Development of the Sterilization Box for Medical Equipment with an Ozone Gas Leak Sensor Feature
Corresponding email: tomy.abuzairi@gmail.com
Published at : 30 Dec 2022
Volume : IJtech
Vol 13, No 8 (2022)
DOI : https://doi.org/10.14716/ijtech.v13i8.6156
Abuzairi, T., Sumantri, N.I., Putri, N.A., Andarini, M.V., Lampung, E.J., Sitinjak, D., 2022. Development of the Sterilization Box for Medical Equipment with an Ozone Gas Leak Sensor Feature. International Journal of Technology. Volume 13(8), pp. 1672-1680
| Tomy Abuzairi | 1. Electrical Engineering, Department of Electrical Engineering, Faculty of Engineering, Universitas Indonesia, Depok, West Java, 16424, Indonesia, 2. Research Center for Biomedical Engineering, Facul |
| Nur Imaniati Sumantri | Biomedical Engineering, Department of Electrical Engineering, Faculty of Engineering, Universitas Indonesia, Depok, West Java, 16424, Indonesia |
| Nadira Alya Putri | Biomedical Engineering, Department of Electrical Engineering, Faculty of Engineering, Universitas Indonesia, Depok, West Java, 16424, Indonesia |
| Mulya Viani Andarini | Biomedical Engineering, Department of Electrical Engineering, Faculty of Engineering, Universitas Indonesia, Depok, West Java, 16424, Indonesia |
| Ebenhaezar Jan Lampung | Biomedical Engineering, Department of Electrical Engineering, Faculty of Engineering, Universitas Indonesia, Depok, West Java, 16424, Indonesia |
| Defrianto Sitinjak | Biomedical Engineering, Department of Electrical Engineering, Faculty of Engineering, Universitas Indonesia, Depok, West Java, 16424, Indonesia |
One of the efforts to control the growth of
microorganisms is sterilization. The sterilization process can use ozone gas,
which is a triatomic form of the element oxygen. Ozone acts as an oxidizing
agent capable of destroying the structure of bacterial cell walls, and their
molecules are unstable and easily decomposed into oxygen, so that ozone can be
applied in sterilization technology for medical devices. In this work, a
sterilization box with an ozone generator has been designed with a MQ-131 ozone
sensor as an indicator if there is a leak in the box. The output voltage of the
ozone generator is 4 kV with a current of 30 mA. We developed an instrument
using an Arduino nano as a microcontroller for reading sensor values and
displaying sensor values on an LCD monitor. For testing the sterilization
effect of the ozone box, a Staphylococcus aureus bacterial sample was
used. For the sterilization object, we designated stethoscopes and thermometers
as medical equipment. The optimum time for sterilizing medical devices on the
sterilization ozone box was 20 minutes, which can reduce the colony of S.
aureus bacteria with an ozone concentration of 4.94 ppm.
Leak sensor; Microcontroller; Medical equipment; Ozone; Sterilization; Staphylococcus bacteria
Ozone (O3) is a strong oxidizing agent and a triatomic
compound of the element oxygen (O). Ozone is also an unstable compound that
quickly decomposes at ambient temperature (Ebbing & Gammon, 2017). The sterilization process
using ozone occurs through a direct oxidation process. Ozone oxidation has the
ability to destroy cell membranes and the outer walls of microorganism cells,
leading to cell dead (Takayama et
al., 2006). Ozone is often used as a sterilization method because it is
oxidative and easy to decompose, among others, to eliminate bacteria in the
soil/soil sterilization. It is applied in the agricultural field because ozone
can kill bacteria, viruses, and fungi in the soil as well as in irrigation
systems, and then ozone
will decompose into oxygen (O2) (Suryawan et al., 2021; Munarso
et al.,
Ozone production can be carried
out in various ways, such as through electrochemical reactions, UV irradiation
methods, and plasma technology. Plasma is the most abundant of these various methods (Suksri et al., 2009).
If the temperature continues to be raised, the atoms will decompose into
charged particles that move freely (electrons and positive ions) and enter the
plasma state (Bellan, 2008).
Plasma also be used as a sterilization method for medical devices using Plasma
Activated Water (PAW) with some modifications to its physicochemical
properties, such as pH, temperature, ORP, and nitrite concentration (Abuzairi et al., 2018).
Moreover,
UVC was employed for room sterilization and disinfection (Rusdinar et al., 2021). ?The authors claim that the UVC
can reduce and kill airborne bacteria; therefore, it can disinfect different
types of rooms (e.g., isolation, operating, and public rooms) contaminated with
hazardous bacteria, such as COVID-19 (Rusdinar et al., 2021; Berawi, 2021).
Recently, ozone has also been
applied in some hospitals as a method for sterilizing medical equipment and
other equipment in hospitals because it has been proven effective against
vegetative bacteria, fungi, mycobacteria, and bacterial spores (Dufresne et al., 2004). In the
food sector, ozone is produced in dielectric barrier discharge plasma (DBDP)
and then applied to maintain the quality of amino acids in fish (Sosiawati et
al., 2014). In addition, ozone could be a hand sanitizer using air
and tap water enriched by hydrogen peroxide (El Shaer et al., 2017). Ozone can be used to combat
coronaviruses both inside and outside the body (Manjunath et al., 2021).
Ozone is very
useful for sterilization; however, at high concentrations, it is also harmful
to humans. From previous studies on the design of sterilization instrumentation
using ozone gas, no study has developed sterilization instrumentation with an
ozone gas leak sensor. Therefore, in this study, we developed a sterilization
box for medical equipment with an ozone gas leak sensor feature.
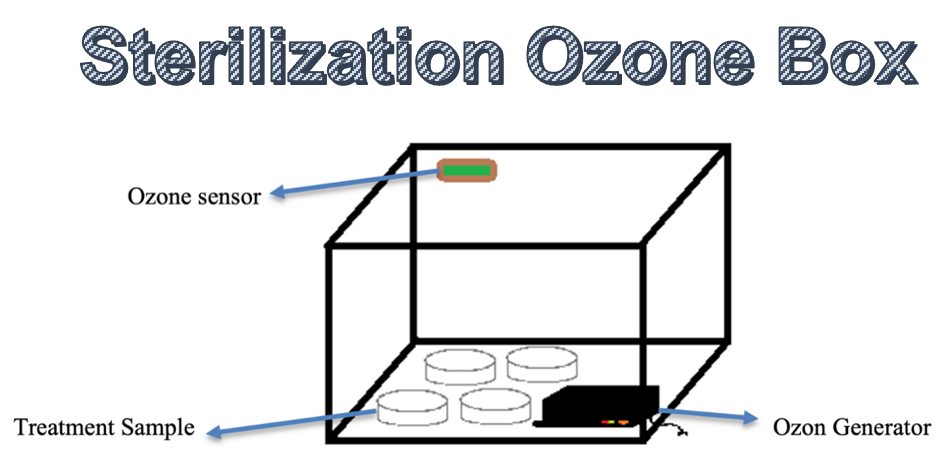
2.1. Ozone sterilization box
The sterilization box is shown in
Figure 1. The ozone generator uses a Dielectric Barrier Discharge (DBD)
configuration. The ozone generator will be put in a sterilization box, which
functions as a safety so that ozone is not inhaled or contaminates other objects.
The ozone
generator and samples will be put into the storage box, and then a hole will be
made as an outlet for the cable. This ozone sterilization box is also connected
to a timer relay and switch to make it easier for users to set the duration of
the ozone generator’s activation.
Figure 1 Sterilization box with ozone gas leak sensor feature
2.2. Ozone gas leak sensor circuit
The ozone sensor MQ-131 is
connected to the Arduino nano, as shown in the schematic in Figure 2. The sensitive
material of the MQ-131 ozone sensor is a metallic oxide semiconductor, which
has high conductivity in clean air. When ozone gas is present, the sensor’s
conductivity decreases as the gas concentration increases. Aside from sensitivity to specific ozone, the MQ-131
sensor also has a sensitivity to strong oxides, Cl2 and NO2.
In order to work optimally, it requires preheating for approximately 48 hours.
The MQ-131 sensor can be applied as a domestic or industrial ozone
concentration alarm and can also be used as a portable ozone concentration
detector. The MQ-131 ozone sensor has a long life, easy to apply and is
flexible enough to be connected to a microcontroller for various applications.
The MQ-131 ozone sensor circuit is added outside the sterilization box and then connected to the LCD to be able to monitor the level of ozone that comes out/leaks during the treatment process. This is done as a safety system when a leak occurs or the box is not tightly closed. This sensor was also used in other Covid-19 research for controlling ozone concentration (Dave et al., 2020).
Figure 2 Schematic of ozone gas leak sensor circuit
2.3. Ozone concentration measurement
A plasma
generator is connected to a high voltage generator at the DBD to see the glow discharge and
ozone produced. Ozone concentration is calculated quantitatively with an air
quality monitor the Zuidid DM502-O3 where there is one feature that can measure
ozone concentration in ppm units.
2.4. Validation of ozone sterilization
In this study, we used a Staphylococcus aureus bacterial sample to validate the sterilization effectiveness of ozone. S. aureus is a Gram-positive bacterium in the form of a coccus. When the ozone generator circuit has been designed, the next step is to test the effectiveness against staphylococcus bacteria on medical equipment, which is stethoscopes and thermometer guns (Abuzairi et al., 2021). Bacterial samples were taken from a stethoscope and thermometer, which were then swabbed and planted in Mannitol Salt Agar (MSA) and Plate Count Agar (PCA) media. Samples that have been planted are then treated using an ozone generator, which is designed with a treatment duration of 5 minutes, 10 minutes, and 20 minutes. Samples that have been treated will then be cultured for two days, and after counting the bacterial colonies formed, they will be counted and analyzed. In the treatment method, the device is put into a sterilization box to be treated according to the optimal time obtained in the first experiment, then cultured in the media for 2 days, and the bacterial colonies that form will be counted and analyzed. Figure 3 shows the stages of bacterial sterilization.
Figure 3 Stages of
bacterial sterilization with an ozone sterilization box. (a)-(b)
Medical equipment is swabbed, and the sample is stored in a transport medium.
(c) The sample is planted on a PCA/MSA plate. (d) the sample-containing plate
is treated with ozone. (e) The plate is incubated at 36°C for 48 hours. (f)-(g)
Colony counting and cell observation under the microscope
3.1. Ozone generator
Figure 4(a) shows the
device before it is electrified, and Figure 4(b) shows it after it is
electrified. When electrified, a glow discharge appears on the dielectric
barrier, indicating a plasma discharge process. This is in accordance with the
parameters for the ozone generator, which are indicated by the presence of a
glow discharge when electrified. In addition, there is a distinctive smell of
ozone. Based on the technical specifications in the circuit and the simulation
results, the output voltage is 4 kV with a current of 30 mA. This circuit will
be measured ozone concentration and designed for a sterilization box.
Figure 4 (a) Ozone generator not electrified. (b)
Ozone generator electrified
3.2. Ozone sensor
The ozone gas leak sensor circuit is attached to the upper of the sterilization box, as shown in Figure 5(a). The MQ-131 sensor could detect the ozone concentration up to 1000 ppm, with a sensitivity (ozone/air) is 2 in 50 ppm ozone (Zhengzhou Winsen Electronics Technology, 2021). The side of the storage is perforated for cable output, which will be connected to switches and timers to make it easier to turn off and turn on the device because it would be better if during the treatment process, there were no people in the treatment room and there is air circulation in the room to anticipate if something happened like an ozone gas leak. On the outside of the front sterilization box, a series of sensors MQ-131 are provided with an LCD Figure 5(b) to monitor during treatment if there is ozone leaking from inside the box, as shown in Figure 5(c). This is added because, according to WHO regulations and some countries limits, the ozone concentration in a room should not be more than 0.1 ppm. Human exposure to ozone is primarily by inhalation, but reactions on the skin are also reported (Salonen et al., 2018). Acute and chronic health effects and the contributions of ozone to morbidity and mortality are summarized in WHO documents (WHO, 2021).
Figure 5 (a) Inside of the sterilization box with the
generator. (b) Sensor MQ-131 outside the box. (c) Ozone sensor circuit MQ-131
3.3. Ozone concentration
Figure 6 shows ozone concentrations for (a) 5 minutes, (b) 10 minutes, and (c) 20 minutes in the sterilization ozone box. In Figure 6(a), the duration of the ozone generator is 5 minutes, the concentration of ozone formed reaches 1.65 ppm, and the duration of the decomposition of ozone into O2 takes 3 minutes. For Figure 6(b) with the generator running for 10 minutes, every minute the ozone concentration increases linearly until it reaches its maximum point for 10 minutes with an ozone concentration of 4.01 ppm after the ozone generator is turned off and it takes 4 minutes to decompose all the ozone. Furthermore, in Figure 6(c), when the ozone generator is turned on for 20 minutes, the ozone concentration gradually increases until it reaches a concentration of up to 4.92 ppm. Then, to be able to decompose as a whole it takes about 6 minutes. Previous research conducted by (Ma’ruf et al., 2017) and (Waluyo et al., 2015) also showed this trend, in which ozone concentration will increase with the duration of treatment.
Figure 6 Ozone Concentration for: (a)
5 minutes; (b) 10 minutes; and (c) 20 minutes
In
the three variations of duration, the increase in ozone concentration was
stable until it reached a concentration of 4 ppm and slowed down. The
concentration of ozone is known to be in line with duration and the increase of
voltage to produce ozone (Vezzu et al., 2009). On the other hand, the
concentration of ozone produced also refers to the oxygen around the generator.
A previous study said ozone generators with a dry air source and an oxygen
source directly experienced differences in the concentrations produced. Ozone
produced by oxygen sources is more and more concentrated, as much as 7–10% (Teke et al., 2014). In this
research, the source in the form of ambient air is not supplied with oxygen
directly, and when it reaches a concentration of 4 ppm and a duration of 17
minutes, the increase in ozone concentration slows down. This is because the
amount of oxygen that was in the sterilization box is decreasing, and because
the box is closed, there is no incoming oxygen supply, so the oxygen
concentration produced is stuck at four ppm.
3.4. Results of sterilization treatment of medical
equipment
The results of the treatment
process are presented in Table 1 that sterilizes several
medical equipment’s, namely a stethoscope and thermometer gun. The
results showed that when each of these devices was not treated, bacterial
colonies formed above 300 CFU, or it can be said to be Too Numerous to Count
(TNTC). After that, each medical device was treated and put into a
sterilization box for 20 minutes according to the optimal time in the previous
experiment, then planted in two different media (PCA and MSA) and cultured; the
number of bacterial colonies was reduced to below 50 CFU. It can be concluded
that within 20 minutes, with the concentration of ozone produced, ozone can
damage the walls of bacteria in each medical device and cause the bacteria to
lyse or die. Previous research by (Botelho-Almeida
et al., 2018), (Verma et
al., 2016), and (Fu et al., 2020) with ozone
treatment to reduce bacteria in medical devices was also successful.
Table 1 Results of sterilization
treatment of the medical equipment
|
Medical
equipment |
Duration
(minutes) |
Colonies
formed (CFU) | |
|
PCA |
MSA | ||
|
Stethoscope |
0 |
TNTC |
32 |
|
5 |
126 |
26 | |
|
10 |
64 |
18 | |
|
20 |
36 |
10 | |
|
Thermometer gun |
0 |
TNTC |
7 |
|
5 |
134 |
6 | |
|
10 |
76 |
4 | |
|
20 |
36 |
1 | |
Ozone is considered to be a disinfectant agent in the medical-related
field for its bactericidal activity (Botelho-Almeida et al., 2018; Giuliani et al., 2018). The way it kills the
bacteria is by disrupting the bacteria's cell membrane. Glycoprotein and
glycolipid components of the cell membrane are the target of ozone properties,
resulting in cell rupture. Protein and nucleotide disturbances are the other mechanism
that occurs due to ozone exposure. Cellular enzymatic activity is affected by
the ozone attack on the sulfhydryl groups of certain enzymes, leading to a loss
of function. Ozone also attacks nucleic acid bases,
resulting in DNA damage (Megahed et al., 2018). The previous study
revealed the effect of ozone on membrane cell disruption leading to cell lysis
in both Gram-positive and Gram-negative bacteria (Thanomsub et al., 2002).
Figures 7-8
show samples in PCA and MSA media after incubation. In PCA media, the
growth of general bacterial colonies can be seen, while MSA media show certain
bacterial colonies. MSA media is useful for growing bacterial colonies of S.
aureus (Sharp
& Searcy, 2006). There was a very significant decrease in colonies in the treatment
with a duration of 20 minutes, Petri dishes given treatment in 5 minutes had
the number of colonies TNTC (>300) on PCA media, while in MSA media colonies
ranged under 50 colonies, this is because MSA is a selective and differential
growth medium for Gram-positive bacteria, so that Gram-negative bacteria could
not grow. When the duration of exposure is increased to 10 minutes, the number
of colonies again decreases until only tens or dozens remain. At 20 minutes of
exposure, it can be seen that the number of colonies decreases to near zero.
|
|
|
|
Figure 7 Bacterial colonies on PCA media
after treatment for 5, 10, and 20 minutes (a,b,c)
|
|
|
|
Figure 8 Bacterial colonies on MSA media
after treatment for 5, 10, and 20 minutes (a,b,c)
The sterilization box for
medical equipment with an ozone gas leak sensor feature has been successfully
developed. The sterilization box with an ozone generator has been designed with a
MQ-131 ozone sensor as an indicator if there is a leak in the box. In testing the effectiveness of ozone treatment
against S. aureus,
the optimal duration was 20 minutes with an ozone concentration of 4.92 ppm,
which significantly reducing the number of colonies compared to a shorter
duration. Further testing was carried out on two medical equipment, namely a
stethoscope and a thermometer gun, with a duration of 20 minutes, and the
results showed very significant sterilization, with bacterial colonies formed
after the treatment process being below 50 CFU.
This research was supported by Kedaireka Grant
2022 from Ministry of Education, Culture, Research and Technology Republic of
Indonesia and Research Grant PUTI Q3 2020 (NKB-4567/UN2.RST/HKP.05.00/2020)
from the Directorate Research and Development Universitas Indonesia.
Abuzairi,
T., Ramadhanty, S., Puspohadiningrum, D.F., 2018. Investigation on
Physicochemical Properties of Plasma activated Water for The Application of
Medical Device. In AIP Conference Proceedings, Volume 1933, p. 040017
Abuzairi,
T., Sumantri, N.I., Irfan, A., Mohamad, R.M., 2021. Infrared Thermometer on the
Wall (Ithermowall): An Open Source and
3-D Print Infrared Thermometer for
Fever Screening. HardwareX, Volume 9, p. e00168
Bellan,
P.M., 2008. Fundamentals of Plasma Physics. Cambridge University Press,
Cambridge, England
Berawi,
M.A., 2021. World Agenda on Sustainable Recovery from the Covid-19 Pandemic:
Recover Together, Recover Stronger. International Journal of Technology,
Volume 12(4), pp. 671–675
Botelho-Almeida,
T.d.S., Lourenço, F.R., Kikuchi, I.S., Awasthi, R., Dua, K., Pinto, T.d.J.A.,
2018. Evaluating the Potential, Applicability, and Effectiveness of Ozone
Sterilization Process for Medical Devices. Journal of Pharmaceutical
Innovation, Volume 13, pp. 87–94
Dave,
N., Pascavis, K.S., Patterson, J., Kozicki, M., Wallace, D., Chowdhury,
A., Abbaszadegan, M., Alum, A., Herckes,
P., Zhang, Z., Chang, J., Ewell, C., Smith, T., Naufel, M., 2020.
Characterization of a Novel, Low-Cost, Scalable Ozone Gas System for
Sterilization of N95 Respirators and Other COVID-19 Related Use Cases. MedRxiv, https://doi.org/10.1101/2020.06.24.20139469
Dufresne,
S., Hewitt, A., Robitaille, a.S., 2004. Ozone Sterilization: Another Option for
Healthcare in the 21st Century. American Journal of Infection Control,
Volume 32(3), pp. 3–4
Ebbing,
D., Gammon, S. D., 2016. General Chemistry. Cengage Learning, Boston, USA
El
Shaer, M., Mobasher, M., Zaki, A., 2017. Low-Cost Dielectric Barrier Discharge
Plasma Hand Sanitizer Using Air and Tap Water Enriched by Hydrogen Peroxide. Plasma
Medicine, Volume 7(3), pp. 273–282
Fu,
L., Le, T., Liu, Z., Wang, L., Guo, H., Yang, J., Chen, Q., Hu, J., 2020.
Different Efficacies of Common Disinfection Methods Against Candida
Auris and Other Candida Species. Journal of
Infection and Public Health, Volume 13(5),
pp. 730–736
Giuliani,
G., Ricevuti, G., Galoforo, A.,
Franzini, M., 2018. Microbiological Aspects of Ozone: Bactericidal Activity and
Antibiotic/Antimicrobial Resistance in Bacterial Strains Treated with Ozone. Ozone
Therapy, Volume 3(3), p. 7971
Manjunath,
S.N., Sakar, M., Katapadi, M., Balakrishna, R.G., 2021. Recent Case Studies on
the Use of Ozone to
Combat Coronavirus: Problems and perspectives. Environmental Technology
& Innovation, Volume 21, p. 101313
Ma’ruf,
A., Dewi, S., Wardoyo, F., 2017. Time of Exposure to Ozone Gas on the Growth of
Escherichia Coli Bacteria. In: Seminar Nasional Pendidikan, Sains dan
Teknologi, pp. 1–5
Megahed,
A., Aldridge, B., Lowe, J., 2018. The Microbial Killing Capacity
of Aqueous and Gaseous Ozone on Different Surfaces Contaminated with Dairy
Cattle Manure. The Microbial Killing Capacity of Aqueous and Gaseous Ozone on
Different Surfaces Contaminated with Dairy Cattle Manure. PLoS One,
13(5), p. e0196555
Munarso,
S.J., Kailaku, S.I., Arif, A.B., Budiyanto, A., Mulyawanti, I., Sasmitaloka,
K.S., Setyawan, N., Dewandari, K.T., Widayanti, S.M., 2020. Quality Analysis of
Chili Treated with Aqueous Ozone Treatment and Improved Transportation and
Handling Technology. International Journal of Technology, Volume 11(1),
pp. 37–47
Paw?at,
J., Stryczewska, H.D., Ebihara, K., 2010. Sterilization Techniques for Soil
Remediation and Agriculture Based on Ozone and AOP. Journal of Advanced
Oxidation Technologies, Volume 13, pp. 138–145
Rusdinar,
A., Purnama, I., Fuadi, A.Z., Adiluhung, H., Wicaksono, M., Risnanda, N., 2021.
Automated Ultraviolet C Light Mobile Robot for Room Sterilization and
Disinfection. International Journal of Technology, Volume 12(4), pp. 854–864
Salonen,
H., Salthammer, T., Morawska, L., 2018. Human Exposure to Ozone in School and
Office Indoor Environments. Environment International, Volume 119, pp.
503–514
Sharp,
S.E., Searcy, C., 2006. Comparison of Mannitol Salt Agar and Blood Agar Plates for Identification and Susceptibility Testing of Staphylococcus Aureus in Specimens from
Cystic Fibrosis Patients. Journal of
Clinical Microbiology,
Volume 44(12), pp. 4545–4546
Suksri,
A., Karnchanalekha, K., Tonmitra, K.,
Apiratikul, P., 2009. A comparative study on suitable high voltage sources for
ozone generation. In: 2009 6th International Conference on
Electrical Engineering/Electronics, Computer, Telecommunications and
Information Technology, IEEE, Volume 1, pp. 296–299
Suryawan,
I.W.K., Septiariva, I.Y., Helmy, Q., Notodarmojo, S., Wulandari, M., Sari,
N.K., Sarwono, A., Pratiwi, R., Lim, J., 2021. Comparison of Ozone
Pre-Treatment and Post-Treatment Hybrid with Moving Bed Biofilm Reactor in
Removal of Remazol Black 5. International Journal of Technology, Volume 12(4),
pp. 728-738
Takayama,
M., Ebihara, K., Stryczewska, H., Ikegami, T., Gyoutoku, Y., Kubo, K.,
Tachibana, M., 2006. Ozone Generation by Dielectric Barrier Discharge for Soil Sterilization. Thin Solid Films,
Volume 506, pp. 396–399
Thanomsub,
B., Anupunpisit, V.,
Chanphetch, S., Watcharachaipong, T., Poonkhum, R., Srisukonth, C., 2002.
Effects of Ozone Treatment on
Cell Growth and Ultrastructural Changes in Bacteria. The Journal of General
and Applied Microbiology, 48(4), pp. 193–199
Teke,
S., Nur, M., Winarni, T., 2014. Ozone Generator by Using Dielectric Barrier
Discharge Plasma Technology with Spiral-Cylinder Configuration: Comparison
Between Oxygen and Air as Sources. Berkala Fisika, Volume 17, pp. 25–32
Verma,
K., Gupta, D., Gupta, A., 2016. Optimization of Ozone Disinfection and Its
Effect on Trihalomethanes. Journal of Environmental Chemical Engineering,
Volume 4(3), pp. 3021–3032
Vezzu,
G., Lopez, J., Freilich, A., Becker, K., 2009. Optimization of Large-Scale Ozone Generators. IEEE Transactions
on Plasma Science, Volume 37(6),
pp. 890–896
World Health Organization (WHO),
2021. WHO Global Air Quality Guidelines: Particulate Matter (?PM2.5 And PM10)?,
Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. World Health Organization
Waluyo,
W., Permana, D.A., Saodah, S., 2015. Design and Realization of Ozone Generator
Using Alternating High Voltage Generation Method. Jurnal Teknik Energi
Elektrik, Teknik Telekomunikasi, & Teknik Elektronika, Volume 3, p. 38
Zhengzhou
Winsen Electronics Technology, 2021. MQ131 High Concentration Ozone Gas Sensor.
Avalaible Online at: https://www.winsen-sensor.com/sensors/o3-gas-sensor/mq131-h.html,
Accessed on 16 March 2022
