Cognitive Measurement in Vascular Dementia Patients with Prefrontal Cortex Activation Analysis
Corresponding email: adrianirizka.amalia@gmail.com
Published at : 30 Dec 2022
Volume : IJtech
Vol 13, No 8 (2022)
DOI : https://doi.org/10.14716/ijtech.v13i8.6142
Zakaria, H., Amalia, A.R., Hadiyoso, S., Ashadi, M.F.R., 2022. Cognitive Measurement in Vascular Dementia Patients with Prefrontal Cortex Activation Analysis. International Journal of Technology. Volume 13(8), pp. 1681-1691
| Hasballah Zakaria | School of electrical engineering and informatics, Bandung Institute of Technology, Jl. Ganesa 10, Bandung, West Java 4013 Indonesia |
| Adriani Rizka Amalia | School of electrical engineering and informatics, Bandung Institute of Technology, Jl. Ganesa 10, Bandung, West Java 4013 Indonesia |
| Sugondo Hadiyoso | School of electrical engineering and informatics, Bandung Institute of Technology, Jl. Ganesa 10, Bandung, West Java 4013 Indonesia |
| Muhammad Fakhrul Rozi Ashadi | School of electrical engineering and informatics, Bandung Institute of Technology, Jl. Ganesa 10, Bandung, West Java 4013 Indonesia |
Stroke is one of the world‘s second leading causes of death,with a prevalence of 10.9% in
2018. In Indonesia, strokes have increased over the last five years. Epidemiology suggests that small
strokes in the prefrontal cortex (PFC) can cause cognitive impairment, leading to vascular dementia.
The prefrontal cortex is a structure in the brain that is located in the frontal lobe. Accurate detection
or diagnosis becomes important for therapeutic management because, it is difficult to identify at an
early stage. Therefore, in this study, an analysis of differences in brain activation in healthy elderly
(non-stroke) and post-stroke patients with vascular dementia was conducted when performing
memory recall work. This study involved seven elderly non-stroke and seven stroke patients with
vascular dementia. Brain activity was recorded using a 19-channel clinical electroencephalogram
(EEG). The study compared prefrontal cortex activity during an attention test. Standardized lowresolution brain electromagnetic tomography (sLORETA) was used to analyze active brain areas.
Then the analysis of differences in prefrontal cortex activity between non-stroke patients and those
with vascular dementia used a paired T-test. The results of the paired T-test (with p<0.05) showed
that elderly non-strokes produced significant differences in activity when repeating numbers
correctly (remember the numbers) and incorrectly (forget the numbers), while in stroke patients
with vascular dementia, there was no significant difference when repeating numbers correctly and
incorrectly. Another implication is that there is a decrease in the prefrontal cortex activity in stroke
patients with dementia compared to elderly non-stroke patients. This study is expected to support
the early detection of vascular dementia, especially in post-stroke patients.
EEG; Prefrontal Cortex; sLORETA; Stroke; Vascular Dementia
A stroke is a medical emergency because brain cells can die in a few minutes. An ischemic stroke is caused by a blockage along the path of an artery leading to the brain. One type of ischemic stroke is an atherothrombotic stroke. An atherothrombotic stroke occurs due to the blockage of blood vessels by plaque in the walls of the arteries (Alway et al., 2009). Epidemiologists show that small strokes in the PFC can result in cognitive impairment (Zhou, 2016). Research on prefrontal cortex activity has been carried out on the human brains of stroke patients, including (Hermand et al., 2019; Mori et al., 2018; Takeuchi et al., 2016; Al-Yahya et al., 2016).
Several studies have reported that dual tasks activate the prefrontal cortex, which plays an
important role in executive functions such as attention and multi-tasking (Miller & Cohen, 2001). Dual tasks, in this case, employ both cognitive and
physical treatment. PFC activity in older subjects was lower than in younger
individuals, which emphasizes the association between lower PFC activity and an
increased risk of falls in the elderly. Therefore, changes in PFC activity may
affect the risk of falls in stroke patients. However, the difference in PFC
activation during dual tasks
between stroke patients and healthy subjects is still unclear (Takeuchi
et al., 2016). A study conducted by (Mori
et al., 2018) found that the effect of PFC activation on dual tasks was different between
young and old subjects. However, no studies have investigated the relationship
between dual-task and PFC
activation in stroke patients. The correlation between PFC activity and the
decrease in the dual tasks was
carried out by the Analysis of
Variance (ANOVA) test. This can be useful for determining the risk of
falling in stroke patients.
Several studies have used sLORETA
to analyze problems related to brain damage. In the
study of Cao et al. (2009), the functional connectivity
properties of 29 student-athletes were compared under resting conditions and after
sport-related mild traumatic brain injury (MTBI). The method
used Independent Component Analysis (ICA) to remove noise and then applied the
source reconstruction algorithm (sLORETA) to identify the cortical region of
interest (Cao et al., 2009). sLORETA
analysis revealed that patients with psychosis had decreased scores in the
alpha band and patients with apathy had higher scores, especially in the right
frontal and temporal regions (Shim & Shin, 2020).
In contrast to previous studies, this study used a single-task treatment, cognitive.
Then, selected non-stroke patients and vascular dementia stroke patients were
to perform Electroencephalography
(EEG) recording for 20 minutes with the Montreal
Cognitive test (MoCA) and hyperventilation tests. Furthermore, to
determine the active brain areas in vascular dementia stroke patients by
performing the inverse problem
using sLORETA, then to find out whether or not there is a difference in prefrontal cortex activity between
non-stroke and vascular dementia stroke patients when repeating numbers
correctly and incorrectly, the paired
T-test was used statistically.
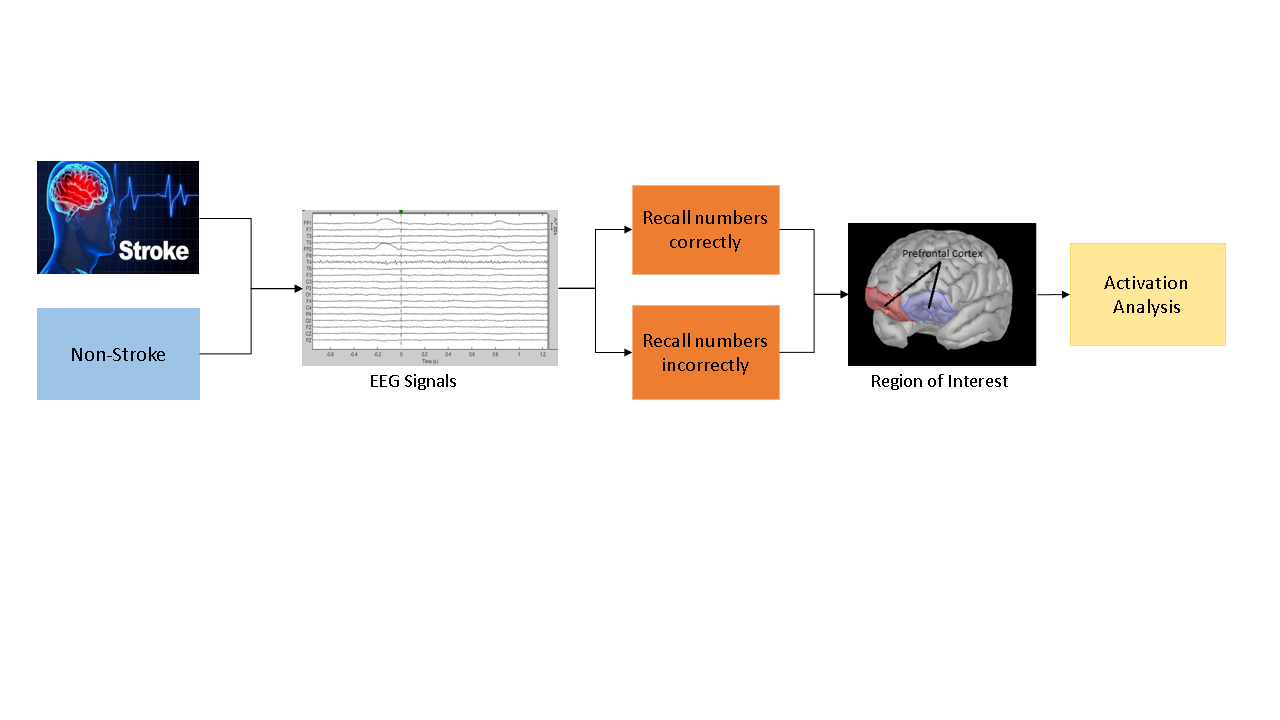
As part of the design of this system, a signal analysis was performed to
compare the activation of the prefrontal
cortex between non-stroke and stroke patients. The signal analysis
scheme is illustrated in Figure 1. The
first step is EEG recording. The next step is data grouping. The authors
collected data from non-stroke patients and stroke patients with vascular
dementia. Then, researcher labels the data when the patient receives treatment
and at the time of memory recall.
Independent Component Analysis (ICA) was used to eliminate noise and
artifacts. After obtaining a clean signal, data segmentation was carried out,
followed by converting the EEG signal into a source signal using the inverse
problem and identifying the cortical region of interest using the sLORETA
method. Lastly, a significance test analysis was performed using the paired
T-test. Stages of data processing and statistical tests using Brainstrom.
Figure 1 Scheme of signal analysis
2.1. Data Retrieval
EEG recording experiments in this study were
carried out for 20 minutes at Hasan Sadikin Hospital, with details as shown in
Table 1. This study has received ethical approval from the ethics committee of
the Hasan Sadikin hospital with number LB.02.01/X.6.5/272/2019. All subjects
agreed to participate in this study by filling out informed consent forms.
Subjects will be given
two types of tests, the MoCA testand the hyperventilation test. Furthermore,
this study focuses on the attentional MoCA test, specifically when testing the
forward and backward digits.
Table 1 Experimental design of EEG data recording
|
No |
Description of recording |
Recording Duration |
|
1 |
Resting condition with eyes closed |
5 minutes |
|
2 |
Resting condition with eyes open |
3 minutes |
|
3 |
Montreal Cognitive Test (MoCA) |
5 minutes |
|
4 |
Resting condition with eyes closed |
5 minutes |
|
5 |
Hyperventilation Test |
2 minutes |
|
Total recording time |
20 minutes | |
The MoCA test aims to determine the presence of Mild
Cognitive Impairment (MCI) (Julayanont & Nasreddine, 2017). MCI is a clinical condition intermediate between normal
cognitive aging and dementia; in many cases, MCI can lead to dementia in the
future (Nasreddine, 2005).
Husein et al. (2010) have tested the validity and reliability of the Indonesian version of
the MoCA instrument, which aims to adapt the application of the test to the
Indonesian people. The validity test of the MoCA-Ina test was carried out using
the Transcultural World Health Organization (WHO) method, and the reliability
test was carried out using the K statistic (Kappa).
The Indonesian version of MoCA consists of 30
points that will be tested by assessing several cognitive domains:
a. Executive
function can be defined as a person's complex process of solving a new problem
or problems. Executive function was assessed by trail-making B (1
point), the phonemic fluency test (1 point), and two-item
verbal abstraction (1 point).
b. Visuospatial is a
constructional ability such as drawing or imitating various kinds of images and
also arranging blocks. Visuospatial was assessed by a clock drawing test (3 points) and by depicting a
three-dimensional cube (1 point).
c. Language is
assessed by mentioning three animal names (lion, camel, rhino; 3 points),
repeating two sentences (2 points), and language fluency (1 point).
d. A delayed recall is assessed by
mentioning five words (5 points) and mentioning them back after 5 minutes (5
points).
e. Attention by
assessing alertness (1 point), subtracting sequentially (3 points), forward and
backward digits (1 point each).
f. Abstraction
assesses the similarity of an object (2 points).
g. Orientation is
assessed by mentioning the date, month, year, day, place, and city (1 point
each).
2.2. Independent Component Analysis
It is implicit that the electrical signals measured on the scalp are a
combination of brain activity as well as different artifacts. An EEG signal is
an electrical impulse that propagates through all layers of the cortex, skull,
and tissues, and is ultimately present at every location of the scalp. This ICA
is the stage for removing noise and artifacts from the recording (Michel
et al., 2019).
2.3. Data Segmentation
The next stage is data segmentation. This study was
divided into two categories, non-stroke and stroke patients. Seven non-stroke
and seven vascular dementia stroke patients were selected to obtain attentional
MoCA data. Short-term memory can store seven items of information in 15 to 30
seconds. The memory retrieval process requires a search of short-term memory,
with each item checked individually. This serial short-term memory search works
at a speed of 35 to 40 milliseconds per item, which is too fast for humans to
realize (Vergauwe, 2014). The
segmentation process takes 0.75 seconds before the forward and backward digit
recall, until 1.25 seconds after.
During the attention test, the patient is given verbal
instructions. The time delay between the patient being given instructions and
when the patient recalls them is approximately 12 seconds. During the time
delay, the patient is resting. The author chooses only 2 seconds of data by
throwing away the first and last 5 seconds of recording on the resting state
data.
Based
on the results of the EEG recording, it was found that not all patients
recalled numbers correctly, but there were also patients who recall numbers
incorrectly. Some patients recall the forward and backward numbers correctly,
and some patients only repeat one of the numbers correctly. Therefore, the data
is divided into two categories, repeating true numbers and repeating wrong
numbers. The results of the data segmentation process were obtained in as many
as 42 parts, with 11 data for non-stroke patients and 10 data for stroke
vascular dementia patients, respectively, on MoCA attention and resting state.
In non-stroke patients, 7 patients recall numbers correctly, and 4 patients
recall numbers incorrectly. In contrast, fewer stroke patients
correctly recalled the numbers; 5 patients recalled the correct numbers and 5
patients recalled the incorrect numbers.
2.4. sLORETA
Standardized Low-Resolution
Electromagnetic Tomography (sLORETA) is the most effective solution for the
inverse EEG/MEG problem in three-dimensional head shape modelling (Shim & Shin, 2020). The inference of the current source position
from the electrode potential is known as the “inverse EEG problem”, An
illustration of the inverse problem can be seen in Figure 2. This method
converts the EEG signal (electrode potential) into a source signal.
sLORETA produces a
linear imaging method with precise zero-error localization under ideal
conditions, as shown (Tong, 2009).
Figure 2
Illustration of forwarding problem
and inverse problem
where is related to noise in the measurement, and SJ to the biological source of variability, i.e.,
covariance for current density. When
is considered to be proportional to the
identity matrix. Under these conditions, the current density covariance is
given by
2.5. Region of Interest (RoI)
Figure 3 Region of interest (prefrontal cortex)
2.6. Statistical Paired T-test
The last stage in this research is the significance test.
The significance test was used to determine whether or not there was an average
difference between non-stroke and stroke patients in the prefrontal cortex in memory recall
activity. The statistical test used is the paired t-test. The Paired
t-test is a parametric test that can be used on two paired data sets (Widiyanto,
2013). The purpose of this test is to see if there
is a difference in the mean between two paired or related samples. Because it
is a pair, the data from both samples must have the same amount or come from
the same source. For example, if the two samples are not related or do not have
the same data, then the independent
sample t-test can be used. Data criteria for paired t-test: 1) the data is
normally distributed or Gaussian; 2) the same number of samples; 3) the
variance values may or may not be the same; 4) The scale requires an interval
or ratio.
In this test, several stages must be completed, including
(Santoso, 2010):
a. Determining
the null hypothesis (Ho) and alternative hypothesis (Ha), whose principle is to
test the characteristics of the population based on the information received
from a sample. Example: Ho = Both population means are identical (µ1=µ2 or
µ1-µ2 = 0); Ha = Both population means are not identical (µ1?µ2 or µ1-µ2?0).
b. Determine
the level of significance (?), which is the probability of an error rejecting
the hypothesis that turns out to be true. If it says 5%, it means that the risk
of making a wrong decision is 5%. The smaller the level of significance, the
lower the risk of error.
c. Paired T-Test Formula:
where:
t is T-value; is Average sample measurements 1 and 2;
d. Define
table statistics and test statistics. In this test, the T-table and T-value will be searched. For conditions of
unequal standard deviation get the T-value and calculate the formula
used as follows (Sudjana,
2005).
Where,
S2 represents the sample variance, n is the number of
samples, x1
e. Draw conclusions based on the results of T-tables and T-value. Accept Ho if T-value is less than T-table and reject Ho if T-value is greater than T-table (Widarjono, 2015).
This chapter is organized as follows: First, we discuss the results of
sLORETA, a particular technique we use to find ROI. Then we will discuss paired
t-test results, a particular technique we use to compare the prefrontal
cortex of non-stroke and those with vascular dementia stroke patients.
3.1. sLORETA Result
Figure 4
Transformation of sLORETA
3.1.1. Anatomical modeling
Anatomical modeling followed the ICBM152 template with 15002 vertices and 29984 faces (Do et al., 2021). The appearance of the cortex can be seen in Figure 5.
Figure 5 Anatomical model
3.1.2. Head
modeling
In
this research, the head model chosen is OpenMEEG BEM. This forward model uses Symmetric Boundary Element
Method (Symmetric BEM)
and was developed by the French public research institute INRIA. This model uses three layers; scalp, inner skull,
and outer skull, with details of 1082 vertices on the scalp and 642 vertices (inner skull and outer skull).
3.1.3. Noise covariance
Minimum Norm Estimate (MNE) and dipole modeling are best with an accurate
noise covariance model, generally calculated from experimental data. As such, these estimates are prone to errors arising from
relatively few data points, weak sensors, and data that can cause the eigen
spectra of the covariance matrix to become unconditioned (i.e. a large spread
of eigenvalues or matrix condition
numbers). To “stabilize” or “adjust” the noise covariance matrix,
the L2 matrix norm is defined
as the largest eigenvalue of its eigen spectrum. This adds a diagonal matrix
whose entries are fractional norms of the matrix to the covariance matrix. The
norm value of the matrix is used at 0.1, so the covariance matrix is stabilized
by adding it to the identity matrix which is scaled to 10% of the largest
eigenvalues.
3.2. Paired T-test
Result
The non-zero
values show a significant difference between the two conditions. This
analysis focuses on when the patient will repeat numbers or recall memories, precisely before the
zero point in the prefrontal cortex.
There are four paired T-test
statistical tests;
- · Non-stroke patients were compared when they correctly
repeated numbers and when resting.
- · Non-stroke
patients were compared when they repeated numbers incorrectly and were in their resting state.
- · Compared to
stroke patients when repeating numbers correctly and at rest
- · Compared to
stroke patients when repeating numbers incorrectly and at rest
3.2.1. Paired t-test results for non-stroke patients
3.2.2. Paired t-test results for vascular dementia
stroke patients
Figure 7 (a) Stroke patients when repeating numbers correctly, (b) Stroke patients when repeating numbers incorrectly
3.3. Analysis
In the attention MoCA experiment, two conditions were
applied, non-stroke and vascular dementia stroke
patients during memory recall.
As explained in the previous chapter, for the MoCA attention test, the patients
were asked to repeat two different sequences of numbers, one sequence of
numbers was ordered from the front, and one sequence of numbers was ordered
from the back.
Looking at the results of the EEG recording, the authors
want to see if there is a significant difference in the prefrontal cortex between non-stroke
and vascular dementia stroke patients. As well as observing the effect of
activation in non-stroke patients and vascular dementia stroke patients on the
success of memory recall.
·
In assessing the
different cognitive domains, i.e., attention, executive functions, memory,
language, visuospatial skills, conceptual thinking, calculation, and orientation, with a total score of 30 points; 26 or above is
considered normal. Subjects with a MoCA-Ina score less than 26 (<26)
indicate impaired cognitive functions. The result of the MoCA score is shown in
Table 2.
Table 2 MoCA score in non-stroke and vascular
dementia stroke patients
|
Subject |
Class |
MoCA Score |
Subject |
Class |
MoCA Score |
|
1 |
Non-stroke |
27 |
8 |
Stroke |
19 |
|
2 |
Non-stroke |
27 |
9 |
Stroke |
24 |
|
3 |
Non-stroke |
26 |
10 |
Stroke |
22 |
|
4 |
Non-stroke |
28 |
11 |
Stroke |
24 |
|
5 |
Non-stroke |
26 |
12 |
Stroke |
20 |
|
6 |
Non-stroke |
26 |
13 |
Stroke |
24 |
|
7 |
Non-stroke |
27 |
14 |
Stroke |
25 |
3.3.1. Analysis of activation in non-stroke and vascular dementia stroke
patients
The following will explain the meaning of brain
activation as seen from non-zero
values. If the non-zero values are further away from
the zero point, both positive and negative, and there are more
non-zero values before memory recall (dotted vertical line), it means that the
brain shows a high activation condition.
The condition of brain activation in non-stroke patients can be seen in the patterns in Figures 6 and 7. So
some descriptions of the analysis can be taken as follows:
- · There is a
significant difference between the conditions of the brain during recalled memory activity with resting state conditions in non-stroke patients.
- · There is a
difference, but not significant, between the condition of the brain during memory recall activity and the resting state in stroke patients.
- · When it comes to repeating numbers correctly
versus incorrectly, non-stroke patients have significantly higher activation.
- · When stroke
patients correctly repeat numbers, their brains are more activated than when
they incorrectly repeat numbers, but the difference is not statistically
significant.
3.3.2. Analysis
of prefrontal cortex activity in non-stroke and vascular dementia stroke
patients
The distinct
prefrontal areas have
extensive interconnections with the dorsomedial
nucleus of the thalamus. Playing an important role in the work of this
cortical area, lesions of the dorsomedial nucleus have effects that are in some
ways similar to prefrontal damage.
Areas exposed to the lateral convexity (dorsolateral
prefrontal cortex) have massive interconnections with the parietal
multimodal cortex and somatosensory, visual, and auditory association areas.
Patients with damage to this prefrontal area have problems with planning,
problem-solving, and maintaining attention (Vanderah,
2018).
It is seen that in non-stroke patients, there
is a significant difference between repeating numbers correctly and incorrectly. In contrast, in
vascular dementia stroke patients, there is no significant difference when
repeating numbers correctly and incorrectly. This means a decrease in activity
in the prefrontal cortex in
vascular dementia and stroke patients.
We acknowledge the support received from Hasan Sadikin
hospital. In addition, the author wants to thank, in particular, the patience,
care, and support of the laboratory assistant.
Alway, D., Cole, J.W., 2009. Stroke Essentials For Primary Care: A Practical Guide. USA: Springer Science & Business Media.
Al-Yahya, Johansen-Berg, H., Kischka, U., Zarei, M., Cockburn, J., Dawes, H., 2016. Prefrontal Cortex Activation While Walking Under Dual-Task Conditions In Stroke: A Multimodal Imaging Study. Neurorehabilitation and Neural Repair, Volume 30(6), pp. 591–599
Cao, C., Slobounov, S., 2009. Alteration of Cortical Functional Connectivity as a Result of Traumatic Brain Injury Revealed by Graph Theory, ICA, and Sloreta Analyses of EEG Signals. IEEE Transactions on Neural Systems and Rehabilitation Engineering, Volume 18(1), pp. 11–19
Daniel, R., Pandey, V., Bhat, K.R., Rao, A.K., Singh, R., Chandra, S., 2018. An Empirical Evaluation of Short-Term Memory Retention Using Different High-Density EEG Based Brain Connectivity Measures. In: 2018 26th European Signal Processing Conference (EUSIPCO). pp. 1387–1391
Do, T.T.N., Jung, T.P., Lin, C.T., 2021. Retrosplenial Segregation Reflects the Navigation Load During Ambulatory Movement. IEEE Transactions on Neural Systems and Rehabilitation Engineering, Volume 29, pp. 488–496
Hermand, E., Tapie, B., Dupuy, O., Fraser, S., Compagnat, M., Salle, J.Y., Daviet, J.C., Perrochon, A., 2019. Prefrontal Cortex Activation During Dual Task With Increasing Cognitive Load in Subacute Stroke Patients. Frontiers in Aging Neuroscience, Volume 11, p. 160
Husein, N., Lumempouw, S., Herqutanto, Y.R., 2010. Validity and reliability test of the Indonesian version of the Montreal Cognitive Assesment (MoCA-Ina) for screening cognitive function disorder. Neurona, Volume 27(4), pp. 15–22 (Please translate the title to English)
Julayanont, P., Nasreddine, Z.S., 2017. Montreal Cognitive Assessment (Moca): Concept and Clinical Review. Cognitive screening instruments. Springer, Cham. pp. 139–195
Michel, C.M., Brunet, D.. 2019. EEG Source Imaging: a Practical Review of The Analysis Steps. Frontiers In Neurology, Volume 10, p. 325
Widiyanto, M.A., 2013. Applied Statistic. Elex Media Komputindo.
Miller, E.K., Cohen, J.D. , 2001. An Integrative Theory of Prefrontal Cortex Function. Annual Review of Neuroscience, Volume 24(1), pp. 167–202
Mori, T., Takeuchi, N., Izumi, S.I., 2018. Prefrontal Cortex Activation During A Dual Task In Patients With Stroke. Gait & Posture, Volume 59, pp. 193–198
Nasreddine, Z.S., Phillips, N.A., Bédirian, V., Charbonneau, S., Whitehead, V., Collin, I,, 2005. The Montreal Cognitive Assessment, MoCA: a Brief Screening Tool For Mild Cognitive Impairment. Journal of the American Geriatrics Society, Volume 53(4), pp. 695–699
Pascual-Marqui, R.D., 1999. Review of Methods For Solving The EEG Inverse Problem. International Journal of Bioelectromagnetism, Volume 1(1), pp. 75–86
Pascual-Marqui, R.D., 2002. Standardized Low-Resolution Brain Electromagnetic Tomography (sLORETA): Technical Details. Methods Find Exp Clin Pharmacol, Volume 2002, pp. 5–12
Santoso, S., 2010. Parametric Statistic. Jakarta. Indonesia. Elex Media Komputindo.
Shim, Y.S., Shin, H.E., 2020. Analysis of Neuropsychiatric Symptoms in Patients With Alzheimer’s Disease Using Quantitative EEG and sLORETA. Neurodegenerative Diseases, Volume 20(1), pp. 12–19
Štolc, S., Krakovská, A., Teplan, M., 2003. Audiovisual Stimulation of Human Brain Linear and Nonlinear Measures. Measurement Science Review 3, Volume 2003
Sudjana, N., 2005. Statistical Method. Bandung: Tarsito
Takeuchi, N., Mori, T., Suzukamo, Y., Tanaka, N., Izumi, S.I., 2016. Parallel Processing Of Cognitive and Physical Demands in Left and Right Prefrontal Cortices During Smartphone Use While Walking. BMC Neuroscience, Volume 17(1), pp. 1–11
Tong, S,, Thankor, N.V., 2009. Quantitative EEG Analysis Methods and Clinical Applications. Boston, London: Artech House
Vanderah, T., 2018. Nolte's The Human Brain in Photographs and Diagrams. Philadelphia: Elsevier Health Sciences.
Vergauwe, E., Nelson, C., 2014. A Common Short-Term Memory Retrieval Rate May Describe Many Cognitive Procedures. Frontiers in Human Neuroscience, Volume 8, p. 126
Widarjono, A., 2015. Applied Statistic With Excel & SPSS. Yogyakarta: UPP STIM YKPN
Zhou, L.Y., Wright, T.E., Clarkson, A.N., 2016. Prefrontal Cortex Stroke Induces Delayed Impairment In Spatial Memory. Behavioural Brain Research, Volume 296, pp. 373–378
