Surface Coating Effect on Corrosion Resistance of Titanium Alloy Bone Implants by Anodizing Method
Corresponding email: eva-oktavia@chem-eng.its.ac.id
Published at : 28 Jun 2023
Volume : IJtech
Vol 14, No 4 (2023)
DOI : https://doi.org/10.14716/ijtech.v14i4.6146
Ningrum, E.O., Khoiroh, I., Nastiti, H.I., Affan, R.A., Karisma, A.D., Agustiani, E., Surono, A., Suroto, H., Suprapto, S., Taji, L.S., Widiyanto, S., 2023. Surface Coating Effect on Corrosion Resistance of Titanium Alloy Bone Implants by Anodizing Method. International Journal of Technology. Volume 14(4), pp. 749-760
| Eva Oktavia Ningrum | Department of Industrial Chemical Engineering, Faculty of Vocational Studies, Institut Teknologi Sepuluh Nopember, Kampus ITS Sukolilo, Surabaya 60111, Indonesia |
| Ianatul Khoiroh | Department of Chemical & Environmental Engineering, Faculty of Science and Engineering, University of Nottingham Malaysia, Jalan Broga 43500 Semenyih, Selangor, Malaysia |
| Hanifah Inas Nastiti | Department of Industrial Chemical Engineering, Faculty of Vocational Studies, Institut Teknologi Sepuluh Nopember, Kampus ITS Sukolilo, Surabaya 60111, Indonesia |
| Ryan Anindya Affan | Department of Industrial Chemical Engineering, Faculty of Vocational Studies, Institut Teknologi Sepuluh Nopember, Kampus ITS Sukolilo, Surabaya 60111, Indonesia |
| Achmad Dwitama Karisma | Department of Industrial Chemical Engineering, Faculty of Vocational Studies, Institut Teknologi Sepuluh Nopember, Kampus ITS Sukolilo, Surabaya 60111, Indonesia |
| Elly Agustiani | Department of Industrial Chemical Engineering, Faculty of Vocational Studies, Institut Teknologi Sepuluh Nopember, Kampus ITS Sukolilo, Surabaya 60111, Indonesia |
| Agus Surono | Department of Industrial Chemical Engineering, Faculty of Vocational Studies, Institut Teknologi Sepuluh Nopember, Kampus ITS Sukolilo, Surabaya 60111, Indonesia |
| Heri Suroto | Orthopedic and Traumatology Department, Faculty of Medicine, Airlangga University/Dr. Soetomo General Academic Hospital, Jl. Mayjen Prof. Dr. Moestopo No.47, Surabaya, Jawa Timur 60132, Indonesia |
| S. Suprapto | Department of Industrial Chemical Engineering, Faculty of Vocational Studies, Institut Teknologi Sepuluh Nopember, Kampus ITS Sukolilo, Surabaya 60111, Indonesia |
| Lulu Sekar Taji | Department of Industrial Chemical Engineering, Faculty of Vocational Studies, Institut Teknologi Sepuluh Nopember, Kampus ITS Sukolilo, Surabaya 60111, Indonesia |
| Sinung Widiyanto | Magister of Ocean Engineering, Faculty of Engineering and Marine Sciences, Hang Tuah University, Jl. Arief Rahman Hakim No.150, Keputih, Kec. Sukolilo, Surabaya 60111, Indonesi |
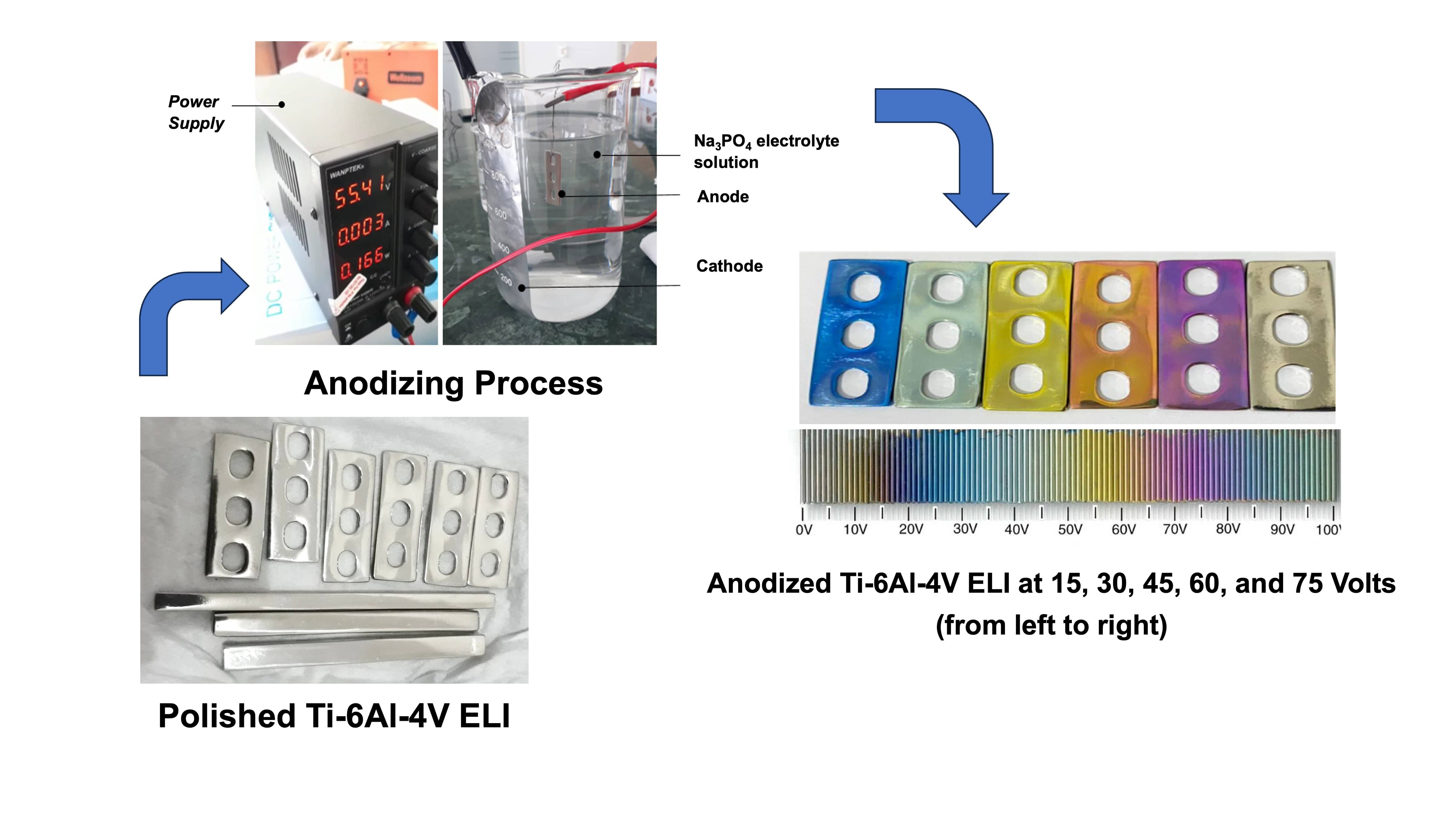
In the presented work, the
formation of anodic oxide film on Ti-6Al-4V ELI (Extra Low Interstitial) alloy
in 0.02 M trisodium phosphate (Na3PO4) electrolyte solution using various voltages were investigated. The color produced by the anodizing, the intensity of TiO2 content, the thickness of the oxide layer, and the corrosion rate were examined. It was obtained that the color appearance of Ti-6Al-4V ELI could be changed easily by altering the applied voltages. The higher the voltage applied in the anodizing process, the thicker the titanium oxide layer formed. The corrosion resistance analysis in a Simulated Body Fluid revealed that the non-anodized specimen showed a higher corrosion rate compared to the anodized specimen. The increase of oxide layer thickness leads to a significant decrease in corrosion rate and consequently increases the corrosion resistance. In addition, the anodized sample achieved the highest corrosion resistance at 15 V.
Anodizing; Corrosion resistance; Titanium oxide; Ti-6Al-4V alloy
A bone
implant is a medical device used to strengthen the existing bone
structure or supports an injured bone structure. In this case,
approximately 90% of implants in Indonesia imports. Therefore, given
the rising demand for implants, the development of bone implants is a crucial
debate issue. Based on the biomedical viewpoint, implant stability and the
osseointegration process, which has the potential for rehabilitation, are the
most important internal factors in the implantation of medical devices. Therefore,
it is essential to create bone implants that have high-quality and
effective when placed within the body (Dewi et
al., 2020; Genisa et al., 2020; Izmin et al., 2020). Orthopedic implants are now
made from various materials, including polymers, ceramics, metals, and
composites. The majority of metals are bio tolerant, although titanium and its
alloys have a bioinert nature under
specific circumstances (Koju, Niraula and Fotovvati, 2022).
Titanium Ti-6Al-4V ELI is a commonly used implant material in the
medical field due to its mechanical and corrosion resistance properties
Implant surface modification is necessary to obtain good implant
properties and performance for the body. This surface modification is important
to increase surface energy by providing surface roughness and chemical
composition. This will increase tissue adhesion and implant integration as well
as reduce bacterial reactions and inflammatory responses in the body
According to prior research, some inorganic
ions, such as molybdate and metavanadate, can passivate titanium in solutions
of sulfuric and hydrochloric acids
Research concerning the anodizing process of Ti-6Al-4V alloy has been
numerously conducted; however, it is only limited to the use of corrosion
resistance test solution, causing its inability to simulate the body's
physiological condition. Therefore, the current research used SBF solution. The
effect of various voltage on the implant color visual, the mass of the oxide
layer formed, oxide film thickness, and implant corrosion resistance were
investigated in this study. Moreover, the correlation between film thickness
and its corrosion resistance was also elucidated. In addition, the trisodium
phosphate electrolyte solution was chosen because it provides better corrosion
resistance compared to the acidic electrolyte solution (Karambakhsh et al., 2015).
2.1.
Materials
The main material used in this research was Ti-6Al-4V ELI metals act as
an anode that would be layered by TiO2 using an anodizing method. An
aluminum foil sheet was also used as a cathode. The electrolyte used was 2 g of
trisodium phosphate dodecahydrate purchased from Merck, which initially
dissolved in 1000 mL aquadest. Titanium wire 28 AWG 0.3 mm and alligator clip
were also employed as the supporter of both Ti-6Al-4V ELI metal and aluminum
foil during the anodizing process. The electrical power source was obtained
from DC Power Supply WANPTEK of NPS 1203W 120V/3A type. In addition, a SBF
solution was used as an electrolyte during the potentiodynamic polarization
analysis to investigate the corrosion rate of anodized Ti-6Al-4V ELI metal
material.
2.2. Methods
Anodizing method was done in Na3PO4
(base solution) electrolyte solution. In this case, the Ti-6Al-4V
specimen obtained pre-treatment to make the implant surface shiny so that the
anodizing color produced can be seen clearly. This pre-treatment process used
Ti-6Al-4V ELI metals, previously polished using langsol or green stone to obtain a mirror-like surface. The
solution used for anodizing was Trisodium Phosphate solution with a
concentration of 0.02 M of 250 mL volume. The cathode was aluminum foil, and
the anode was the Ti-6Al-4V specimens. Anodizing process was performed within
30 s for each sample, the variable parameter being the anodizing voltage.
Anodizing was performed in the 15-75 V voltage range, and with the steps of 15
V. The schematic apparatus for an anodizing process is shown in Figure 1.
2.2.1. XRD Analysis
XRD was
used to identify the titanium oxide crystal component formed after anodizing.
2.2.2. Spectrophotometry
Analysis
Spectrophotometry
Analysis was carried out to determine the quantitative parameters of the
specimen surface color (Konica Minolta CM-5 spectrophotometer). In order to
measure the color difference of each specimen, this tool would obtain data
values of L*, a*, and b*, where chromaticity was obtained using the CIELAB
color space method based by using Equation (1)
|
No |
Reagent |
Composition (g/L) |
|
1. |
NaCl |
8.00 |
|
2. |
KCl |
0.40 |
|
3. |
CaCl2 |
0.18 |
|
4. |
NaHCO3 |
0.35 |
|
5. |
Na2HPO4.2H2O |
0.48 |
|
6. |
MgCl2.6H2O |
0.10 |
|
7. |
KH2PO4 |
0.06 |
|
8. |
MgSO4.7H2O |
0.10 |
|
9. |
Glucose |
1.00 |
3.1. Analysis of Anodizing
Results in Trisodium Phosphate Electrolyte
3.1.2. Effect of Voltage on the Mass of Oxide Layer Formed
Based on Faraday’s Law I, the mass formed on the anode after the
anodizing process can be obtained using Equation (3)
As data comparison, in addition to using a theoretical equation, data
collection was also conducted by weighing the titanium specimen mass, both
before and after the anodizing process, to investigate the mass of the oxide
layer formed after the anodizing process. Figure 3 indicates the addition of
mass in each anodizing voltage variety based on Faraday’s Law I equation. In
this case, there was an increase in titanium oxide mass from 15 V voltage to 75
V voltage. Such results are in accordance with the previous literature that the
higher the voltage for the anodizing process, the thicker the titanium oxide
layer formed obtained from the experimental method using
an analytical scale that mass addition resulting from 15 V to 75 V voltage
shows an increasing trend. Hence, based on the comparison between these data,
the higher voltage given during anodizing process, the thicker the oxide layer
formed on the titanium specimen.
Based on the theoretical calculation conducted, the addition
anomaly occurred at 45 V to 60 V, indicating the most significant increase
compared to the other voltages. In this case, the
increase occurred was
more than 100% from 45 to 60 V. This occurred due to the current formed during
the anodizing process. Furthermore, it was known that from 45 V to 60 V the
increased current was quite high from 0.090 A to 0.48 A. This current increase
occurred due to the instability of electrode distance during the anodizing
process, where the closer the distance of the electrode, the higher the current
) obtained from both theoretical and experimental calculations.
Figure 3 Effect of Voltage on TiO2 Mass Formed
3.2. Analysis of XRD
The peak of
Figure 4 XRD Analysis spectrum of
Ti-6Al-4V specimen (a) Titanium Oxide Reference of ICDD Standard No.
98-009-9784 and XRD Analysis spectrum after Anodizing Process at (b) 75 Volt,
(c) 45 Volt, (d) 15 Volt
3.3. Analysis of Spectrophotometry Results
Table 2 L*, a*, b* Parameter and Chromaticity
|
No |
Variable |
L* |
a* |
b* |
Chromaticity |
|
1. |
15
Volt |
46.75 |
-0.47 |
-0.44 |
0.207 |
|
2. |
30 Volt |
49.25 |
-0.36 |
-7.51 |
28.264 |
|
3. |
45
Volt |
49.61 |
-1.03 |
-3.83 |
7.864 |
|
4. |
60 Volt |
50.67 |
-1.67 |
-2.04 |
3.475 |
|
5. |
75
Volt |
64.47 |
5.40 |
9.65 |
61.141 |
Table 2 above shows that the
highest lightness was obtained at a voltage of 75 Volt, which produced goldish
yellow. This lightness parameter would be further used to know the brightness
level of the color. Meanwhile, a high chromatic score was obtained at a voltage
of 30 V by 28.264 and at 75 V by 61.141. In this case, chromaticity is defined as the quality of light
characterized by a dominant wavelength. Based on this description, L*,
a*, and b* parameters on CIELAB color space were used to investigate color
quality produced by the anodizing process, which has no correlation with the
oxide layer thickness produced.
3.3.1. Effect of Voltage on Oxide
Film Thickness
Table 3 Film Thickness of Titanium Oxide
(TiO2)
|
No |
Voltage |
Wavelength (nm) |
Refractive index (n0) |
Thickness of Oxide Layer (nm) |
|
1. |
15 V |
380 |
1.448 |
65.596 |
|
2. |
30 V |
469 |
1.438 |
81.543 |
|
3. |
45 V |
535 |
1.429 |
93.626 |
|
4. |
60 V |
583 |
1.421 |
102.580 |
|
5. |
75 V |
608 |
1.416 |
107.307 |
Table 3 shows that the oxide layer
thickness is directly related to anodizing voltage. As shown above, the thickness of the oxide layer
increased by increasing voltage from 15-75 V. In this case, the highest oxide
layer was obtained at 75 V by 107.307 nm, while the lowest was at 15 V by
65.596 nm. This is in accordance with the report by (Karambakhsh et al., 2011) that the addition of voltage in the anodizing process produces an
increase in the oxide layer, which leads to an increase in
mass produced. This
difference further provides different interference color visuals as well, according to the voltage variety.
The increase in TiO2 film thickness was caused by the greater the
applied voltage, so the greater the current delivered. This causes more TiO2
to be deposited at the Ti-6Al-4V anode.
3.4. Effect of Voltage on Implant
Corrosion Resistance
Table 4 Corrosion Resistance of non-anodized and anodized specimens
|
No |
Variable |
Ecorr |
Icorr |
Corrosion Rate | |
|
(Volts) |
(Amps/cm2) |
(mmpy) | |||
|
1 |
Non-anodized |
-1.31E-01 |
1.21E-06 |
9.27E-02 | |
|
2 |
15 V |
1.97E-03 |
4.38E-08 |
3.35E-03 | |
|
3 |
30 V |
-1.76E-02 |
4.46E-08 |
3.42E-03 | |
|
4 |
45 V |
-6.61E-02 |
1.04E-07 |
7.94E-03 | |
|
5 |
60 V |
1.83E-01 |
1.13E-07 |
8.68E-03 | |
|
6 |
75 V |
1.87E-01 |
1.25E-07 |
9.56E-03 | |
Based on the potentiodynamic
analysis obtained, the Tafel curve is shown in Figure 5. From this Tafel curve,
it can be seen the effect of Ecorr and Icorr on the corrosion rate. The higher
the I corr, the higher the corrosion rate. It was also
found that the higher the voltage or the current, the greater the corrosion
rate, according to Equation 4.
where
CR =
corrosion rate (mm/yr) for icorr
K1 =
0.1288 (mpy.g/A.cm2)
Icorr = exchange current
density
=
density (g/cm3)
EW =
equivalent weight
The corrosion rate is directly
proportional to its Icorr. Even though anodizing may reduce the corrosion rate
when compared to without anodizing, there still be a percentage increase in
corrosion rate as the anodizing voltage increases.
Figure 5 Tafel Curve in Each Voltage
Variety (a) 15V, (b) 30V, (c) 45V, (d) 60V, (e) 75V, and (f) non-anodized
specimen
Table 5
shows that the effect of voltage on corrosion rate was not quite
significant, where there was an increased corrosion rate by 2% at 15 V to 30 V,
9% at 45 V to 60 V, and 10% 60 V to 75 V. This indicates that voltage in a
fixed anodizing time variable was not quite significant in affecting the
corrosion rate, where the corrosion rate tends to be constant at 15-30 V and at
45-75 V. However, an anomaly occurred in the corrosion rate increase from 30V
to 45V by 57%. Based on the testing on the anodized titanium specimen, there
was an insignificant increase in corrosion rate in each voltage variable. This
occurred because TiO2 layer thickness had not yet been complete during the anodizing process
at 45-75 V in 30 seconds because the higher the voltage, the higher the current
given. When the current provided during the anodizing process is higher, the
time spent on the anodizing process also increases. In this case, the anodizing
process is considered complete when the current value (A-COARSE) on the power
supply shows a decrease approaching zero. In addition, this increase can
also cause higher porosity than the surface layer as the voltage increases,
hence increasing the corrosion rate
Table 5 Layer Thickness and Corrosion
Rate for anodized and non-anodized Ti-6Al-4V specimen
The
anodizing process using Na3PO4 electrolyte solution
produces different colors in various voltages by the interference color
phenomenon. Based on spectrophotometry analysis revealed that a high chromatic
score was obtained at a voltage of 75 V. From the refractive index indicates
that the higher the voltage, the longer the wavelength obtained. Higher voltage
in the anodizing process produces an increase in the oxide layer, which leads
to an increase in mass produced. Moreover, based on the potentiodynamic
analysis obtained that increasing the voltage or the current result in a higher
corrosion rate. The XRD analysis indicates the presence of TiO2
based on the ICDD standard in all voltages employed. Increasing the anodizing
voltage increased the oxide film thickness, consequently increasing the TiO2
content in the specimen. Furthermore, the corrosion resistance analysis
in a Simulated Body Fluid revealed the non-anodized specimen showed a higher
corrosion rate compared to the anodized specimen. The increase of oxide layer
thickness leads to a significant decrease in corrosion rate and consequently
increases the corrosion resistance. Therefore, in future work, the anodized
implant of Ti-6Al-4V ELI in this work will be further evaluated by comparing it
with other electrolytes such as hydroxide, organic acid, sulfuric acid, and
friendly inorganic salt electrolytes in various concentrations.
The current study was supported by a Research Grant of “Penelitian Produk Vokasi Unggulan
Perguruan Tinggi 2022” from The Ministry
of Education, Culture, Research, and Technology (KEMENDIKBUDRISTEK
TAHUN 2022 (1919/PKS/ITS/2022).
Al-Mobarak,
N.A., Al-Swayih, A.A., 2014. Development of Titanium Surgery Implants for
Improving Osseointegration Through Formation of a Titanium Nanotube Layer. International
Journal of Electrochemical Science, Volume 9(1), pp. 32–45
Alphanoda, A.F.,
2016. Effect of Anode-Cathode Distance and Coating
Duration on Corrosion Rate on Hard Chrome Electroplating Results (Pengaruh Jarak Anoda-Katoda dan Durasi Pelapisan Terhadap Laju Korosi
pada Hasil Electroplating Hard Chrome). Jurnal Teknologi Rekayasa, Volume 1(1),
pp. 1–6
Atmani, D.,
Saoula, Nadia., Abdi, A., Azzaz, M., Wang, Y., Mohamedi, M., 2018.
Structural, Morphological, and Electrochemical Corrosion Properties of TiO2
Formed on Ti6Al4V Alloysby Anodization. Crystal Research and Technology,
Volume 53(12), pp. 1–7
Bakr, M.,
Thabet, H., Ghandour, N., Farrag, A, Mohamed, R.E., 2021. Histological and
Ultrastructural Changes of White Blood Cells in Vitro Storage of Human Blood at
Different Time Intervals. Egyptian Journal of Histology, Volume 45(3),
pp. 849–862
Bhola, R.,
Bhola, M., Mishra, B., Ayers, R.A., Olson, D.L., Ohno, T., 2011.
Surface Characterization of Anodically Treated - Titanium Alloy for
Biomedical Applications.
Volume 47(4),
pp. 75–82
Dewi, A.H., Yulianto, D.K.,
Ana, I.D., Rochmadi, Siswomihardjo, W., 2020. Effect of Cinnamaldehyde, an
Anti-Inflammatory Agent, on the Surface Characteristics of a Plaster of Paris –
CaCO3 Hydrogel for Bone Substitution in Biomedicine. International
Journal of Technology, Volume 11(5), pp. 963–973
Fadl-allah,
S.A., Quahtany, M., El-Shenawy, N.S., 2013. Surface Modification of Titanium
Plate with Anodic Oxidation and Its Application in Bone Growth. Journal of Biomaterials and
Nanobiotechnology. Volume 4(1), pp. 74–83
Gabor, R.,
Doubkove, M., Gorosova, S., Malanik, K., Vandrovcoca, M., Cvrcek, L.,
Drobikova, K., Kutlakova, K.M., Bacakova, L., 2020. Preparation of Highly
Wettable Coatings on Ti–6Al–4V ELI Alloy for Traumatological Implants Using
Micro-Arc Oxidation in An Alkaline Electrolyte. Scientific Reports,
Volume 10(1), pp. 1–16
Genisa, M., Shuib, S., Ahmad
Rajion, Z.A., Mohamad, D., Arief, M.E., 2020. Dental Implant Monitoring Using
Resonance Frequency Analysis (RFA) and Cone Beam Computed Tomography (CBCT)
Measurement. International Journal of Technology, Volume 11(5), pp. 1015–1024
Indira, K.,
Mudali, K.U., Rajendran, N., 2013. Corrosion Behavior of Electrochemically
Assembled Nanoporous Titania for Biomedical Applications. Ceramics
International. Volume 39(2), pp. 959–967
Izmin, N.A.N., Hazwani, F., Abdullah, A.H., Todo,
M., 2020. Risk of Bone Fracture in Resurfacing Hip Arthroplasty at Varus
and Valgus Implant Placements. International Journal of Technology.
Volume 11(5), pp. 1025-1035
Izmir,
M., Ercan, B., 2019. Anodization of Titanium Alloys for Orthopedic
Applications. Frontiers of Chemical Science and Engineering, Volume
13(1), pp. 28–45
Jaafar, A.,
Hecker, C., Arki, P., Joseph, Y., 2020. Sol-Gel Derived Hydroxyapatite
Coatings for Titanium Implants: A Review. Bioengineering, Volume 7(4),
p. 127
Kahar, S., Singh,
A., Patel, V., Kanetkar, U., 2020. Anodizing of Ti and Ti Alloys for Different
Applications: A Review. International Journal for Scientific Research &
Development, Volume 8(5), pp. 272–276
Karambakhsh,
A., Afshar, A., Ghahramani, S., Malekinejad, P., 2011. Pure Commercial
Titanium Color Anodizing and Corrosion Resistance. Journal of Materials
Engineering and Performance, Volume 20(9), pp. 1690–1696
Karambakhsh,
A., Afshar, A., Malekinejad, P., 2012. Corrosion Resistance and Color
Properties of Anodized Ti-6Al-4V. Journal of Materials Engineering and
Performance, Volume 21(1), pp. 121–127
Karambakhsh,
A., Ghahramani, S., Afshar, A., Malekinejad, P., 2015. Comparison of the
Corrosion Resistance of Alkaline- and Acid Anodized Titanium. Materials
Performance, Volume 54(1), pp. 51–55
Kashyap, N.,
Rashid, R.A., Khanna, N., 2022. Carbon Emissions, Techno-Economic and
Machinability Assessments to Achieve Sustainability in Drilling Ti6Al4V ELI for
Medical Industry Applications. Sustainable Materials and Technologies,
Volume 33, pp. 175–194
Kiel-Jamrozik,
M., Szewczenko, J., Basiagi, M., Nowinska, K., 2015. Technological Capabilities of Surface Layers
Formation on Implant Made of Ti-6Al-4V ELI Alloy. Acta of Bioengineering and
Biomechanics, Volume 17(1), pp. 31–37
Koju, N.,
Niraula, S., Fotovvati, B., 2022. Additively Manufactured Porous Ti6Al4V for Bone
Implants: A Review, Metals, 12(4), p. 687
Lestari, F.P.,
Sari, Y.R., Rokhmanto, F., Asmaria, T., Pramono, A.W., 2020. Surface
Modification of Ti-6Al-4V Alloy By Anodization Technique at Low Potential to
Produce Oxide Layer. Journal of Electronics, Volume 2(3), pp. 93–102
Martinez, A.L.,
Flamini, D.O., Saidman, S.B., 2022. Corrosion Resistance Improvement of
Ti-6Al-4V Alloy by Anodization In The Presence of Inhibitor Ions. Transactions
of Nonferrous Metals Society of China, Volume 32(6), pp. 1896–1909
Mogoda, A.S.,
Ahmad, Y.H., Badawy, W.A., 2004. Corrosion Inhibition of Ti-6Al-4V Alloy in
Sulfuric and Hydrochloric Acid Solutions Using Inorganic Passivators. Materials
and Corrosion, Volume 55(6), pp. 449–456
Nagy, P.M., Ferencz, B.,
Kálmán, B., Djuricic, B., Sonnleitner, R., 2005.
Morphological Evolution of Ti Surfaces During Oxidation Treatment. Materials
and Manufacturing Processes, Volume 20(1), pp. 105–114
Napoli, G.,
Paura, M., Vela, T., Schino, A.D., 2018. Colouring Titanium
Alloys By Anodic Oxidation. Metalurgija, Volume 57(1–2), pp. 111–113
Narayanan,
R., Seshadri, S.K., 2007. Phosphoric Acid Anodization of Ti-6Al-4V - Structural
and Corrosion Aspects. Corrosion Science, Volume 49(2), pp. 542–558
Rani, S.,
Jatolia, S.N., 2018. Electrodeposited Hap Coatings on Titanium Alloys: A
Review. International Journal of Research and Analytical Reviews, Volume
847(4), pp. 847–853
Roessler,
S., Zimmermann, R., Scharnweber, D., Werner, D., Worch, H., 2002.
Characterization of Oxide Layers on Ti6Al4V and Titanium by Streaming Potential
and Streaming Current Measurements. Colloids and Surfaces B: Biointerfaces,
Volume 26(4), pp. 387–395
Saraswati,
W.C., Anawati, A., Jujur, I.N., Gumelar, M.D., 2020. Effect of Coloring by
Anodizing on the Corrosion Behavior of Ti-6Al-4V Alloy. In: AIP
Conference Proceedings, Volume 2232(1). pp. 1–5
Swain, S.,
Misra, R.D.K., You, C.K., Rautray, T.R., 2021. TiO2 Nanotubes
Synthesised on Ti-6Al-4VELI Exhibits Enhanced Osteogenic Activity: A potential
Next-Generation Material to Be Used as Medical Implants. Materials
Technology, Volume 36(7), pp. 393–399
Szewczenko,
J., Basiaga, M., Kiel-Jamrozik, M., Kaczmarek, M., Grygiel, M., 2015. Corrosion
Resistance of Ti6Al7Nb Alloy After Various Surface
Modifications. Solid State Phenomena, Volume 227, pp. 483–486
Szewczenko,
J., Walke, W., Nowinska, K., Marciniak, J., 2010. Corrosion Resistance of
Ti-6Al-4V Alloy After Diverse Surface Treatments. Materialwissenschaft und
Werkstofftechnik, Volume 41(5), pp.
360–371
Szymczyk-Ziolkowska,
P., Hoppe, V., Gasiorek, J., Rusinska,
M., Keszycki,
D., Szczepanski, L., Dudek-Wicher, R., Detyna,
J., 2021. Corrosion Resistance Characteristics
of a Ti-6Al-4V ELI Alloy Fabricated by Electron Beam Melting After the Applied
Post-Process Treatment Methods. Biocybernetics and Biomedical Engineering,
Volume 41(4), pp. 1575–1588
Szymczyk-Ziolkowska,
P., Ziolkowski, G., Hoppe, V., Rusinska, M., Kobiela, K., Madeja, M.,Dziedzic, R., Junka, A., Detyna, J., 2022.
Improved Quality and Functional Properties of Ti 6Al-4V ELI Alloy for
Personalized Orthopedic Implants Fabrication with EBM Process. Journal of Manufacturing Processes, Volume
76(1), pp. 175–194
Tamilselvi,
S., Raman, V., Rajendran, N., 2006. Corrosion Behaviour of Ti-6Al-7Nb and
Ti-6Al-4V ELI Alloys in the Simulated Body Fluid Solution by Electrochemical
Impedance Spectroscopy. Electrochimica Acta, Volume 52(3), pp. 839–846
Van-Gils, S.,
Mast, P., Stijins, E., Terryn, H., 2004. Colour Properties of Barrier
Anodic Oxide Films on Aluminium and Titanium Studied with Total Reflectance and
Spectroscopic Ellipsometry. Surface and Coatings Technology, Volume 185(2–3), pp. 303–310
Wadhwani,
C., Brindis, M., Kattadiyil, M., O'Brien, R., Chung, K.H., 2018. Colorizing
Titanium 6Aluminum-4Vanadium Alloy Using Electrochemical Anodization:
Developing a Color Chart. Journal of Prosthetic Dentistry, Volume
119(1), pp. 26–28
Ziebowicz,
A., Ziebowicz, B., Baczkowski, B., 2015. Electrochemical Behavior of Materials
Used in Dental Implantological Systems. Solid State Phenomena, Volume
227, pp. 447–450
