Design of a Transforaminal Lumbar Interbody Fusion (TLIF) Spine Cage
Corresponding email: yudan.whulanza@ui.ac.id
Published at : 30 Dec 2022
Volume : IJtech
Vol 13, No 8 (2022)
DOI : https://doi.org/10.14716/ijtech.v13i8.6152
Faadhila, A., Rahman, S.F., Whulanza, Y., Supriadi, S., Tampubolon, J.Y., Wicaksana, S.I., Rahyussalim, A.J., Kurniawati, T., Abdullah, A.H., 2022. Design of a Transforaminal Lumbar Interbody Fusion (TLIF) Spine Cage. International Journal of Technology. Volume 13(8), pp. 1663-1671
| Afrah Faadhila | Biomedical Engineering Study Program, Department of Electric Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, West Java 16424, Indonesia |
| Siti Fauziyah Rahman | 1. Biomedical Engineering Study Program, Department of Electric Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, West Java 16424, Indonesia, 2. Research Center for Biomedic |
| Yudan Whulanza | 1. Research Center for Biomedical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, West Java 16424, Indonesia, 2. Department of Mechanical Engineering, Faculty of Engineer |
| Sugeng Supriadi | 1. Research Center for Biomedical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, West Java 16424, Indonesia, 2. Department of Mechanical Engineering, Faculty of Engineer |
| Joshua Yoshihiko Tampubolon | PT Trafas Dwi Medika, Jakarta 13920, Indonesia |
| Septian Indra Wicaksana | PT Trafas Dwi Medika, Jakarta 13920, Indonesia |
| Ahmad Jabir Rahyussalim | Department of Orthopaedic and Traumatology, Cipto Mangunkusumo National Central General Hospital and Faculty of Medicine, Universitas Indonesia, Jakarta 10430, Indonesia |
| Tri Kurniawati | Stem Cell and Tissue Engineering Cluster, Indonesian Medical Education and Research Institute, Faculty of Medicine, Universitas Indonesia, Jakarta 10430, Indonesia |
| Abdul Halim Abdullah | Biomechanical & Clinical Engineering Research Group, College of Engineering, Universiti Teknologi Mara, Malaysia |
Lumbar Interbody Fusion is a technique used to treat
various spinal disorders, which has many types, such as the Transforaminal
Lumbar Interbody Fusion (TLIF) Technique. With TLIF being one of the most
well-known techniques, which many spinal surgeons are trained and skilled at,
there are various types of TLIF Spine Cages available on the market. In this
paper, we designed a TLIF Cage and compared the simulation's analysis with the
prototype's experimental testing. The design was developed using the reverse
engineering method, and findings on the jaws profile and other design
considerations through literature review. The design was then analyzed through
a simulated compression test using Ansys Software. The simulation showed that
the designed TLIF spine cage in this paper can withstand the force usually
given to an implanted lumbar spinal cage.
3D Design; Interbody Fusion; Spine Cage; TLIF implant
Patients increasingly suffer spinal injuries due to accidents or incorrect movement positions in athletes. The loss or reduced function of the spinal disc to support the spine and maintain foraminal height is one of the most common injuries. This injury can cause the narrowing of the spinal canal, or degenerative lumbar spinal stenosis, which affects the patient’s movement (Lee et al., 2020). If not properly treated, this disease can lead to ischemia and chronic pain (Lee et al., 2020). Several types of treatment can be given to patients, ranging from therapeutic testing with injections for minor injuries, combining medications with physical therapy, for spine cage implant surgery using the lumbar interbody fusion technique (Hennemann & de Abreu, 2021; Mobbs et al., 2015). The Lumbar Interbody Fusion Technique treats various spinal disorders, including degenerative pathologies, trauma, infection, and neoplasia. This technique is done by inserting an implant, a cage, a spacer, or a structural graft, into the intervertebral space using various approaches (Mobbs et al., 2015).
There are
several spine cage implant options available that differ in their geometry
depending on the approach for insertion. First is Posterior Lumbar Interbody
Fusion (PLIF) which many spinal surgeons are well-trained to use. It provides
better nerve root visualization than other cages. But it also requires high
neural retraction. Then there is Anterior Lumbar Interbody Fusion (ALIF) as the
most efficacious and predominant treatment for discogenic low back pain which
can maximize implant size and surface area. But ALIF insertion surgery can
cause some complications, such as retrograde ejaculation, and visceral and
vascular injury. For sagittal and coronal deformity correction, lateral lumbar
interbody fusion (LLIF) and oblique lumbar interbody fusion (OLIF) are
suitable. Both cages can be performed with rapid postoperative mobilization and
aggressive deformity correction. These cages, unfortunately, can cause lumbar
plexus, psoas, bowel, and vascular injuries. The last cage type is
Transforaminal Lumbar Interbody Fusion (TLIF).
This technique is the best for stabilization and treatment of
degenerative lumbar disease following failed conservative treatment (Rahyussalim et al., 2017).
Despite the disadvantages, such as paraspinal iatrogenic injury from prolonged
muscle retraction, it can still perform direct and unilateral access to the
intervertebral foramen and preserve ligamentous structures. This access can
reduce direct dissection, the prior chance of damaging back muscles and the
thecal sac, minimize bleeding, and improve postoperative recovery (Hammad et al.,
2019; Mobbs et al., 2015).
The neural foramen is also opened on one side only, so damage to the nerves is
less compared to the other technique (Mobbs et al., 2015). With all
those advantages, TLIF has become one of the most commonly used techniques for
Disc Degeneration Disease treatment and is worth developing.
2.1.
Determining the Size and 3D model of the TLIF Spine Cage
where:
UVW : upper vertebral width
UVD : upper vertebral depth
DH : disc height
PH : pedicle height
PDW : pedicle width
The UVW, UVD, DH, PH and PDW will be acquired from
obtained data from previous research.
2.2.
Prototyping of the TLIF spine scage
In order to execute the 3D design of the spine cage, an
additive manufacturing technique commonly known as stereolithography (SLA) was
conducted. A Photon Mono X (Anycubic, Shenzhen, China) with LCD-based SLA
technology that has faster printing speeds and a larger volume was utilized. The previously determined solid model file in.stl
format was now ready to be transferred to the SLA machine. The Photon Mono-X
machine uses a
bio-photopolymer resin mixed with bio-poly lactic acid from eSUN (Shenzhen, China). The PLA-resin has a low viscosity and has
mechanical properties as detailed in Table 1.
Table 1 Mechanical Properties of
eResin-PLA at 25?C
2.3.
Numerical simulation of TLIF spine cage
A finite element analysis (FEA) was conducted using ANSYS
2022 workbench software (2022 R2 version, Canonsburg, Pennsylvania, USA). In
the FEA simulation, the spine cage was assumed to be isotropic (Ahmad et al.,
2020). The material that is being simulated for the spine cage is
polyetheretherketone (PEEK). The data shows that this material has a
compressive strength of 120-300 MPa (depending on the molecular weight) and
elongation of a break at around 1.6-43%.
The environmental simulation needed the mechanical properties
of the PLA-resin materials. Therefore, a compressive test was conducted to
acquire the mechanical parameters of the PLA-resin material. A 3D printed block
with the size of 20mm x 10mm x 10mm was prepared as the testing material. The
compression was conducted using the universal testing machine MCT-2150 from
A&D Company (Tokyo, Japan). The compression rate was set at 10 mm/min.
3.1. Size
Determination and 3D Model of the Spine Cage Implant
Figure
1 The image processing result of Indonesian Lumbar
dimension in sagittal plane (a) and axial plane (b)
Table 1 Lumbar Dimension Indonesian Lumbar dimension in sagittal plane (a)
and axial plane (b)
|
Parameters |
Spine Section- L4 |
Spine Section- L5 |
Units |
|
UVW |
46 |
48 |
mm |
|
UVD |
33 |
33 |
mm |
|
DH |
11 |
10 |
mm |
|
PH |
13 |
12 |
mm |
|
PDW |
10 |
13 |
mm |
Spine cage sizes were derived from the dimensions of the
above spine section. Note that the spine cage was not designed to cover all the
spine areas. The spine cage was designed to be as small as possible compared to
that spine section, but the cage must withstand the load from the human body.
Based on our study, we converted the dimension of TLIF spine cages to adapt a
minimum insertion size at around 8 mm. The formula to calculate the length (L),
Width (W), Height (H), and Lordosis Angle (LA) of the TLIF spine cages are
given in Equations 1, 2, 3, and 4 based on L4 sizes. The L was calculated to
adjust the length area of the cages that can cover the spine area. The W was
arranged from the PDW size as the insertion side of the cage. The LA was
formulated from the measure of the lordotic angle between two lumbar bodies.
Besides the dimension and angle of the
cage, it is known that several factors might influence the biomechanical stability of a lumbar
interbody spine cage construct, such as geometry, contact area, and integrated
fixation (Triwardono
et al., 2021). Therefore, in this paper, we designed a
banana-shaped spine cage with a slanted side to facilitate TLIF placement.
Based on the lumbar morphometry, formulas, and those biomechanical stability
factors, geometry, and sizes of the spine cage design are shown in Table 2.
Table 2 Design Fixture of Spine Cage
|
Fixture |
Metrics |
Units |
|
Length |
27.7 |
mm |
|
Width |
8 |
mm |
|
Height |
11 |
mm |
|
Lordosis Angle |
7 |
degree |
|
Jaws Shape |
Pyramid |
-- |
|
Slanted Side |
36.5 |
degree |
|
Window Holes |
3 |
pieces |
By following the lumbar morphometry and biomechanical stability factors in Table 2, the TLIF spine cage design is shown in Figure 2. The spine cage consists of a vertical middle hole and two horizontal holes to insert bone graft materials (Figure 2c). The banana-shaped facilitated the placement of the implant through the posterior side. The designed implant has slightly different measurements compare to those available on the market. The length of the designed implant was 27.7 mm and had an angle of 7.27 degrees.
Figure
2 The 3D design of spine cage considering the
adjustment of size and features of bone graft holes and pyramid jaws: a) top
view; b) side view and perspective view
The fixation for this implant is designed to use a pedicle screw
fixation, as an integrated screw fixation is usually not enough to give the
biomechanical stability needed for a spine cage implant. For the contact area,
the profile of the jaws was made to increase friction and limit the micromotion
of the spine cage. Increasing the surface roughness of the spine cage is suitable
for fixating the implant to the bone (Triwidodo et
al., 2021). Therefore, we designed the surface area equipped with a pyramid
jaws profile to gain a better osteogenic process. with bone graft than a simple one-type jaws
profile design. The pyramid jaws are depicted in Figure 2c.
3.2. Prototyping of the TLIF Spine Cage
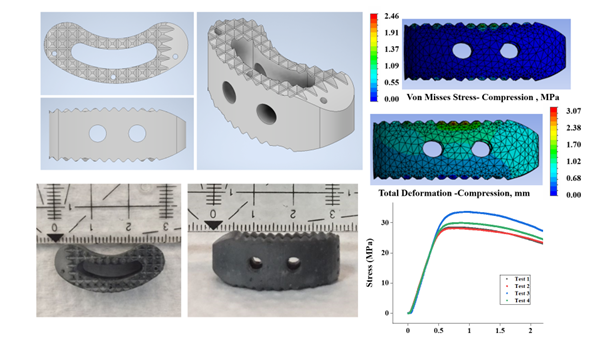
The prototyping was conducted using additive manufacturing technology that involves stereolitography of liquid resin. The machine realized the structure as a predetermined design in the 3D model file. The fabrication result was depicted in Figure 3, with an accuracy of around less than 0.5 mm according to the technical specification. Moreover, the realized geometry indicated that the deviation was less than 1 mm compared to the design dimension. It can be concluded that this additive manufacturing technique can be used as a reference model or a prototype of the implant before its transfer to industrial scale. (Syuhada et al., 2018).
Figure
3 TLIF design printing with PLA Resin: a) top view
and b) side view
3.3.
Numerical Simulation of TLIF Spine Cage
The numerical simulation predicts that our design would withstand applied loading on the spine cage. The simulation calculates the peak of von Mises stress (PVMS) value as the failure criteria of the selected material of the spine cage (Izmin et al., 2020). Moreover, this study also ensures the safety design of the spine cage with our geometrical arrangement. The stress visualizations of the spine cage simulation are shown in Figure 4. Here, a force of 500 N was applied to the spine cage in an axial direction, following the highest possible loading of the human body (Alief et al., 2019).
Figure
4 Finite Element Analysis results for spine cage
implant: a) Total Deformation - Compression and b) Von Misses – Compression
The
simulation results were summarized further in Table 5 to include the maximum
point of the spine case during the loading scenario. As shown in Table 5, the
von misses results are far below the tensile strength of the simulated material,
i.e. PEEK. It suggested that the implant geometry and material successfully
support the spinal movement (figure 4a). Also, in torsion and shear simulations,
the result does not indicate the failure of the spine cage implant (table 5). A
relatively small deformation value from the results also indicate that the
implant will be able to function properly (figure 4b). Since compression,
tensile, shear, and torsion tests only indicate a simple vertical movement of
the spine.
Table 3 Differentiation of Lumbar
Interbody Fusion Implant
3.4. Validation of the
model
A comparison of numerical simulation and the experimental setup is
needed to validate the numerical study in our previous section. We simulate a
compressive test of the realized resin-PLA spine cage in the finite element environment.
This phenomenon was followed by compression using the universal testing
machine. Figure 5 shows the Finite
Element Analysis of the sample block in terms of its total deformation and
calculated von Misses Stress.
Figure
5 Finite Element Analysis results for PLA sample
block: a) Von Misses and b) total deformation in compression testing mode
The compressive test on a 3D printed acquire the young’s modulus value
at around 55.6 MPa. This value was confirmed by our previous study (Supriadi et al., 2021, Saseendran et al.,
2017). A 500 N vertical compression and tensile force were given from
the top surface to calculate Von Mises and deformation of the implant (He et al., 2021). Shear stress with 200 N and 25 Nm torsion was also evaluated in this
implant simulation (Krijnen
et al., 2006; Pitzen et al., 2000). Table 4 shows the mechanical parameters in the Ansys simulation
software.
Table 4 Parameters for Finite Element Analysis
|
Density (g/mm3) |
Young’s Modulus (MPa) |
Poisson’s Ratio |
Reference |
|
1.13 |
55.6 |
0.35 |
(Saseendran et
al., 2017) |
Figure
6 Compressive test result for PLA sample block
compare with the numerical calculation of elastic modulus
Figure 6 presents the experimental result of block test (with
four repetitions of samples). It showed its maximum strength at around 30 MPa.
The numerical simulation gives the dotted line in Figure 6. The line was
projected from the force-displacement relation from the environmental
simulation (Figure 5). It showed a deviation of 9% compared to the experimental
result. Consequently, it can be suggested that the simulation result of spine
cage has a 9% gap mostly in the elastic region (red line area in Figure 6).
A TLIF spine cage based on Indonesian morphometry with a 28 x 9 x
11 mm dimension was designed with a pyramid-shaped jaw profile, multiple holes
as a space for bone graft, and a thread hole for insertion of the implant into
the disc space. We performed a finite
element analysis simulation with the Ansys software, performing compression and
tensile tests to stimulate the stress impacted on the implant. Shear and
torsion test was also simulated in this research. This simulation was done to
test the strength of the design using PLA Resin material. The results showed
that the design is strong enough to withstand the force given. Our numerical
study also showed that a deviation around 9% between experimental loading and
numerical calculation might occurred. However, it is believed that this gap
between experimental and realization might give important information when the
candidate material, PEEK, will be used in the future application.
The authors would like to acknowledge the Matching
Fund Grant No.279/PKS/WRIII-DISTP/UI/2022 from Kementerian Pendidikan,
Kebudayaan, Riset, dan Teknologi.
Ahmad, M.A., Zulkifli, N.N.M.E., Shuib, S., Sulaiman, S.H., Abdullah,
A.H., 2020. Finite Element Analysis of Proximal Cement Fixation in Total Hip
Arthroplasty. International
Journal of Technology, Volume 11(5), pp. 1046–1055
Alief, N.A., Supriadi, S., Whulanza, Y., 2019. Modelling The Shape
Memory Properties of 4D Printed Polylactic Acid (PLA) for Application of Disk
Spacer in Minimally Invasive Spinal Fusion. In: AIP Conference
Proceedings, 2092, 020005
Burnard, J.L., Parr, W.C.H., Choy, W.J., Walsh, W.R., Mobbs, R.J., 2020.
3D-printed Spine Surgery Implants: A Systematic Review of The Efficacy and
Clinical Safety Profile of Patient-Specific and Off-The-Shelf Devices. European Spine
Journal,
Volume 29(6), pp. 1248–1260
Genisa, M., Shuib, S., Ahmad Rajion, Z.A., Mohamad, D., Arief, M.E.,
2020. Dental Implant Monitoring Using Resonance Frequency Analysis (RFA) and
Cone Beam Computed Tomography (CBCT) Measurement. International Journal of Technology, Volume 11(5), pp. 1015–1024
Hammad, A., Wirries, A., Ardeshiri, A., Nikiforov, O., Geiger, F., 2019.
Open Versus Minimally Invasive TLIF: Literature Review and Meta-Analysis. Journal of
Orthopaedic Surgery and Research, Volume 14(1), p. 229
He, L., Xiang, Q., Yang, Y., Tsai, T.Y., Yu, Y., Cheng, L., 2021. The Anterior
and Traverse Cage Can Provide Optimal Biomechanical Performance for Both
Traditional and Percutaneous Endoscopic Transforaminal Lumbar Interbody Fusion.
Computers in Biology and Medicine, Volume 131, p. 104291
Hennemann, S., de Abreu, M.R., 2021. Estenose Degenerativa Do Canal
Lombar (Degenerative Stenosis of The Lumbar Canal). Revista Brasileira de Ortopedia, Volume 56(01), pp. 009–017
Izmin, N.A.N., Hazwani, F., Todo, M., Abdullah, A.H., 2020. Risk of
Bone Fracture in Resurfacing Hip Arthroplasty at Varus and Valgus Implant
Placements. International Journal of Technology, Volume 11(5), pp. 1025–1035
Krijnen, M.R., Mensch, D., van Dieen, J.H., Wuisman, P.I., Smit, T.H., 2006.
Primary Spinal Segment Stability with a Stand-Alone Cage: In Vitro Evaluation of
a Successful Goat Model. Acta Orthopaedica, Volume 77(3), pp. 454–461
Lee, B.H., Moon, S.H., Suk, K.S., Kim, H.S., Yang, J.H., Lee, H.M., 2020.
Lumbar Spinal Stenosis: Pathophysiology and Treatment Principle: A Narrative
Review. Asian Spine Journal, Volume 14(5), pp. 682–693
Mobbs, R.J., Phan, K., Malham, G., Seex, K., Rao, P.J., 2015. Lumbar Interbody
Fusion: Techniques, Indications and Comparison of Interbody Fusion Options
Including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. Journal of Spine
Surgery,
Volume 1(1), pp. 2–18
Peck, J.H., Kavlock, K.D., Showalter, B.L., Ferrell, B.M., Peck, D.G.,
Dmitriev, A.E., 2018. Mechanical Performance of Lumbar Intervertebral Body
Fusion Devices: An Analysis of Data Submitted to The Food and Drug
Administration. Journal of Biomechanics, Volume 78, pp. 87–93
Pitzen, T., Geisler, F.H., Matthis, D., Müller-Storz, H., Steudel, W.I.,
2000. Motion of Threaded Cages in Posterior Lumbar Interbody Fusion. European Spine
Journal,
Volume 9(6), pp. 571–576
Rahyussalim, A.J. Kurniawati, T., Aprilya, D., Anggraini, R., Ramahdita,
G., Whulanza, Y., 2017. Toxicity and Biocompatibility Profile of 3D Bone
Scaffold Developed by Universitas Indonesia: A Preliminary Study. AIP Conference
Proceedings, Volume 1817, p. 020004
Saseendran, S., Wysocki, M., Varna, J., 2017. Cure-state Dependent
Viscoelastic Poisson’s Ratio of LY5052 Epoxy Resin. Advanced Manufacturing:
Polymer & Composites Science, Volume 3(3), pp. 92–100
Supriadi, S., Rachman, P., Saragih, A.S., Whulanza, Y., Rahyussalim,
A.J., Triwidodo, A., 2021. Design, Development, and Finite Element Study on The
Novel Biomimetic Lumbosacroiliac Prosthesis. In: AIP Conference Proceedings, AIP Publishing LLC, Volume
2344(1), p. 050021
Syuhada, G., Ramahdita, G., Rahyussalim, A.J., Whulanza, Y.
Multi-material Poly (Lactic Acid) Scaffold Fabricated Via Fused Deposition
Modeling and Direct Hydroxyapatite Injection as Spacers in Laminoplasty. AIP Conference
Proceedings, Volume 1933, p. 020008
Triwidodo, A., Rahyussalim, A.J., Yulisa, N.D., Pandelaki, J., Huraiby,
L.S., Hadi, I.A.N., Liosha, F.Y., Dilogo, I.H., 2021. Sacrum Morphometry and
Spinopelvic Parameters Among The Indonesian Population Using Computed
Tomography Scans. Medicine, Volume 100(47)
Triwardono, J., Supriadi, S., Whulanza, Y., Saragih, A.S., Novalianita
D.A., Utomo, M.S., Kartika, I., 2021. Evaluation of The Contact Area in Total
Knee Arthroplasty Designed for Deep Knee Flexion. International Journal of Technology, Volume 12(6), pp.
1312–1322
Walter, C.,
Baumgärtner, T., Trappe, D., Frantz, S., Exner, L., Mederake, M., 2021.
Influence of Cage Design on Radiological and Clinical Outcomes in Dorsal Lumbar
Spinal Fusions: A Comparison of Lordotic and Non-Lordotic Cages. Orthopaedic Surgery, Volume 13(3), pp. 863–875
