Optimization of Irgacure® 2959 Concentration as Photo-Initiator on Chitosan-Alginate Based Hydrogel for Colon Tissue Sealant
Corresponding email: nugrahenips@ugm.ac.id
Published at : 30 Dec 2022
Volume : IJtech
Vol 13, No 8 (2022)
DOI : https://doi.org/10.14716/ijtech.v13i8.6164
Puspitasari, D., Budhijanto, W., Purnomo, E., Nugraheni, P.S., 2022. Optimization of Irgacure® 2959 Concentration as Photo-Initiator on Chitosan-Alginate Based Hydrogel for Colon Tissue Sealant. International Journal of Technology. Volume 13(8), pp. 1704-1714
| Diah Puspitasari | Department of Fisheries, Faculty of Agriculture, Universitas Gadjah Mada, Jl. Flora Gd. A4, Bulaksumur, Yogyakarta, 55281, Indonesia |
| Wiratni Budhijanto | Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Senolowo, Jl. Farmako, Sekip Utara, Depok, Sleman, Yogyakarta, 55281, Indonesia |
| Eko Purnomo | Department of Chemical Engineering, Faculty of Engineering, Universitas Gadjah Mada, Sendowo, Sinduadi, Sleman, Yogyakarta, 55281, Indonesia |
| Prihati Sih Nugraheni | Department of Fisheries, Faculty of Agriculture, Universitas Gadjah Mada, Jl. Flora Gd. A4, Bulaksumur, Yogyakarta, 55281, Indonesia |
Tissue sealant is a material that is used as an
adhesive to bond two tissue surfaces used during surgery. Chitosan and alginate
are natural macromolecules used to manufacture hydrogels as tissue sealants
because these two materials can strengthen bonds and the mechanical properties
of tissue sealants.
Irgacure®
2959, as a photo-initiator, can strengthen the cross-linking between
chitosan-alginate to produce a tissue sealant with good mechanical strength.
Therefore, this study aims to determine the effect of adding Irgacure® 2959
to chitosan-alginate hydrogel with different concentrations as a tissue sealant
designed for the colon. The experimental design used a completely randomized
design (CRD) with the addition of Irgacure® 2959 0; 1; 2; 3; 4%. The
characteristics of the hydrogel as a tissue covering observed in this study
include the degree of swelling, water resistance, simulated adhesion, degree of
crystallinity, and ability to inhibit bacteria. The results of this study
showed that the addition of 4% Irgacure® 2959 reduced the degree of
swelling, increased the hydrogel's resistance to water, and increased adhesion.
The interaction of chitosan and alginate with the addition of Irgacure®
2959 produces cross-links, as evidenced by a degree of crystallinity of 26.21%.
Based on these results, the chitosan-alginate hydrogel with the addition of
Irgacure® 2959 showed good potential in tissue sealant formulations,
even though the inhibition of bacteria in all treatments showed low results,
namely < 5 mm.
Alginate; Chitosan; Hydrogel; Irgacure® 2959; Photo-initiator
Chitosan
is a polymer with a polycationic linear chain consisting of one primary amine
and two free hydroxyl groups (Bakshi et al., 2020).
Chitosan is used in the biomedical field in the form of a hydrogel, which can
be used as a tissue sealant to combine tissue surfaces during the operation
process (Jarret et al., 2013). Hydrogels are
hydrophilic and can absorb and release water. These properties make hydrogels
usable as sealants because they have a moist environment, are flexible, have
mechanical muscle strength, and are non-toxic, so they have the potential to be
applied in the large intestine (Ehterami et al., 2019). Hydrophilic properties of hydrogels can
influence cellular attachment between two surfaces (Dewi
et al., 2020). Tissue
sealants are more advantageous than sutures and clamps because the wound closure process becomes more
accessible, faster, and less painful to minimize problems during the
application and removal of sutures (Nayeb et al., 2011).
Chitosan is one of
the biomacromolecules utilized. Chitosan has biocompatible, biodegradable
properties, good tensile strength properties, is easily modified when applied
to physiological pH, can act as an adhesive, is antibacterial, can help heal
wounds quickly, and is non-toxic. As a result, it is suitable for use as a
material in the production of hydrogels (Asadpour
et al., 2020; Bagher et al., 2020; Bektas et al., 2020). Chitosan
requires a crosslinker to increase its cross-linking strength so that the
resulting hydrogel has strong adhesion and is not easily brittle. Alginate can
play the role of a crosslinker because it has good biocompatibility, low
toxicity, and can be modified easily via photocrosslinking (Lee & Mooney, 2012). According to Wang et al. (2017), who tried to make hydrogels
from chitosan-alginate by physically cross-linking methods applied to olfactory
nerve cells, which shows that the hydrogels were able to stimulate cell
proliferation. Based on this study, chitosan-alginate can be used as a tissue
sealant.
The
hydrogel can use both materials in the form of chitosan-alginate to make
hydrogels through the photocrosslinking method. Making hydrogels using the
photocrosslinking method requires a photo-initiator. These compounds can form
good mechanical bonds, maintain hydrogel properties in the long term, obtain
uniform properties, and help form pores as a place for cell attachment (Qi et al., 2013). Irgacure® 2959 is
the most commonly used photo-initiator because of its low cytotoxicity and
possible immunogenicity, making it safe to make hydrogels as tissue sealants (Tomal et al., 2020). The added concentration of
Irgacure® 2959 affects the cross-linking between polymers and the
mechanical properties of the hydrogel as a tissue sealant. Therefore, this
study aims to determine the effect of the addition of Irgacure® 2959
on the characteristics of the chitosan-alginate hydrogel. Its parts will be
observed so that it can assess its potential as a tissue covering designed for
the large intestine.
2.1. Hydrogel Preparation
The chitosan comes from Bio
Chitosan Indonesia with a DD 98%, alginate from Leyoung Int, and Irgacure®
2959 from Jinan Huijinchuan Chemical Co., Ltd). The manufacture of the hydrogel
begins with the manufacture of a 2% chitosan solution, which refers to the
research of Baysal et al. (2013) with
modifications. 2 grams of powdered chitosan were dissolved in a 0.5% (v/v)
acetic acid solution. Stirred the solution using a hot plate stirrer (Arec Heating Magnetic Stirrer), at a
speed of 400 rpm for 1 hour at a temperature of 40-45?C. Furthermore, making a
2% alginate solution refers to the research of Bagher
et al. (2020) with modifications. 2 grams of powdered alginate were
dissolved in 100 ml of distilled water. Stirred the alginate solution using a
hot plate stirrer at a speed of 400 rpm for 1 hour at a temperature of 40-45?C.
The selection of alginate chitosan concentration was carried out through a
preliminary study by observing the viscosity of the solution visually. According to Sularsih (2013), when used as a wound healer in a
chitosan solution thick enough to facilitate application, its mucoadhesive
properties are expected to be better for wound healing and closure. The choice
of 2% alginate solution is because, according to Chandramouli
et al. (2004), the use of alginate concentrations of more than 2% is not
possible to obtain homogeneous results due to an increase in solution viscosity
and mass diffusivity, and higher alginate concentrations give heavier and
thicker membrane results. Still, the porosity, strength, and elongation are
decreasing.
2.2. Optimization of
Chitosan-Alginate Solution Mixing Method and Concentration of Irgacure®
2959
Mixing chitosan and alginate solutions refers to the research of Baysal et al. (2013) with modifications. Chitosan solution and alginate solution were mixed in a 1:1 ratio. The selection of hydrogel from the mixing method is chosen based on its homogeneity. At this stage, optimization is carried out with five different methods, namely as follows:
2% chitosan solution plus 2% alginate solution added dropwise at
room temperature
2% chitosan solution plus 2% alginate powder at 50oC
2% chitosan solution at 40?C plus 2% alginate solution at room
temperature directly
2% chitosan solution plus 1% alginate solution directly at a temperature
of 40-45oC
2% chitosan solution plus 2% alginate solution directly at a temperature
of 40-45oC
2.3. Method of
Mixing Irgacure® 2959 into Chitosan-Alginate Solution
Optimization of the
Irgacure® 2959 addition method was carried out in 2 ways: directly and added 30
minutes after the chitosan-alginate solution was mixed. Furthermore,
optimization of the concentration of Irgacure® 2959 was carried out
referring to the research by Qi et al. (2013)
and study by Han et al. (2020), used
concentrations of 0.5%, 0.75%, 1%, 1.25%, 2%, 3%, 4%, 5%, and 6%. The addition
of Irgacure® 2959 was added in powder form to the chitosan-alginate
solution. The choice of concentration used is based on the homogeneity of the
hydrogel and its suitability for application as a tissue sealant.
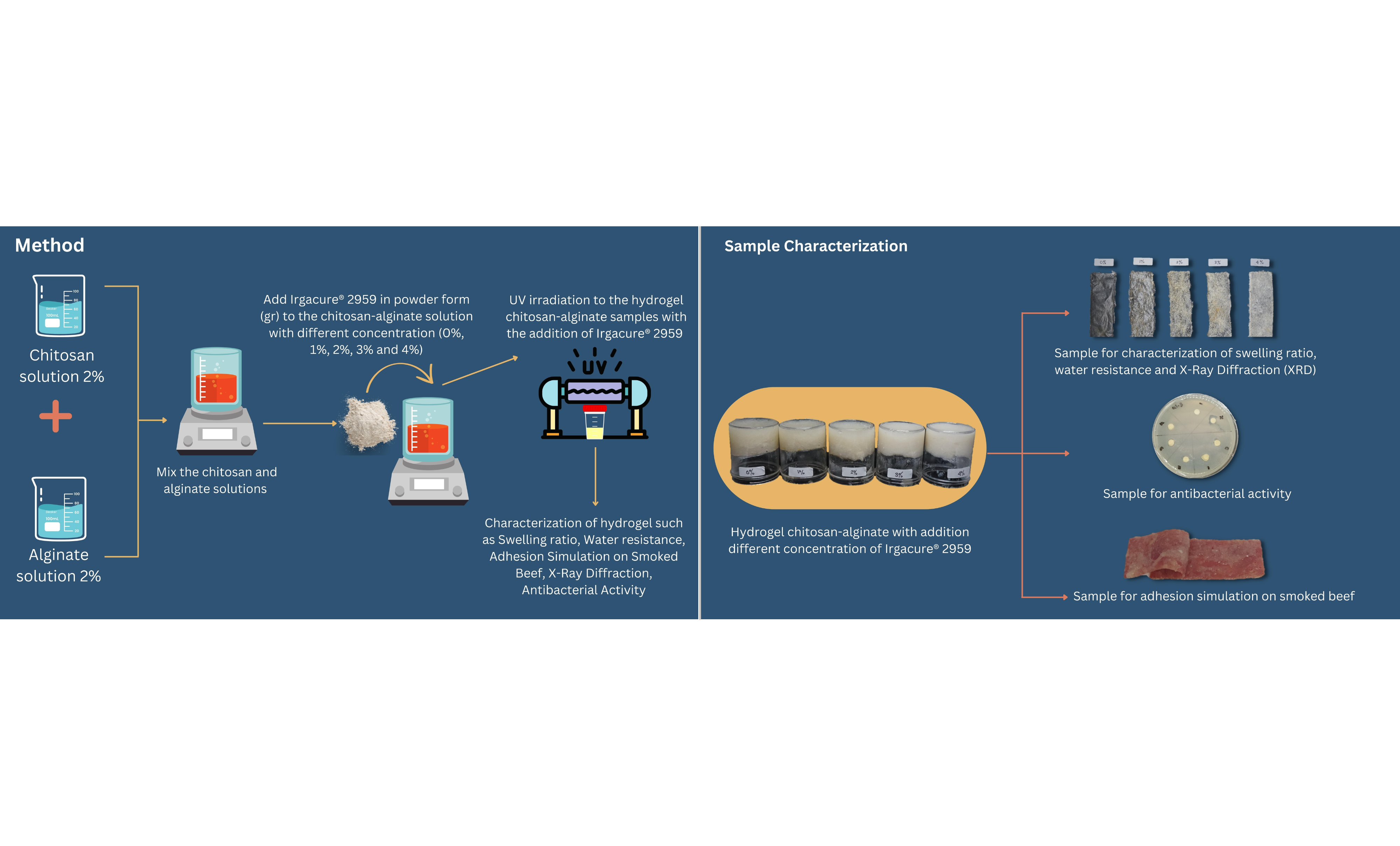
After doing all the optimization, the next step is to make chitosan-alginate hydrogel with the addition of Irgacure® 2959. The scheme for the illustration of the preparation of the composite can be seen in Figure 1.
Figure 1 Illustration of the preparation of the composite
2.4. Analysis and
Characterization of Hydrogels as Tissue Sealant
2.4.1. Swelling ratio
The swelling test refers to
the research of Khan et al. (2020), which
was modified. The swelling test was carried out using a 1x1 cm dried hydrogel
sample that was immersed in 10 ml of phosphate buffer saline (PBS) solution at
37?C at 130 rpm for 24 hours on a water bath shaker. The water still on the
surface of the hydrogel was removed using a tissue. The swelling ratio is
calculated as the difference between the final weight after immersion and the
initial weight, divided by the final weight of immersion, and multiplied by
100%.
Where Wd is the sample was weighed, and Ws is the initial weight
2.4.2. Water
resistance
The water resistance test
refers to the research of Sumarni et al. (2017).
The sample used is a dry hydrogel measuring 1 x 1 cm. Then, put the sample into
a beaker containing 10 ml of distilled water for 1 minute. After 1 minute,
remove the sample and wipe away any adhering water with a tissue. Then repeat
the process until you reach a constant weight. Then the percentage of water
resistance is calculated as 100% minus the difference in the final weight with
the initial weight divided by the initial weight multiplied by 100%.
Where W0 is the initial
weight of the sample, and W has finally weighed the sample
2.4.3. Adhesion
simulation on smoked beef
This test was conducted using smoked
beef media, which refers to the research of Ono et
al. (2000). The adhesive simulation test is done by preparing two pieces
of smoked beef measuring 2x5 cm with a thickness of 2mm. The created hydrogel
is then applied to one part of the smoked beef and glued together. After that,
the smoked beef is stored in the refrigerator for three days. The results are
observed by opening the two smoked beef that have been glued together and then
qualitatively observing whether the two smoked beef are glued together or not.
2.4.4. X-ray
Diffraction (XRD)
The test was carried out by cutting the
sample of 1.9x1.4 cm with a thickness of 1mm according to the size of the
sample holder. Sample pieces are placed on the sample holder. The sample holder
is then placed on the XRD tool for analysis. The analysis took six minutes
using an X-ray diffraction device. The results will appear on the monitor
screen with readings ranging from 5? to 40?. After obtaining the peak data, the
percentage of sample crystallinity was calculated by dividing the area of the
??crystalline fraction by the area of the ??crystalline fraction plus the area
of the ??amorphous fraction multiplied by 100. The formula for calculating the
percent crystallinity is as follows:
2.4.5. Antibacterial activity
The procedure for testing
film samples was modified using the paper disc diffusion method on escherichia
coli bacteria. Antibacterial testing using the disc method refers to the
research of Mahdavinia et al. (2019) with
modifications. Escherichia coli bacteria were grown on NB medium, and the
density was measured through OD testing using a spectrophotometer. The
bacterial suspension was taken in amounts as much as 0.1 ml and put into a
petri dish that already contained a solid NA medium. The paper dish that will
be used is immersed in the sample in each treatment for 5 minutes, then put
into a petri dish. After that, the media filled with bacterial suspension is
incubated for 24 hours at 37?C, then the inhibition zone formed is measured
using a ruler. The diameter of the inhibition zone included was calculated as
the difference from the diameter of the paper disk.
3.1. Optimization of The Method of Mixing
Chitosan Solution and Alginate Solution
Mixing chitosan and alginate solutions
was carried out to obtain a homogeneous solution. The mixing method's
optimization results are given in Table 1. Based on the optimization
results, the more plus signs indicate the thicker the solution obtained, and
the more lumps, precipitates, fibers formed, and smoother the texture, so the
method chosen for the next process is the one that has the fewest and most
homogeneous fibers, namely the method with code E using a 2% chitosan solution
plus a 2% alginate solution directly at a temperature of 40-45?C.
Table
1 Mixing Method of
Chitosan and Alginate
|
Code |
Method |
Physical Observation of Solution | ||||||
|
Chitosan (%) |
Alginate (%) |
Material condition |
Temperature |
Viscosity |
Lumps |
Fiber |
Texture | |
|
A |
2 |
2 |
S+S |
27oC |
++ |
+++ |
+++ |
+ |
|
B |
2 |
2 |
S+P |
50oC |
+ |
+++ |
+ |
+ |
|
C |
2 |
2 |
S+S |
40oC 27oC |
++ |
++ |
++ |
+ |
|
D |
2 |
1 |
S+S |
40oC-45oC |
++ |
++ |
++ |
++ |
|
E |
2 |
2 |
S+S |
40oC-45oC |
+++ |
+ |
+ |
+++ |
Information: +:
low; ++: medium; +++: high; S: Solution; P: Powder
3.2. Optimization of Mixing Method and
Concentration of Irgacure® 2959
Table 2 Irgacure® 2959 mixing
method
|
Method |
Physical
Observation of Solution |
|
Added directly |
The solution is not mixed, the
texture is rough, and there are clumps of fibre |
|
Added after the
chitosan-alginate solution was stirred for 30 minutes |
The solution is well mixed; the
texture is smooth and compact |
The method of mixing
Irgacure® 2959 into the chitosan-alginate hydrogel was carried out
in two ways: directly added and added 30 minutes after the chitosan-alginate
solution was stirred. The results of mixing are given in Table 2. Based on
these results, the method of adding Irgacure® 2959 was chosen by
adding it after the chitosan-alginate solution was stirred for 30 minutes. The
choice was based on the fact that when Irgacure® 2959 was directly
added together with chitosan-alginate, it could cause its bonding with alginate
and chitosan to become less regular, resulting in an inhomogeneous solution,
while the addition of Irgacure® 2959 30 minutes after the
chitosan-alginate was mixed could provide space for the chitosan and alginate
to be mixed beforehand, which made the bond between the chitosan-alginate and
the Irgacure® 2959 more regular and more homogeneous.
3.3 Chitosan-Alginate Hydrogel Analysis and Characterization
3.3.1. The effect of
variations in the concentration of Irgacure® 2959 on the swelling
ratio
Based on the study's results, the addition of Irgacure® 2959
caused a lower degree of development. This follows the research of Qi et al. (2013) that the addition of Irgacure®
2959 at higher concentrations causes the degree of swelling to be lower due to
an increase in cross-linking, which can cause the cross-linking to become
stronger so that the structure will be tighter, and less water will enter. The
percentage of development produced from chitosan-alginate hydrogel with the
addition of Irgacure® 2959 as a photo-initiator is given in Figure 2.
Based on these results, this composite has
potential as a tissue sealant because, Mukherjee et
al. (2018), in its application as a wound healer, state that low
swelling is needed to heal wounds because hydrogels with too high swelling can
inhibit the rate of healing, and too much fluid absorption that occurs can
cause dehydration in the wound, thereby slowing down the wound healing process.
And hydrogels with good hydrophilicity can provide soft physical properties
when applied to living tissue (Barleany et al.,
2019).
Figure 2 Swelling ratio of chitosan-alginate
hydrogel with the addition of Irgacure® 2959 at different concentrations
3.3.2. The
effect of variations in the concentration of Irgacure® 2959 on
hydrogel resistance to water
The adhesive's resistance to water makes the tissue sealant not easily crushable or detached when attached to surfaces with a lot of water content, such as intestines. The addition of Irgacure® 2959 with a higher concentration causes its ability to bind water to increase. These results indicate that the addition of Irgacure® 2959 significantly affects the water resistance of the chitosan-alginate hydrogel as a tissue sealant. According to Rudyardjo (2014), when there is no addition of Irgacure® 2959 as a photo-initiator, the O-H group possessed by chitosan will bind less, so that its water resistance will decrease. On the other hand, with the addition of Irgacure® 2959, the O-H group will bind more and more, so its ability to hold water will increase. The average value of hydrogel resistance to water are given in Figure 3.
Figure 3 Percentage of hydrogel
resistance to water
Based on the findings, this composite has the
potential to be used as a tissue sealant due to its high-water resistance
characteristics. This result is suitable as a tissue sealant. According to Briawan et al. (2011) the body contains 75% water
and 25% solid matter, so a tissue sealant that has an elevated level of water
resistance is needed so that when it is used as a tissue sealant in the
intestine, it is not easily brittle but can still help the process of
attachment and cell growth.
3.3.3. The
effect of variations in the concentration of Irgacure® 2959 on the
simulation results of adhesion to smoked beef
Adhesion simulation was carried out using
smoked beef media because its structure is like tissue. Adhesion simulation was
carried out to qualitatively determine the adhesive strength of
chitosan-alginate hydrogel with the addition of Irgacure® 2959. The
results of the simulation of adhesion can be seen in Table 3. The simulation
results indicate that the higher addition of Irgacure® 2959 means
that smoked beef can stick together. Based on Table 3, the more signs the
smoked beef has, the stronger the bond and the more difficult it is to
separate. In connection with these results, the addition of Irgacure®
2959 at a higher concentration can produce hydrogels with more potent adhesive
abilities.
Table 3
Simulation results of adhesion with variations of Irgacure® 2959
with qualitative methods
|
Concentration of Irgacure® 2959 |
Adhesive Strength Level |
Description |
|
0% |
+ |
Smoked
beef can stick to each other, but when you take it off, it's still easy to
separate |
|
1% |
++ |
Smoked
beef can stick to each other, but when you take it off, it's still easy to
separate |
|
2% |
+++ |
Smoked
beef can stick to each other, but when you take it off, it's getting a little
difficult to separate |
|
3% |
++++ |
Smoked
beef can stick to each other, but when you take it off, it's hard to separate |
|
4% |
+++++ |
Smoked
beef can stick to each other, but when you take it off, it's hard to separate |
Information: +: less sticky; ++: slightly
sticky; +++: a bit sticky; ++++: paste; +++++: very sticky
3.3.4. The effect of variations in the concentration of Irgacure®
2959 on the degree of crystallinity (XRD)
XRD
is used to identify crystallites in materials using X-ray diffraction radiation
to identify crystallite structures that appear as sharp or amorphous peaks (Hakim et al., 2019). The results of the XRD graph
can be seen in Figure 4. Based on Figure 4, the graphic pattern of
chitosan and alginate does not have a crystal peak, indicating that chitosan
and alginate are amorphous materials. Furthermore, the chitosan-alginate graph
pattern shows amorphous results with comprehensive graphs without clear crystal
peaks. In contrast, the Irgacure® 2959 graph pattern shows the
presence of crystallinity indicated by sharp peaks, and based on calculations,
Irgacure® 2959 has a crystallinity percent of 63.39%. The addition
of Irgacure® 2959 to chitosan-alginate showed a crystal peak seen at
a sharp peak, indicating that the presence of Irgacure® 2959 caused
cross-linking between chitosan and alginate. The percentage of crystallinity
obtained after the calculation is 26.21%.
This
value is lower than Irgacure® 2959 because of the cross-linking that
occurs with chitosan-alginate. The percent crystallinity was obtained by
calculating the area fraction of the crystalline material divided by the total
area of ??the amorphous and crystalline materials. The results of mixing
chitosan-alginate hydrogel with Irgacure® 2959 decreased
crystallinity, indicating that there had been an interaction between
chitosan-alginate and Irgacure® 2959, which was supported by the
research of Cervera et al. (2004), which
states that the decrease in intensity can indicate that there is an interaction
that occurs between chitosan-alginate. Based on the XRD results, the addition
of Irgacure® 2959 to the chitosan-alginate hydrogel causes
crystallinity to occur and indicates that there has been a bond between
chitosan-alginate and Irgacure® 2959.
Figure 4 Graph of Crystallinity Degree (XRD) Pattern (a) Alginate; (b) Chitosan; (c) Irgacure® 2959; (d) Chitosan-Alginate; (e) Chitosan-Alginate-Irgacure® 2959
3.3.5. Antibacterial
activity testing aims to determine the ability of the hydrogel to inhibit
bacterial growth.
Characteristics
of antibacterial activity were carried out only to find out whether the
hydrogel still had antibacterial properties derived from chitosan after being
mixed with alginate and Irgacure® 2959. According to Surjowardojo
et al. (2015), the diameter of the inhibition zone formed at 5mm falls
into the weak category. The results of the inhibition zone with the addition of
Irgacure® 2959 treatment ranged from 0% to 4%, were included in the
weak category because of < 5 mm. The mean value of the diameter of the
bacterial inhibition zone on the chitosan-alginate hydrogel with the addition
of Irgacure® 2959 is presented in Table 4.
Table 4 Average
zone of inhibition formed on Escherichia coli bacteria
|
The concentration of Irgacure®
2959 |
Inhibitor Zone (mm) |
|
Control negative |
0b |
|
Control positive |
4a |
|
0% |
1.7ab |
|
1% |
1.3ab |
|
2% |
1.3ab |
|
3% |
1.7ab |
|
4% |
1.7ab |
abcat each concentration, for different letters
showed statistically significant differences with a 95% confidence level
(p-value <0.05)
These results suggest that the addition of
Irgacure® 2959 influences the inhibitory ability of bacteria in
chitosan-alginate gel used as a tissue sealant. Still, between treatments,
Irgacure® 2959 with different concentrations did not produce a
significantly different effect. This happens because chitosan is bound to
alginate and Irgacure® 2959, so there is a reduction in the positive
charge on the chitosan group. Thus, the electrostatic interaction with the
negative amount on the bacterial cell wall decreases (Pasaribu,
2020). The NH3+ glucosamine content in chitosan,
which has a positive charge, will interact with the negative charge-forming
proteins on the bacterial cell membrane, which can cause damage to the outer
cell membrane and leakage of intracellular constituents of the bacteria so that
the bacteria will die (Nurainy et al., 2008).
The additional concentration of Irgacure®
2959 to the chitosan-alginate hydrogel can be used as a tissue sealant.
Increasing the concentration of Irgacure® 2959 results in a lower
swelling percentage and increases the hydrogel's resistance to water, so the
hydrogel is not easily brittle. Based on the characteristics, the best
concentration is Irgacure® 2959 4%.
This research is part of the thesis of
the first author. We would like to thank the
Faculty of Agriculture, Universitas Gadjah Mada for the funding that has been
provided through Lecturer Research Grant 2021.
Asadpour, S., Kargozar, S.,
Moradi, L., Ai, A., Nosrati, H., Ai, J., 2020. Natural Biomacromolecule Based Composite Scaffolds from Silk
Fibroin, Gelatin and Chitosan Toward Tissue Engineering Applications. International
Journal of Biological Macromolecules, Volume 154, pp. 1285–1394
Bagher, Z.I., Ehterami, A.,
Safdel, M.H., Khastar, H., Semiari, H., Asefnejad, A., Davachi, S.M., Mirzaii,
M., Salehi, M., 2020. Wound Healing with Alginate/Chitosan
Hydrogel Containing Hesperidin in Rat Model. Journal of Drug Delivery
Science and Technology, Volume 55, pp. 1–8
Bakshi, P.S., Selvakumar, D.,
Kadirvelu, K., Kumar, N.S., 2020. Chitosan as an
Environment Friendly Biomaterial – A Review on Recent Modi?cations and
Applications. International Journal of Biological Macromolecules, Volume
150, pp. 1072–1083
Barleany, D.R., Ananta, C.V., Maulina, F., Rochmat,
A., Alwan, H., Erizal, E., 2020. Controlled Release of Metformin Hydrogen
Chloride from Stimuli-responsive Hydrogel based on Poly (N-
Isopropylacrylamide)/Chitosan/Polyvinyl Alcohol Composite. International
Journal of Technology, Volume 11(3), pp. 511–521
Baysal. K., Aroguz, A.Z., Adiguzel,
Z., Baysal, B.M., 2013. Chitosan/alginate Cross-Linked
Hydrogels: Preparation, Characterization and Application for Cell Growth
Purposes. International journal of biological macromolecules, Volume 59,
pp. 342–348
Bektas, N., ?enel, B., Yenilmez,
E., Özatik, O., Arslan, R., 2020. Evaluation of Wound Healing Effect of
Chitosan-Based Gel Formulation Containing Vitexin. Saudi pharmaceutical
journal, Volume 28(1), pp. 87–94
Briawan, D., Sedayu, T.R., Ekayanti, I., 2011. Drinking Habits and
Fluid Intake of Adolescents in Urban Areas. Indonesian Journal of Clinical
Nutrition, Volume 8(1), pp. 36–41
Cervera, M.F., Heinämäki, J.,
Krogars, K., Jörgensen,
A.C., Karjalainen, M., Colarte, A.I., Yliruusi, J., 2004. Solid-state and Mechanical Properties of Aqueous
Chitosan-Amylose Starch Films Plasticized with Polyols. AAPS PharmSciTech, Volume
5(1), pp.109–114
Chandramouli, V., Kailasapathy, K.,
Peiris, P., Jones, M., 2004. An Improved Method
of Microencapsulation and Its Evaluation to Protect Lactobacillus Spp. in Simulated
Gastric Conditions. Journal of Microbiological Methods, Volume 56(1),
pp. 27–35
Dewi, A.H., Yulianto, D.K., Ana, I.D., Rochmadi,
Siswomihardjo, W., 2020. Effect of Cinnamaldehyde, an Anti-Inflammatory Agent,
on the Surface Characteristics of a Plaster of Paris – CaCO3 Hydrogel for Bone
Substitution in Biomedicine. International Journal of Technology, Volume
11(5), pp. 963–973
Hakim, L., Dirgantara, M., Nawir,
M., 2019. Characterization of The Material Structure of Group C Excavated Sand
Blocks Using X-Ray Diffraction (XRD) in The City of Palangkaraya. Jurnal
Jejaring Matematika dan Sains (Journal of Mathematics and Science Network),
Volume 1(1), pp 44–51
Han, W.T., Jang, T., Chen, S.,
Chong, L.S.H., Jung, H.D., Song, J., 2020. Improved Cell Viability for Large-Scale Biofabrication with
Photo-Crosslinkable Hydrogel Systems Through a Dual-Photo-Initiator Approach. Biomaterials
Science, Volume 8(1), pp. 450–461
Jarret, P, Coury, A., 2013.
Joining and Assembly of Medical Materrials and Devices. Woodhead publishing limited. USA, pp. 449–490
Khan, Y.A., Ozaltin, K.,
Bernal-Ballen, A., Di Martino, A., 2020. Chitosan-Alginate Hydrogels for Simultaneous and Sustained
Releases of Ciprofloxacin, Amoxicillin and Vancomycin for Combination Therapy. Journal
of Drug Delivery Science and Technology, Volume 61(5), pp 1–13
Lee, K.Y., Mooney, D.J., 2012.
Alginate: Properties and Biomedical Applications. Progress in polymer
science, Volume 37, pp. 106–126
Mahdavinia, G.R., Karimi,
M.H., Soltaniniya, M., Massoumi, B., 2019. In Vitro Evaluation of Sustaine
Ciproflfloxacin Release from ?-Carrageenan-Crosslinked Chitosan/Hydroxyapatite
Hydrogel Nanocomposites. International Journal of Biological Macromolecule, Volume
126, pp. 443–453
Mukherjee, D., Azamthulla, M.,
Santhosh, S., Dath, G., Ghosh, A., Natholia, R., Anbu, J., Teja, B.V.,
Muzammil, K.M., 2018. Development and Characterization
of Chitosan-Based Hydrogels as Wound Dressing Materials. Journal of Drug
Delivert Science and Technology, Volume 46, pp. 498–510
Nayeb, H.F., Salahshoor, K.S., Afshar, T.F.,
Shariatinia, Z., 2011. A Novel Topical
Biocompatible Tissue Adhesive Based on Chitosan-Modified Urethane Pre-Polymer. Irian
Polymer Journal, Volume 20(8), pp. 671–680
Nurainy, F., Rizal, S.,
Yudiantoro, 2008. Effect of Chitosan Concentration on Antibacterial Activity
Using the Agar Diffusion Method (Wells). Journal of Industrial Technology
and Agricultural Products, Volume 13(2), pp. 117–125
Ono, K., Saito, Y., Yura, H.,
Ishikawa, K., Kurita, A.,
Akaike, T., Ishihara, M., 2000. Photocrosslinkable Chitosan as a Biological Adhesive. Journal of
Biomedical Materials Research: An Official Journal of The Society for
Biomaterials and The Japanese Society for Biomaterials, Volume 49(2), pp. 289–295
Pasaribu, S.P., 2020. Synthesis of N-Maleoyl Chitosan Hydrogel - Dialdehyde Alginate for
Retentive Drug Delivery Systems. Disertation, Graduate Program, Faculty of Mathematics and Natural
Sciences, University of Northern Sumatra, Indonesia
Qi, Z., Xu, J., Wang, Z.,
Nie, J., Ma, G., 2013. Preparation and Properties of
Photo-Crosslinkable Hydrogel Based on Photopolymerizable Chitosan Derivative. International
Journal of Biological Macromolecules, Volume 53, pp. 144–149
Rudyardjo, D.I., 2014.
Effect of The Addition of Glycerol Plasticizer on The Characteristics of
Chitosan-Glutaraldehyde Hydrogel for Wound Dressing Applications. Jurnal
Ilmiah Sains (Scientific Journal of Science), Volume 14(2), pp.18–28
Sularsih. 2013. Effect of
The Viscosity of Chitosan Gel on Its Use in The Wound Healing Process. Journal
of Dentistry Materials, Volume 2(1), pp. 60–67
Sumarni, W., Prasetya, A.T.,
Rahayu, E.F., 2017. Effect of Glycerol Sumarni, W., A.T.
Prasetya, Dan E.F. Rahayu. 2017. Effect of Glycerol on Physical Properties of
Biofilms Gembili Starch (Dioscorea Esculenta)–Chitosan. In Proceeding
of Chemistry Conferences, Volume 2, pp. 56–65
Surjowardojo, P.,
Susilorini, T.E., Sitrait, G.R.B., 2015. Decoction Inhibition of Manalagi Apple
Skin (Malus Sylvestrs Mill.) on The Growth of Staphylococcus Aureus and Pseudomonas Sp. Causes of
Mastitis in Dairy Cows. Journal of Tropical Livestock, Volume 16(2), pp.
40–48
Tomal, W., Ortyl, J., 2020.
Water-soluble Photo-Initiators in Biomedical Applications. Polymers,
Volume 12(5), p. 1073
Wang, G., Wang, X., Huang,
L., 2017. Feasibility of Chitosan-Alginate (Chi-Alg) Hydrogel Used as Scaffold
for Neural Tissue Engineering: A Pilot Study in Vitro. Biotechnology &
Biotechnological Equipment, Volume 31(4), pp. 766–773
