Integrated Electrochemical Dopamine Sensing with Finger Priming Pump on a Chip
Corresponding email: yudan.whulanza@ui.ac.id
Published at : 30 Dec 2022
Volume : IJtech
Vol 13, No 8 (2022)
DOI : https://doi.org/10.14716/ijtech.v13i8.6140
Whulanza, Y., Antory, A.D., Warjito, W., Rahman, S.F., Gozan M., Utomo, M.S., Kassegne, S., 2022. Integrated Electrochemical Dopamine Sensing with Finger Priming Pump on Chip. International Journal of Technology. Volume 13(8), pp. 1735-1744
| Yudan Whulanza | 1. Department of Mechanical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, West Java 16424 Indonesia, 2. Research Center for Biomedical Engineering, Faculty of Engineerin |
| Abram Dion Antory | Department of Mechanical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, West Java 16424 Indonesia |
| Warjito Warjito | Department of Mechanical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, West Java 16424 Indonesia |
| Siti Fauziyah Rahman | Research Center for Biomedical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, West Java 16424 Indonesia 3Biomedical Engineering Study Program, Department of Electric Eng |
| Misri Gozan | 1. Research Center for Biomedical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, West Java 16424 Indonesia, 2. Department of Chemical Engineering, Faculty of Engineering, |
| Muhammad Satrio Utomo | National Research and Innovation Agency (BRIN), Tangerang Selatan, 15314, Indonesia |
| Samuel Kassegne | Department of Mechanical Engineering, College of Engineering, San Diego State University, San Diego 5500, USA |
Development in microfluidic technology has
contributed to increased understanding in neural tissue engineering through the
in vitro observation of cell-on-chip (CoC) systems. This has been further
helped by the integration with the broader MEMS (micro mechanical and
electromechanical systems) technology that offers external devices such as
detectors or biosensors to show the characteristics of the observed object. An
on-chip microsystem microfluidic platform for dopamine detection is presented
here. The microfluidic platform integrates electrochemical detection with
finger pumping and a valve system as means to control the fluid flow. This
microenvironment offers a quicker result in observing the phenomena related to
the neural cell activities with a relatively small specimen volume of 50-100
µL, eases the handling of movement, and consequently reduces the cost of
consumable items. The microfluidic platform presented here showed that the pump module that also serves
as a mixing point was able to deliver at maximum of 121.36 µL with 2-3 strokes
of normal finger pressure priming. A series of valves aids in the termination
or isolation of fluid flow in a specific zone for further processing.
Ultimately, the microfluidic platform is also equipped with a portable
electrochemical detection module that allows us to measure the dopamine
concentration up to 1 mM. This development showed that the on-chip testing of
dopamine could be conducted easier and be more portable to handle.
Cell-on-chip; Dopamine; MEMS; Neural tissue engineering; On-chip testing
The use of conductive carbon matrix to culture
neuronal cells has been demonstrated recently (Whulanza et al., 2022; Sagita
et al., 2018). To optimize neuron growth across the matrix, a
scaffold was used as a substrate to enhance nerve cell interaction in vitro.
The substrate, together with the in-vitro platform was analyzed for cellular
viability and electrically controlled and release of neuromodulators
(Nimbalkar et al., 2019; Vomero et al., 2018). The characterization of
neuromodulator, i.e., dopamine ions, in release and uptake studies on the
conducting substrate for neural application is ultimately the main interest of
this research. Successful optimization of these characteristics will be
important for treating injuries to the nervous system. Interactions between
neuron cells and extracellular matrix are key factors in studying cell
migration, proliferation, differentiation, and apoptosis, all of which are
critical functions for a neural-engineered construct (Pavesi et al., 2015; Kotwal &
Schmidt, 2001). These requirements are not fully understood by
researchers; therefore, study of the complete set of biological requirements
must be employed.
The availability of commercial
microfluidic devices such as microreactor systems is growing rapidly to carry
out various industrial and laboratory processes (Nadhif
et al., 2019; Nadhif et al., 2017; Whulanza et al., 2017). The system
typically integrates input processes, chemical reactions, outputs, and analyses
on a laboratory scale that are carried out continuously. Early developments in
microreactor technology mainly focused on the design of microreactor chips with
silicon, glass, or polymer materials such as PDMS (polydimethylsiloxane). The
design aims to add a small amount of reagent to the reaction zone in a volume
of microliters under controlled conditions. Such flows can be clearly observed
through the precise control of reaction parameters (Nunut
et al., 2020; Whulanza et al., 2019; Whulanza et al., 2014). This recent
advancement demonstrates the incorporation of significant microfluidic device
work into a functional Lab-on-Chip that addresses the required result in a
portable device with minimal laborious work.
The process of developing microfluidic systems
has resulted in successful product commercialization. However, some challenges
still lie ahead for a Lab-on-Chip (LoC). The most basic thing is in the concept
of end-to-end integration. But currently, off-device development research will
soon be grafted into a full LoC system. LoC has the same problem in the chip
world, namely the interface problem. A Micro-Electro-Mechanical-System (MEMS)
sensor package is needed in such a way that it can provide accurate
readings. Therefore, this preparation
system requires additional systems such as microscopes, pumps, computers, and
spectrometers (Charmet et al., 2020; Whulanza et al., 2015; Suwandi et al., 2014).
Finger-powered micropump has been widely
developed for variety of application, including operational of finger-actuated,
one-way, positive micropump for pathogens detection system (Qi et al., 2022; Jo et al., 2020),
finger-actuated, negative pressure chamber for electrochemical detection of
ascorbic acid (Liu et al., 2022) and
microreactor (Lee et al., 2022; Park et al., 2019; Whulanza et al., 2019). The
lab-on-a-chip that is being developed in this study is the integration of
microreactor processes such as cell culture or adsorption with the microfluidic
component, i.e., finger priming pump and manual valve. Ultimately, the dopamine
sensing package is also coupled with the portable electrochemical detection
module.
2.1. Design Consideration
The process of designing this lab-on-a-chip
device is based on previously introduced lab-on-a-chip devices, which miniaturized the space of a laboratory onto a single
75 mm x 25 mm x 6 mm chip. This current platform is designed to have a channel
width of 1 mm, with its larger contact surface area facilitating the mixing
process. To improve the practicality and flow conditions of our previously
designed 200 ?m
x 200 ?m
channel, the current channel is designed to have a width and height of 1 mm x 1
mm. There
are three main working divisions of this chip, which are the preparation
region, the pumping and mixing region, and the detection region.
The preparation
region contains two inlets and one intersection merging the inlets. The inlets
have a diameter of 1.68 mm to accommodate the Microfluidic Chipshop GmbH. Luer
connection, which is used to transfer fluid samples from a syringe pump or
micropipette onto the channel of the chip. Two inlets are designed to allow
simultaneous insertion of two samples, if necessary.
The pumping and mixing regions contain the
liquid sample reservoir, which serves also as the micropump, the meander
channel section, and a pneumatic valving mechanism. The reservoir pumping
section is a cylindrical space with 6.51 mm in diameter and 1.5 mm high. It is
also designed to accommodate the pushing of fingers. Since the microfluidic
chip is designed to be 2 mm thick, the ratio of thickness and diameter of
pumping reservoir is expected to be capable of pumping operation. The meander
section is designed to allow two liquid samples to interact and mix by means of
diffusion. Two pneumatic valve points are used to control fluid entering and
exiting the pumping and mixing region.
A reservoir covers the
area of three electrodes used in the electro-chemical detecting process in the
detection region. It has an 8.00 mm in diameter to accommodate the 3 working
electrodes designed for electro-chemical detection (Christian
et al 2022). On top of it lies the sample outlet twice the size of the
inlet.
2.2. Fabrication
There are four important steps to fabricate the lab-on-a-chip device:
mold building, polydimethylsiloxane (PDMS) casting and curing, silicone rubber
casting and curing, and product assembly. Mold is designed by using Solidworks
2016® software. To minimize the cost of production of the mold, FDM/FFF (fused
deposition modeling/fused filament fabrication) was the first method that was adopted,
but results show that DLP-SLA (digital light processing stereolithography) is a
better fit for the final prototype.
After
the mold was completed, PDMS substrate was added to the mold, which is PDMS
casting, along with a curing agent equal to 10% of the PDMS weight. The mold will
then be put into a vacuum chamber, as air is not wanted in the PDMS mix, which
may cause gas bubble formation for 45 minutes, Due to the mold's poor heat resistance, it will then be heated in a
heating bed for about 6 hours at 50°C before peeling. On the other hand,
similar steps were performed on silicone rubber casting, but it needs about 4%
of the curing agent. Also, silicone rubber casting uses a blank aluminum mold,
so heating it to 120? would not be a problem, thereby, speeding up the curing
process to about 1-2 hours.
The last step for lab-on-a-chip fabrication is assembling PDMS and
silicone rubber to become a single integrated chip. This process requires a
plasma bonder from Blackhole Lab., which will be used to stick two different
layers, so that they will become one piece of a device.
2.3. Flow Testing
This chip will be tested by its ability to contain and displace fluids, as
well as create space to be able to work side by side with the electro-chemical
detector. Three tests and three measurements will be conducted to see if the
chip is doing its designated job. A flow test will be done by inserting fluid into
the chip all the way from the inlet to the outlet to see how well those two
layers are attached together so that there will be no leakage. A valve test, on
the other hand, is needed to see how well the valves, are inspired by a water
gate- work by stopping fluid flow. A mixing test will be conducted to see if the
two fluids are mixed consistently.
Finger-pumping force measurement is done as a complementary to see how
much force human fingers exerted on the PDMS chip, which will be useful for the
next measurement. It is also done because of the fluctuation level on the force
gauge meter; hence, measurement will be done 100 times to see the average value
of a human finger's force. Force vs. volume measurement is the main thing to do
in this research because it shows the characteristics of the finger-pump that
is used to displace fluid. It is done by inserting a certain controlled amount
of fluid by using a 10-100 ?l micropipette, pumping that fluid, and extracting
the fluid on the electro-chemical detector section by using a syringe. The
fluid will then be put into micro-tubes to be weighed down, where the
difference between a liquid-inside-micro-tube and an empty micro-tube is the
weight of the water, and hence from its density, the volume of the water can be
found.
2.3.1. Measurement
of Dopamine Ions Using Electrochemical Detection
The dopamine concentration was monitored in situ by measuring the electrochemical detection of the specimen right after the pumping process. Here, the electrodes were connected to an open-source potentiostat (Rodeostat, Pasadena, USA) with a cyclic voltameter (CV) mode. The detection chamber included three electrodes that were screen printed together with the carbon matrix (Arafat et al., 2021; Istiyanto et al., 2019). During the CV tests, the working electrode potential alternated between ?1.0 and 1.0 V with a scan rate of 100 mV/s.
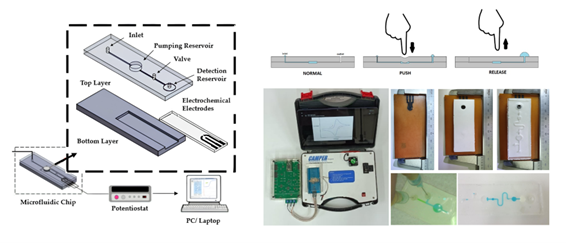
Figure
1 Experiment set-up and apparatus
Figure 2 a) Integration of an electrochemical biosensor, a
microfluidic platform, and a processor; (b) arrangement of carbon-based
electrodes on a PCB substrate in three steps; (c) the silicone rubber as base
layer for microfluidic platform as the inlet (d) top layer of microfluidic
platform from PDMS as pump priming with a stop valve.
3.1. Flow Test
The
flow test is a part of complementary tests to ensure that the chip does what it
is supposed to do, especially the most important one as it should not produce
any fluid leakage. The test uses a syringe pump,
which is set to 7.2 mL/h (a similar value to the peristaltic pump used on the mixing test
later). Strength-wise, the bonding of these two layers is excellent, and no
leakage is present as the fluid flows until the electro-chemical detector
section.
3.2. Valve Test
A
valve test is also included in complementary tests to ensure that two valves
work properly at the designated valve points. These valve points only exist on
the Trial 4 mold and are designed to be a perfect match dimension-wise to the
Microfluidic Chipshop Gmbh. Luer, whose original function is to insert a
syringe tip onto the chip. While using the Luer in place on the valve point, a
syringe pump begins to insert fluid at a normal rate of 7.2 mL/h.
3.3. Finger Pumping Measurement
Figure 3 a) Pressure measurement of finger pumping (n=100)
and b) the liquid delivered into the sensing zone
3.4. Force vs. Volume Measurement
The
characteristic of the finger pump used on this lab-on-a-chip device will be
shown here by how much volume of water is displaced from the mixing point to
the electro-chemical detector region to be extracted using a syringe., But
before any of that, there is one effect that is vital to the pump’s usage, which
is the flow’s velocity difference. Below is the different velocity caused by
pumping the mixing point region of the chip while fluid is constantly flowing.
The
velocity generated by a 7.2 mL/h flow from the syringe pump is 1.11 mm/s; it is measured by
measuring a certain linear distance on the chip and how much time is needed for
the water to reach out that distance. The next step is to measure the linear
channel after the mixing point region and determine how much time is needed for
it to cover that distance. After 4 measurements, the velocity generated by
pumping is 24.00 mm/s with a standard deviation of 2.79. On average, the
velocity rise made from this measurement is about 22.89 mm/s.
Figure
4a shows the mapping of points along the pumping region, which corresponds, to
the x-axis of Figure 4b. Point 5 and 6 are the points where pumping is
happening, and after that, the velocity rises as in Figure 4b. Moving on to the
next task, which is to see how much pressure, is needed to displace a certain amount
of fluid, Pumping it once will not get the job done, so on average, based on
this measurement, it took about 4-5 pumps to displace fluid to the
electro-chemical detector region. 30 measurements are taken to determine how
the data differs from one another in order to generate a linear graph on its
own. Another thing to consider is how well the micropipette works, which in
this case is not so precise. Its 100 ?L of liquid reading is equivalent to an average of 121.36 ?L of liquid by 4 measurements with
a standard deviation of 2.46. It is critical to compare the percentage of
liquid extracted to the percentage of liquid inserted.
From
Figure 3b, the total volume delivered by the pump is extracted when the fluid
is in the electro-chemical detector zone and is extracted by a syringe. The
maximum average volume delivered is 102.31 ?L in class 130 – 135 N, and the
minimum average volume delivered is 83.75 ?L in 90 – 95 N class. This
represents a directly proportional relationship force and volume.
Figure
3b shows a histogram of displaced volume distribution based on the experimental
data. As much as 36.67% out of 30 measurements are in the 90 – 95 class. The
second highest frequency of displaced volume is in the 85 – 90
and 95 – 100
class with 20% each, as it is combined for 76.67% of the total in the 85 –
100
range.
Figure 4 a) The position of measuring point in the pumping
zone and b) measurement of velocity in the pumping zone
3.5. Dopamine Detection Using in Situ Measurement
Figure 5 Electrochemical detection plot result
Figure 5(b) shows that the peak dopamine concentration
was linearly dependent on the concentration that was prepared (from 0.05 mM to
1 mM). Figure 5(b) also shows the regression plot of the
dopamine solution, which is expressed as I (µA) = 2.7752*Concentration
Dopamine (µM) + 0.0742 with a Pearson correlation of 0.986. The sensitivity and
linear detection range of the dopamine solution were specifically calculated
at 0–1 mM. It can be estimated that the limit of detection was around 0.20 mM.
The linear model is used since the most
suitable model for measurement or detection is when the parameters are linearly
related. The linearity analysis suggested that the measurement using this setup
has a plausible result provided by the calculated detection limit. The margin
error compared to the linear model showed a lowest value of 0.3% at a
concentration of 1 mM, whereas at a concentration of less than 0.1 mM showed an
error of 24%. his high
error was consistent with the calculated limit detection at 0.2 mM. Hence, this
finding showed that our setup enables us to work with a dopamine detection
range of 0.2 – 1.0 mM.
It can be concluded from this research that the appropriate rapid
prototyping method for the current chip mold is DLP-SLA. Although its
production cost is more expensive than FFF, the result is much better. DLP-SLA
shows a little deviation among the design, the mold, and the product. Moreover,
combining PDMS and silicone rubber is a perfect match to create one whole
lab-on-a-chip. For the channel size, a 1 mm x 1 mm channel produces a good
mixing with two simultaneous flows. For the finger pump, it creates a rise of
around 22.89 mm/s to the fluid flow velocity. Overall fluid displacement can be
concluded by an average of 75.43% of liquid being displaced from the mixing
point region.
Authors gratefully thanks for the
funding from Kementerian Pendidikan, Kebudayaan, Riset, dan Teknologi through
Penelitian Dasar Unggulan Perguruan Tinggi (PDUPT) 2022 No.
NKB-860/UN2.RST/HKP.05.00/2022.
Arafat, Y.B.,
Whulanza, Y., 2021. Realizing Textured Electrode for Electrochemical Biosensor
Using Homemade CNC Desktop. In: AIP Conference Proceedings, Volume 2344(1),
p. 050019
Ahmed, I.,
Sullivan, K., Priye, A., 2022. Multi-Resin Masked Stereolithography (MSLA) 3D
Printing for Rapid and Inexpensive Prototyping of Microfluidic Chips with
Integrated Functional Components. Biosensors, Volume 12, pp. 1–17
Charmet, J.,
Rodrigues, R., Yildirim, E., Dallmann, R., Whulanza, Y., 2020. Low-Cost Microfabrication
Toolbox. Micromachines, Volume 11(2), p. 135
Christian,
E.T.H., Basari, B., Rahman, S.F., Whulanza, Y., 2022.
Electrochemical Detection and Spectrophotometry of Dopamine using Commercial
Screen-Printed Electrodes. Journal of Biomedical Science and Bioengineering, Volume 2(1), pp. 1–9
Feng, Q.M., Cai, M., Shi, C.G., Bao, N.,
Gu, H.Y., 2015. Integrated Paper-Based Electroanalytical Devices for
Determination of Dopamine
Extracted from Striatum of Rat. Sensors & Actuators: B. Chemical,
Volume 209, pp. 870–876
Istiyanto, J.,
Lubis, H.F., Adhitama, G., Fadhilah, S., Whulanza, Y., Kassegne, S.M., 2019. Beehive Texturing of Carbon Electrode Biosensor Using Screen Printing
Method. In: AIP
Conference Proceedings, Volume 2092,
p.
020010
Jo, Y., Park,
J., Park, J.K., 2020. Colorimetric Detection of Escherichia coli O157:H7 with
Signal Enhancement Using Size-Based Filtration on a Finger-Powered Microfluidic
Device. Sensors, Volume 20, pp. 1–10
Kotwal, A., Schmidt, C., 2001. Electrical Stimulation Alters Protein Adsoption and
Nerve Cell Interactions with Electrically Conducting Biomaterials. Biomaterials, Volume 22(10), pp. 1055–1064
Hardi, G.W.,
Rahman, S.F.,
2020. Amperometric Detection of Dopamine based on a Graphene Oxide/PEDOT: PSS
Composite Electrode. International Journal of Technology, Volume 11(5), pp.
974–983
Lee, Y.S., Lu,
Y.T., Chang, C.M., Liu, C.H., 2022. Finger-powered Cell-Sorting Microsystem
Chip for Cancer-Study Applications. Sensors & Actuators: B. Chemical,
Volume 370, pp. 1–9
Liu, C., Gomez, F.A., Miao, Y., Cui, P.,
Lee, W., 2019. A Colorimetric Assay System for Dopamine Using Microfluidic
Paper-Based Analytical Devices. Talanta, Volume 194, pp. 171–176
Liu, X., Li, M.,
Zheng, J., Zhang, X., Zeng, J., Liao, Y., Chen, J., Yang, J., Zheng, X., Hu,
N., 2022. Electrochemical Detection of Ascorbic Acid in Finger-Actuated
Microfluidic Chip. Micromachines, Volume 13, pp. 1–13
Liu, Z., Jin, M., Cao, J., Niu, R., Li,
P., Zhou, G., Yu, Y., van den Berg, A., Shui, L., 2018. Electrochemical Sensor
Integrated Microfluidic Device for Sensitive and Simultaneous Quantification of
Dopamine and 5-Hydroxytryptamine. Sensors & Actuators: B. Chemical,
Volume 273, pp. 873–883
Maminski, M., Olejniczak, M., Chudy, M.,
Dybko, A., Brzozka, Z., 2005. Spectrophotometric Determination of Dopamine in
Microliter Scale Using Microfluidic System Based on Polymeric Technology. Analytica
Chimica Acta, Volume 540, pp. 153–157
Manbohi, A., Ahmadi, S.H., 2019. Sensitive and Selective Detection of
Dopamine Using Electrochemical Microfluidic Paper-Based Analytical Nanosensor. Sensing
and Bio-Sensing Research, Volume 23, pp. 1–7
Nadhif, M.H., Whulanza, Y., Istiyanto,
J., Bachtiar, B.M., 2017. Delivery
of Amphotericin B to Candida albicans by using biomachined lab-on-A-chip. Journal of Biomimetics, Biomaterials
and Biomedical Engineering, Volume 30, pp.
24–30
Nadhif, M.H., Hadiputra, A.P., Utomo,
M.S., Whulanza, Y., 2019.
Fabrication and Characterization of an Affordable Conditioned Bio-Specimen
Transporter (Conbiport) for Urban Areas. International Journal of Technology, Volume 10(8), pp. 1626–1634
Nimbalkar, S., Castagnola, E.,
Balasubramani, A., Scarpellini, A., Samejima, S., Khorasani, A., Boissenin, A.,
Thongpang, S., Moritz, C., Kassegne, S., 2018. Ultra-capacitive Carbon Neural
Probe Allows Simultaneous Long-Term Electrical Stimulations and High-Resolution
Neurotransmitter Detection. Scientific Reports, Volume
8(1), p. 6958
Nunut, I., Whulanza, Y., Kassegne, S.,
2020. Testing of Beeswax Printing Technology in the Design of a Paper-Based
Microfluidic System. International Journal of
Technology, Volume 11(5), pp. 1036–104
Park, J., Roh,
H., Park, J.K., 2019. Finger-Actuated Microfluidic
Concentration Gradient Generator Compatible with a Microplate. Micromachines, Volume 10(174), pp. 1–10
Pavesi, A., Adriani, G., Rasponi,
M., Zervantonakis, I.K., Fiore, G.B., Kamm, R.D., 2015. Controlled Electromechanical Cell Stimulation On-A-Chip. Scientific
Reports,
Volume 5(1), p. 11800
Rahman, S.F.,
Min, K., Park, S.H., Yoo, J.C., Park, D.H., 2016. Selective Determination of Dopamine with
an Amperometric Biosensor Using Electrochemically Pretreated and Activated
Carbon/Tyrosinase/Nafion®-Modified Glassy Carbon Electrode. Biotechnology and
Bioprocess Engineering, Volume 21(5), pp. 627–633
Qi, W., Zheng,
L., Hou, Y., Duan, H., Wang, L., Wang, S., Liu, Y., Li, Y., Liao, M., Lin, J.,
2022. A Finger-Actuated Microfluidic Biosensor for
Colorimetric Detection of Foodborne
Pathogens. Food Chemistry,
Volume 381, pp. 1–9
Sagita, I.D.,
Whulanza, Y., Dhelika, R., Nurhadi, I., 2018.
Designing Electrical Stimulated Bioreactors for Nerve Tissue Engineering. In: AIP
Conference Proceedings, Volume 1933, p. 040019
Vomero, M.,
Oliveira, A., Ashouri, D., Eickenscheidt, M., Stieglitz, T., 2018. Graphitic Carbon
Electrodes on Flexible Substrate for Neural Applications Entirely Fabricated
Using Infrared Nanosecond Laser Technology. Scientific Reports, Volume 8, p. 14749
Whulanza, Y.,
Aditya, R., Arvialido, R., Utomo, M. S., Bachtiar, B. M., 2017. Ease Fabrication of PCR Modular Chip for
Portable DNA Detection Kit. In: AIP Conference Proceeding 1817, pp. 040006.
Whulanza Y., Arafat, Y.B., Rahman, S.F., Utomo, M.S., Kassegne, S.K., 2022. On-chip
Testing of a Carbon-based Platform for Electro-adsorption of Glutamate. Heliyon, Volume
8(5), pp. 1–10
Whulanza, Y.,
Hakim, T.A., Utomo, M.S., Irwansyah, R., Charmet, J., 2019.
Design and Characterization of Finger-Controlled Micropump for Lab-on-a-Chip
Devices. Evergreen, Volume 6(2), pp. 108–113
Whulanza, Y.,
Sitanggang, T., Istiyanto, J., Supriadi, S, 2015. Seedless-Electroplating Process
Development for Micro-Features Realization. International Journal of
Technology,
Volume 6(6), pp. 1050–1056
Suwandi, D., Whulanza, Y., Istiyanto, J., 2014. Visible Light Maskless
Photolithography for Biomachining Application. Applied Mechanics and Materials, Volume 493, pp. 552–557
Whulanza, Y.,
Utomo, M.S. Hilman, A., 2018. Realization of a passive micromixer using
herringbone structure. In: AIP Conference
Proceedings 1933, pp. 040003
Whulanza, Y.,
Widyaratih, D.S., Istiyanto, J., Kiswanto, G., 2014. Realization and testing of lab-on-chip
for human lung replication. ARPN Journal of Engineering and Applied Sciences,
2014, 9(11), pp. 2064–2067
Yu, Y., Shamsi, M.H., Krastev, D.L.,
Dryden, M.D.M., Leung, Y., Wheeler, A.R., 2016. A Microfluidic Method for
Dopamine Uptake Measurements in Dopaminergic Neurons. Lab on a Chip,
Volume 16, pp. 543–552
Yue, Y., de Campos, R.P.S., Hong, S.,
Krastev, D.L., Sadanand, S., Leung, Y., Wheeler, A.R., 2019. a Microfluidic
Platform for Continuous Monitoring of Dopamine Homeostasis in Dopaminergic
Cells. Microsystems & Nanoengineering, Volume 5(10), pp. 1–13
