, S., Yudiantoro, 2008. The
Effect of Chitosan Concentrations on The Antibacterial Activity with Gel Diffusion/Well
Method. Jurnal
Teknologi Industri dan Hasil Pertanian (Journal of Industrial Technology and Agricultural
Products), Volume
13(2)
Ono, K., Saito, Y., Yura, H., Ishikawa,
K., Kurita, A., Akaike, T., Ishihara, M., 2000.
Photocrosslinkable Chitosan as
A Biological Adhesive. Journal of Biomedical Materials Research: An Official Journal of
The Society for Biomaterials and The Japanese Society for Biomaterials, Volume
49(2), pp. 289?295
Pei, M., Mao, J., Xu, W., Zhou, Y.,
Xiao, P.,2018. Photocrosslinkable
Chitosan Hydrogels and Their Biomedical
Applications. Journal of Polymer Science Part A: Polymer Chemistry, Volume
57(18), pp.1862?1871
Phuong, P.T.M., Won, H.J., Oh, Y.J.,
Lee, H.S., Lee, K.D., Park, S.Y., 2019.
The Chemistry and
Engineering of
Mussel-Inspired Glue Matrix for
Tissue Adhesive and
Hemostatic. Journal of Industrial and
Engineering Chemistry, Volume 80, pp. 749?756
Qi, Z., Xu, J., Wang, Z., Nie, J., Ma,
G., 2013.
Preparation and
Properties of
Photo-Crosslinkable Hydrogel Based on Photopolymerizable Chitosan
Derivative. International Journal of Biological Macromolecules, Volume 53, pp. 144?149
Sedayu, B.B., Cran, M.J., Bigger, S.W., 2019. A Review of Property
Enhancement Techniques For Carrageenan-Based Films and Coating. Carbohydrate Polymers, Volume 216, pp. 287?302
Sularsih., 2013. The
Effect of Viscosity Chitosan Gel to The Application Wound Healing Process.
Jurnal Material Kedokteran Gigi (Journal of Dentistry Materials), Volume 2(1), pp. 60?67
Sumarni, W.,
Prasetya, A.T., Rahayu
E.F.,
2017. Effect of
Glycerol on
Physical Properties of
Biofilm Gembili Starch (Dioscorea esculenta)-Chitosan. In Proceeding of Chemistry Conference. Volume 2, pp. 5665
Surjowardojo, P.,
Tri, E.,Gabriel, R.,
2015. Inhibitor Power of Manalagi Apple Skin Decook (Malus sylvestrs Mill) on The Growth of Staphylococcus aureus and Pseudomonas sp. Causes of Mastitis in Dairy Cow.
Journal of Tropical Animal Production, Volume 16(2), pp. 40?48
Ulfah, F., Nugraha, I., 2014. Effect
of Montmorillonite Addition to Mechanical Properties of
Carrageenan-Montmorillonite Film Composite. Molekul, Volume
9(2), pp. 155?165
Wang, Y., Yuan, C., Cui,
B.,
Liu,
Y.,
2018. Influence of
Cations on
Texture, Compressive Elastic Modulus, Sol-Gel Transition and Freeze-Thaw
Properties of
Kappa-Carrageenan Gel. Carbohydrate Polymers, Volume 202, pp. 503?535
Warsiki, E.,
Setiawan, I., Hoerudin., 2020. Synthesis
of Cassava Skin Starch-Silica Nanoparticles Bioplastic Composite and Its
Characterization. Jurnal
Kimia dan Kemasan (Journal
of Chemistry and Packaging), Volume
42(2), pp. 37-45
Williams, C.G., Malik, A.N., Kim, T.K.,
Manson, P.N., Elisseeff, J.H., 2005. Variable Cytocompatibility of Six Cell
Lines with Photoinitiators Used for Polymerizing Hydrogels and Cell
Encapsulation. Biomaterials, Volume 26(11),
pp.1211?1218
Yegappan, R., Selvaprithiviraj, V.,
Amirthalingam, S., Jayakumar, R., 2018. Carrageenan
Based Hydrogels for
Drug Delivery, Tissue Engineering and Wound Healing. Carbohydrate Polymer, Volume 198, pp. 385?400
Yu, H.C., Zhang, H., Ren, K., Ying, Z.,
Zhu, F., Qian, J., Ji, J., Wu, Z.L., Zheng, Q., 2018. Ultrathin ?-carrageenan/chitosan
Hydrogel Films with High Toughness and Antiadhesion Property. ACS
applied materials & interfaces, Volume 10(10),
pp.9002?9009
Zhang, Y., Wang, X., Xu, C., Yan, W.,
Tian, Q., Sun, Z., Yao, H., Gao, J., 2018. Fabrication Of Chitosan
Gel Droplets Via Crosslinking Of Inverse Pickering Emulsifications. Carbohydrate Polymers, Volume 186, pp. 1?8
